Abstract
Classical Hodgkin lymphoma (HL) relapses after or is refractory to upfront multiagent chemotherapy in 20%–30% of patients. Effective salvage therapy for relapsed or refractory HL is limited, and advancements are needed. Brentuximab vedotin (BV), an anti-CD30 antibody–drug conjugate, has demonstrated significant activity and manageable toxicities in advanced HL. Currently approved as a monotherapy for patients with HL that is relapsed or refractory to multiple lines of chemotherapy or autologous stem cell transplantation, BV is now being evaluated earlier in the course of disease and in combination with other therapies. This review discusses the successful translation of BV from its conception to the clinical setting and highlights ongoing trials that may ultimately expand its role in relapsed or refractory HL and improve outcomes for patients.
Learning Objectives
BV has expanded the therapeutic options for relapsed or refractory Hodgkin lymphoma
Ongoing studies will refine the role of BV in combination regimens and over the course of relapsed or refractory Hodgkin lymphoma
Introduction
Classical Hodgkin lymphoma (HL) represents one of the major success stories in malignant hematology, yet the treatment of relapsed or refractory (RR) disease remains a significant challenge. Less than one-half of patients with RR HL are cured with conventional salvage chemoradiotherapy followed by high-dose therapy and autologous stem cell transplantation (auto-SCT).1 For those who are not candidates for auto-SCT or experience posttransplantation relapse, options have typically been limited to palliative chemotherapy. Brentuximab vedotin (BV) has recently been proven beneficial in this setting and thus has been added to available therapeutic options; its ongoing study is toward identifying additional roles across stages of RR HL and in combination regimens. This review covers the initial data supporting the approval of BV and discusses the novel applications of this agent for patients with RR HL.
Background
Mechanism of action of BV
BV's origin lies with the identification of CD30, a cell membrane protein that in healthy individuals has limited expression outside of activated T and B lymphocytes.2 CD30 is aberrantly expressed on certain virally infected cells and several types of malignancies, including HL Reed-Sternberg cells. It has long been recognized as an attractive therapeutic target due to this differential expression in health and disease. Pharmaceutical targeting of CD30 dates back more than 2 decades and culminated with the synthesis of the antibody–drug conjugate BV.3 BV is a CD30-specific chimeric monoclonal antibody covalently coupled to several molecules of highly toxic payload, the antimitotic tubulin-inhibitor monomethyl auristatin E (MMAE). After BV's target-cell binding and internalization, the dipeptide linker is cleaved through lysosome-mediated proteolysis and MMAE is released into the cytoplasm, where it is active in its naked form and rapidly induces apoptosis.4,5 A small fraction of MMAE may diffuse into the immediate neighborhood of Reed-Sternberg cells, potentially killing tumor-supportive cells.6 The consequent release of cytokines and inflammatory factors is thought to render a further, systemic, immune-mediated antitumor response.7
The mechanism(s) of RR HL resistance to BV has yet to be elucidated. Nathwani et al examined tumor expression of CD30 in 2 patients before exposure to BV and after documented disease progression.8 In both cases, CD30 expression persisted, arguing against the loss of CD30 expression conferring resistance to BV.
Safety, toxicity, and dosing of BV
The first human trial of BV was a landmark phase 1 study in 45 patients (42 of whom had RR HL) with CD30-positive malignancies.7 A standard 3 + 3 dose-escalation scheme was used to assess the safety profile and maximal tolerated dose (MTD). Doses were increased stepwise from <1.2 mg/kg (n = 16) to 3.6 mg/kg (n = 1) and delivered once every 3 weeks. Pharmacokinetic analysis showed that the maximum concentration occurred immediately after infusion for the antibody–drug conjugate and at ∼2-3 days for the MMAE. Steady-state pharmacokinetics for both components was observed by ∼21 days, supporting the 21-day dosing schedule. Predominant observed toxicities were grade 1-2 in severity and included fatigue, pyrexia, diarrhea, nausea, neutropenia, and neuropathy, resulting in dose delays in 36% of subjects; the MTD was determined at 1.8 mg/kg every 3 weeks. Tumor regression was observed in 39 of 45 treated patients, with 17 classified as having an objective response (OR) including 11 complete responses (CRs). These highly promising phase 1 safety and efficacy results warranted further testing of BV in HL.
Subsequent use of BV in HL and other CD30-positive malignancies has borne out its relatively favorable safety profile. Of the more common and mild toxicities mentioned in the previous paragraph, the most clinically significant is neuropathy, which has been found to be dose dependent and is generally cumulative. It is thought to be due to MMAE's potent antitubulin properties on distal neurons. Peripheral sensory neuropathy is observed in up to 50% of patients, with <10% experiencing grade 3 symptoms; peripheral motor neuropathy is seen in ∼10% of patients, with <5% experiencing grade 3 symptoms. Cessation of therapy leads to complete resolution of neuropathy in approximately one-half and partial improvement in an additional one-third of individuals; dose delay or reduction therefore can be attempted in the event of mild symptoms. Additional rare but serious adverse effects of BV have included severe dermatologic reactions and there have been several case reports of acute pancreatitis and progressive multifocal leukoencephalopathy.9,10 Although the pathogenic mechanisms underlying the development of these potentially fatal complications is not clear, it has been speculated that off-target effects on normal cells expressing CD30 may be responsible, including low-level expression in the pancreas and, in the cases of progressive multifocal leukoencephalopathy, a depletion of T cells enabling reactivation of the JC polyoma virus in the CNS.9,10 Retrospective analysis of BV's tolerability in older patients showed increased frequency of treatment-related anemia (30% versus 10%), and trends toward increased rates of neuropathy (60% versus 46%), fatigue (58% versus 43%), and adverse events >grade 2 (70% versus 56%); overall, its toxicity profile is deemed acceptable in patients >60 years old and with relatively more comorbidities.11
BV after auto-SCT
Treatment of recurrent disease
In a pivotal phase 2, multinational study, the efficacy and safety of BV was evaluated in patients with HL that relapsed after auto-SCT (Table 1).12 A total of 102 medically fit patients with histologically proven CD30-positive HL were treated with BV at a dose of 1.8 mg/kg by intravenous infusion every 3 weeks for up to 16 doses. The median age of participants was 31 years. Seventy-one percent of patients had experienced relapse within a year of auto-SCT, and the median number of prior chemotherapy regimens in addition to auto-SCT was 3.5. In this cohort of heavily pretreated patients, tumor regression was seen in 94%, with an overall response rate (ORR) of 75%, and a median progression-free survival (PFS) of 5.6 months. A median of 9 cycles of BV was administered. Median PFS was 21.7 months in the 34% of patients who experienced a CR, in contrast to 5.1 months in the 40% of patients with a partial response (PR), indicating the potential impact of remission quality on outcome. A subset of patients in the pivotal trial was treated with additional BV on a treatment-extension study. Fifteen patients continued to receive BV after the 16-cycle course for a median of 19 cycles (range 17-29). No additional safety signals were observed.13
Selected studies of BV for HL after auto-SCT
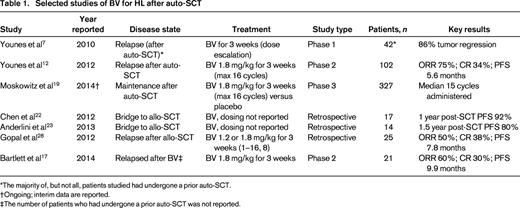
*The majority of, but not all, patients studied had undergone a prior auto-SCT.
†Ongoing; interim data are reported.
‡The number of patients who had undergone a prior auto-SCT was not reported.
The pivotal trial's results led to the accelerated approval in August of 2011 by the U.S. Food and Drug Administration of BV as a single agent in HL after failure of auto-SCT or after failure of at least 2 prior multiagent chemotherapy regimens in patients who are not candidates for auto-SCT. A more recent update of these data at the American Society of Hematology annual conference in 2013 reported that, with a median of 3 years of follow-up, 14 of the 102 patients remained without evidence of disease recurrence; 9 of the 14 had not received any additional anticancer therapy and 5 of the 14 underwent consolidative allogeneic SCT (allo-SCT).14 These encouraging data suggest that single-agent BV may yield long-term remissions in a substantial minority of patients with RR HL.
Since the FDA approval, several published reports have described success comparable to that seen in the pivotal phase 2 study. A retrospective analysis of 65 patients with RR HL treated in nontrial settings at multiple Italian centers showed an ORR of 71%, a CR of 22%, and a total of 9 patients who continued in CR at the time of publication, at which point median follow-up was 14 months (range 10-19).15 The German Hodgkin Study Group reported on 45 patients with RR HL that were treated with BV either in a named patient program or in the context of a safety study.16 Of these, 87% had received prior auto-SCT or allo-SCT and 18% had an Eastern Cooperative Oncology Group performance status of 2. In this cohort of patients with unfavorable prognostic factors, similar rates of OR (60%) and CR (22%) were observed.
Bartlett et al recently reported the results of a phase 2 study of 21 patients with RR HL who were retreated with BV after responding to a first course.17 After a median interval period of 8 months (range 2-45) from completion of the first course of BV and an intervening systemic treatment in 6 patients, BV was re-administered at standard dosing (unless dose reduction had been required during the first course). Twelve of 20 evaluable patients (60%) had an objective response to retreatment with BV; the median PFS was 9.9 months. The observed toxicity profile was comparable to that seen for an initial course, but with a higher rate of peripheral neuropathy (69% of all patients treated). Therefore, retreatment with BV provides a reasonable palliative option for patients with RR HL that responded to a first course and requires careful monitoring for the development or exacerbation of peripheral neuropathy.
Toward refining the use of BV in RR HL, Kahraman et al evaluated the prognostic ability of interim fluorescein di-β-D-galactopyranoside (FDG)-positron emission tomography (PET)/computed tomography (CT) imaging.18 In their retrospective study of 12 consecutive patients, an interim FDG-PET/CT performed after 2-5 cycles of BV and scored visually using a 5-point scale effectively stratified outcomes by 1-year PFS, with a 100% 1-year PFS observed in those with a negative interim scan compared with a 38% 1-year PFS in those with a positive interim scan (P = .03). These data parallel findings from the pivotal trial suggesting that CRs are far more durable than lesser responses and tend to occur early in the treatment course. Nevertheless, in the palliative setting, continuous 3 week dosing of BV for RR HL is considered standard until disease progression, unacceptable toxicity, or a maximum of 16 cycles.
Maintenance therapy after auto-SCT
It has been hypothesized that BV given as maintenance therapy after auto-SCT will reduce the rate of relapse in high-risk patients at the expense of manageable toxicity. The Aethera trial is an ongoing phase 3, randomized, double-blind, placebo-controlled, multicenter study addressing this possibility. HL patients deemed high risk of relapse after auto-SCT (refractory to frontline therapy, first relapse within 1 year of frontline therapy, or extranodal disease at relapse) receive either BV at 1.8 mg/kg every 3 weeks or placebo for up to 16 cycles. A planned interim safety and futility analysis was performed in late 2012 and reported at the BMT Tandem meetings in 2014.19 A total of 327 of 329 enrolled patients received study treatment. A median of 15 treatment cycles were delivered (range 1-15), with 49% receiving all 16 cycles. Sixty-one (19%) patients discontinued treatment due to adverse events. Thirty-five (11%) patients had died at the time of the analysis, with 31 deaths occurring after disease progression. The independent data monitoring committee has recommended continuation of the trial according to protocol and results are anticipated soon.
Bridge therapy before allo-SCT
In the ∼50% of patients with RR HL who suffer relapse after auto-SCT, OS at 5 years is <30%. In this extremely high-risk population, allo-SCT can be curative, but its success is highly dependent on the presence of active disease at transplantation.20,21 Therefore, improving disease control before allo-SCT consolidation may result in improved long-term outcomes. Chen et al retrospectively identified 17 patients treated at the City of Hope and the Seattle Cancer Care Alliance/Fred Hutchinson Cancer Research Center with RR HLwho relapsed after an auto-SCT and received salvage therapy with BV before consolidative allo-SCT with reduced-intensity conditioning.22 The OS at 1 year after allo-SCT was 100% and rates of acute and chronic GVHD of 28% and 56%, respectively, were observed, suggesting that BV has no obvious impact on allo-SCT toxicity and may provide sufficient disease control to enable reduced-intensity conditioning allo-HCT. Several additional retrospective studies have similarly reported a trend toward improved rates and durability of CR after allo-SCT in which BV was used in the bridge-to-transplantation setting.23-25 Although promising, the majority of patients described in these retrospective analyses have had relatively short follow-up times to date and extended follow-up is needed.
BV after allo-SCT
Relapse after an allo-SCT for RR HL occurs in up to 50% of patients and carries an exceedingly poor prognosis, with a median OS estimated at <3 years26,27 ; therefore, there is a great need for novel and effective therapies in this population. A retrospective analysis examined 25 patients with RR HL who relapsed after allo-SCT and were subsequently treated with BV as part of 3 nonrandomized, multicenter, international trials.28 Patients with possibly the highest-risk disease, namely those who were within 100 days after transplantation or who had coincident active GVHD with relapsed disease, were excluded. The median interval time between allo-SCT and the first dose of BV was 42 months and the median total number of prior treatment regimens was 9. BV was given at protocol-specific doses of 1.8 mg/kg (n = 19) or 1.2 mg/kg (n = 6) every 3 weeks, with 4 of the 6 patients in the 1.2 mg/kg cohort escalated to 1.8 mg/kg dosing. In the 24 patients evaluable for response, the ORR was 50% and a CR was achieved in 9 (38%). An additional 10 (42%) had stable disease, with 2 (8%) having progressive disease (PD). The median PFS was 7.8 months and the median OS was not reached at the time of publication. Observed toxicities were similar to those reported previously in separate, larger studies, although more patients had adverse events that led to discontinuation of study treatment or were grade 3 or higher. In addition, cytomegalovirus reactivation was noted in 5 patients. BV may therefore serve a palliative role for patients with HL relapsed after allo-SCT and without active GVHD.
The use of BV in the post-allo-SCT setting is also being explored from the perspective of managing GVHD. Preclinical data suggest that CD30 signaling regulates T-cell-mediated induction of GVHD.29 In a recent study from the German Hodgkin Study Group, 4 patients with HL relapsed after allo-SCT were administered BV as part of a compassionate-use program.30 In an effort to augment a GVL effect, 3 of the 4 subjects were administered BV at 1.8 mg/kg, followed by donor-lymphocyte infusions (DLIs) every 3 weeks for 4 cycles, and then BV alone every 3 weeks thereafter. One of the 4 patients had active GVHD at the time of treatment and so was not deemed a candidate for DLI. Clinical responses were observed in all 4 patients, with a median duration of disease control of 349 days. In the patients who received both BV and DLI, acute GVHD occurred in all 3 and ranged from mild to severe. Any role for BV modulating GVHD and/or GVL therefore remains to be elucidated and, indeed, BV is currently being evaluated in early-phase clinical trials of both the treatment and prevention of GVHD after allo-SCT for various hematologic malignancies.
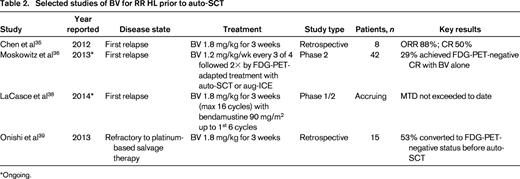
BV use before auto-SCT
Initial salvage therapy
In the 20%–30% of patients with HL who relapse after or are refractory to first-line therapy, response to salvage chemotherapy based on functional imaging assessment predicts the long-term success of auto-SCT.31,32 Retrospective data show that, at the time of auto-SCT, FDG-PET-negative status, defined as FDG uptake less than the mediastinal background, translates into a 5-year posttransplantation OS of 90% compared with just 55% in those for whom FDG-PET normalization is not achieved.33 Moreover, a CR by FDG-PET predicts a favorable response to auto-SCT even after a second salvage regimen.34 Therefore, the incorporation of BV into salvage regimens is being studied in the hope of both lowering exposure to cytotoxic drugs and enhancing the rate of attaining CR in advance of auto-SCT (Table 2).
Chen et al reported a consecutive case-series of 11 patients with RR HL that received BV for first-line salvage, either on an expanded access protocol (n = 3) or on a prospective phase 2 study (n = 8).35 In the 8 patients evaluable for response by PET/CT at the time of the report, an OR was seen in 7 and a CR in 4. Six of the 11 patients had proceeded to auto-SCT, with all 6 patients achieving a CR after transplantation; the remaining 5 patients were undergoing stem cell mobilization or had not completed BV at the time of the report. The prospective phase 2 study continues to enroll patients and additional data are anticipated to be presented later this year.
These encouraging data led to additional prospective evaluation of BV as first-line salvage therapy. Moskowitz et al designed a FDG-PET-adapted treatment strategy for patients with HL relapsed or refractory to 1 prior regimen.36 Two cycles of dose-dense BV (given 1.2 mg/kg weekly for 3 of 4 weeks) was administered as upfront salvage therapy. Restaging FDG-PET imaging was then performed and subsequent treatment adapted accordingly: those who achieved FDG-PET normalization (Deauville score <3) proceeded directly to auto-SCT, whereas those who showed persistent FDG-PET-positive disease received additional chemotherapy with augmented ICE (ifosfamide + carboplatin + etoposide). Interim data from the study were presented at the 2013 ASH Annual Conference and subsequently updated.37 Of 42 patients enrolled, 12 (29%) achieved a CR by FDG-PET after salvage BV alone and 11 of these proceeded directly to auto-SCT. The remaining 30 patients with PET-positive disease after BV received 2 cycles of augmented ICE and, of these, 21 (70%) achieved a CR and then proceeded to auto-SCT. Eight patients did not achieve a CR after augmented ICE and proceeded to further therapy and 1 was lost to follow-up. These results indicate that a FDG-PET-adapted sequential strategy in RR HL using single-agent BV in the upfront salvage setting may protect a subset of patients from receiving conventional cytotoxic chemotherapy and exposure to its cumulative toxicities before auto-SCT, although with the potential for spending additional time receiving therapy for those who do not achieve a CR with BV. Maturation of these data will help to establish the role of BV in this setting.
Combinations with standard salvage chemotherapy
The addition of BV to conventional salvage chemotherapy regimens is being actively investigated. Interim results were presented recently from a multicenter phase 1/2 study of bendamustine combined with BV in first-relapse RR HL.38 Of the 6 patients treated thus far, a maximum of 4 cycles of the combination regimen have been administered and all patients remain on treatment with no dose-limiting toxicities observed. CR has been achieved in all 5 patients assessed for response. Several additional trials are combining BV with conventional chemotherapy regimens in the first-relapse RR HL setting, including gemcitabine + BV in an ongoing phase 1/2 trial in pediatric and young adult patients (NCT01780662), DHAP (dexamethasone + cytarabine + cisplatin) plus BV in an ongoing European multicenter phase 1/2 trial (EudraCT 2012-003097-45), and, in the near future, ICE + BV at our center.
Treatment of platinum-refractory disease
Onishi et al retrospectively evaluated the use of BV in transplant-naive patients relapsed after or refractory to platinum-based salvage chemotherapy.39 Fifteen patients were identified and their responses to platinum-based salvage therapy was categorized as a PR (n = 3), SD (n = 9), and PD (n = 3); all had FDG-PET-positive disease. After receiving a median of 2 cycles of BV, 8 of the 15 (53%) patients converted to FDG-PET-negative status and an additional patient achieved a PR. Of the 8 cases in which BV achieved FDG-PET normalization, none occurred in patients with PD after the prior platinum-based regimen, suggesting that this approach may be less effective in the most chemotherapy-resistant tumors. At the time of the analysis, 13 of 15 patients had proceeded to auto-SCT and 14 of 15 were alive at a median follow-up of 1.3 years. These data support BV as a potential second salvage option to achieve FDG-PET-negative CR, but longer follow-up will be needed to assess the durability of post-ASCT remissions after BV salvage.
Future investigation of BV in RR HL
Ongoing and future trials are aimed at identifying combinations of BV with other novel therapies in RR HL that result in synergistic activity and are well tolerated. Small phase 1 studies for patients with RR HL combining BV with the immune-modulatory anti-CTLA-4 antibody ipilimumab (NCT01896999) and the mTOR-inhibitor temsirolimus (NCT01902160) are in early stages of accrual.
Another area of active research is optimizing the dosing schedule of BV. A phase 1 dose-escalation study in 44 patients with RR CD30-positive hematologic malignancies, 38 of whom had HL, used a dose-dense schedule of BV on days 1, 8, and 15 of a 28-day schedule.40 The MTD was 1.2 mg/kg and the observed rates of efficacy and toxicity were similar to those shown in the standard, every 21-day dosing schedule. This dose-dense strategy was adopted in the ongoing PET-adapted phase 2 study in the first salvage setting and, as described above, has produced promising results to date. It is also being evaluated at the University of Washington/Fred Hutchinson Cancer Research Center as a method to overcome resistance to standard 3-week dosing of BV.
Conclusions
Since its original description by Dr. Thomas Hodgkin in 1832, HL has represented a paradigm for incremental advances in oncology therapies. The antitumor properties of radiation were identified in HL as early as 1902; cure of advanced stage disease with combination chemotherapy was demonstrated in 1964; combined modality therapy with chemotherapy followed by radiation was shown to have increased cure rates and reduced toxicities in studies during the 1990s. BV adds to this legacy of HL and reflects the potential of translational bench-to-bedside research and the future of targeted biological therapies that spare the toxicities of multiagent chemotherapy and radiation.
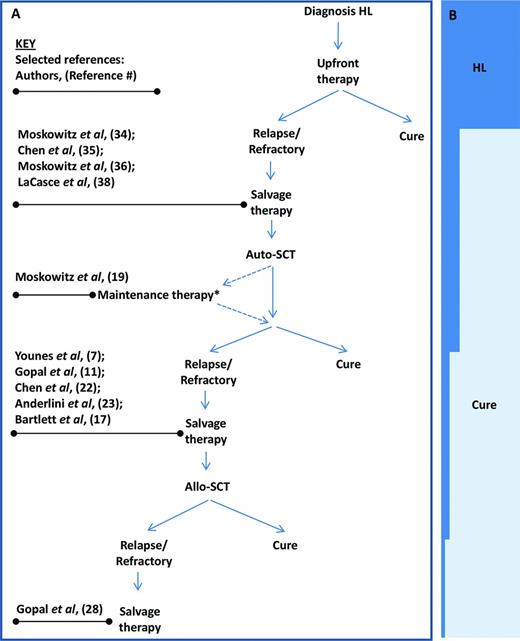
BV is presently FDA approved as monotherapy in HL after failure of auto-SCT or after failure of at least 2 prior multiagent chemotherapy regimens in patients who are not candidates for auto-SCT. It is anticipated that, in the near future, its role in HL will be expanded, possibly to the upfront, first-line salvage, and post-auto-SCT maintenance-therapy settings (Figure 1). Its combination with other cytotoxic and targeted therapies is currently under exploration.
Selected studies of BV informing treatment of RR HL at different disease stage. (A) Algorithm of disease stages of RR HL. *Maintenance therapy is currently under investigation and not standard of care. (B) Relative proportion (approximate) of patients achieving cure.
Selected studies of BV informing treatment of RR HL at different disease stage. (A) Algorithm of disease stages of RR HL. *Maintenance therapy is currently under investigation and not standard of care. (B) Relative proportion (approximate) of patients achieving cure.
Disclosures
Conflict-of-interest disclosures: A.K.G. has received research funding from Spectrum, Gilead, BMS, Pfizer, Janssen, Takeda, Seattle Genetics, and Teva; has consulted for Pfizer and Seattle Genetics; has received honoraria from Takeda and Seattle Genetics; and has been affiliated with the speakers' bureau for Takeda and Seattle Genetics. S.A.G. declares no competing financial interests. Off-label drug use: BV for the treatment of HL.
Correspondence
Ajay K. Gopal, MD, 825 Eastlake Ave E, Seattle, WA 98109; Phone: 206-288-2037; Fax: 206-288-1130; e-mail: agopal@u.washington.edu.