Abstract
Pediatric patients with newly diagnosed, non-Hodgkin Lymphoma (NHL) have an excellent overall survival. However, therapy regimens are associated with acute toxicity and late effects. Furthermore, patients with relapsed or refractory disease have relatively few options with proven clinical benefit. Both histologic and molecular differences exist between adult and pediatric NHL preventing simple translation of adult NHL successes into improvements in pediatric NHL treatment. This review summarizes the introduction of targeted therapies into frontline treatments for patients with anaplastic large-cell lymphoma and CD20–positive tumors, with the goal of improving overall survival while limiting both short- and long-term toxicities. In addition, newer approaches that have limited data in children but may have a significant role in how we treat pediatric NHL in the future are reviewed, which include CD19 directed therapy, Notch inhibition, the tri-functional antibody, FBTA05, and EZH2 inhibition.
Learning Objectives
Identify currently available targeted agents for children with non-Hodgkin lymphoma
Critique novel therapies for pediatric patients with refractory or relapsed non-Hodgkin lymphoma
There will be approximately 1000 new cases of non-Hodgkin lymphoma (NHL) diagnosed in patients under the age of 20 years in the United States this year.1 This disease has a variety of subtypes, with different treatments and outcomes. In 2008, the World Health Organization (WHO) revised its classification system of lymphomas based on cell lineage and derivation of the lymphoma from either precursor or mature cells.2 The four most common NHL subtypes that occur in pediatric patients include 3 derived from mature cells: diffuse large B-cell lymphoma (DLBCL); Burkitt lymphoma (BL), and anaplastic large-cell lymphoma (ALCL) and 1 subtype classified as precursor NHL: lymphoblastic lymphoma.2 Aggressive, high-grade disease makes up the majority of NHL cases in children, compared with a predominance of low- and intermediate-grade lymphomas in adults.
The majority of NHL subtypes have excellent event-free survival (EFS) with ALCL having the lowest, at ∼70%, and BL having the highest survival rates, with EFS reported ∼90%.3 As in all pediatric malignancies, novel therapies are needed in the treatment of pediatric NHL to decrease toxicities of frontline therapy and prevent relapses, as success with salvage therapies for relapsed pediatric lymphoma is not optimal, with a 5-year EFS ranging from 23%-53%, depending on salvage therapy and lymphoma subtype.4-6 Current standard treatment protocols incorporate topoisomerase inhibitors, anthracyclines, and alkylator therapy (Table 1), which predispose patients to organ toxicity, infertility, and secondary malignancies, and patients who relapse often require a hematopoietic stem cell transplant (HSCT) for a curative approach, which adds further long-term side effects.5,6
Standard frontline therapy for advanced stage, nonlymphoblastic, pediatric NHL

C indicates cyclophosphamide; V, vincristine; P, prednisone; M, methotrexate; D, doxorubicin; AraC, cytarabine; E, etoposide; Dex, dexamethasone; I, ifosfamide, Vb, vinblastine; 6MP, mercaptopurine; 0.5, 0.5 g/m2; 3, 3 g/m2; 8, 8 g/m2; CNS, central nervous system.
* Modified EPOCH (E, D, V, C, and P) with rituximab is now favored for the treatment of primary mediastinal B lymphoma in adult patients and being explored in pediatric patients. Lymphoblastic lymphomas are for the most part treated on acute lymphoblastic leukemia trials in pediatric patients.
Although there is significant overlap between NHL that occurs in adults and pediatrics, there are characteristics unique to NHL in children that are important to recognize in order to rationally develop novel therapeutics for this population. For example, there are multiple differences between pediatric and adult DLBCL in both histology and genomics. The majority of DLBCL that is found in pediatrics is of the germinal center B cell (GCB) subtype.7 Adult patients with NHL also have a high incidence of GCB, however a second subtype, activated B cell (ABC), is observed fairly often and accounts for ∼40% of DLBCL in adult patients. These differences in subtypes have a dramatic influence on success of therapy with the ABC form of DLBCL associated with poorer outcomes.8 In addition, the introduction of novel therapies for relapsed or refractory disease is also impacted by this difference in histology. Bruton's tyrosine kinase (BTK) inhibitors, such as Ibrutinib, have been shown to be primarily and perhaps singularly effective in the ABC subtype of DLBCL,9 which is rarely encountered in pediatrics. Therefore, efforts to move BTK inhibitors into pediatric trials have been halted and a success in the adult NHL arena cannot be translated into improvement in therapy for children with NHL.
Identification of genomic differences between adult and pediatric DLBCL have recently been reported as well. A classic translocation between chromosome 14 and 18 involving bcl2 is commonly found in GCB DLBCL of adult patients. In pediatric patients with DLBCL, however, translocations of bcl2 are essentially absent.7 Molecular findings in cases of pediatric DLBCL have revealed complex chromosomal aberrations that vary from typical adult cases of DLBCL. These differences signal a different biology of this tumor based on the age of the patient and emphasize that responses in adult patients may not have significance for pediatric therapy.10
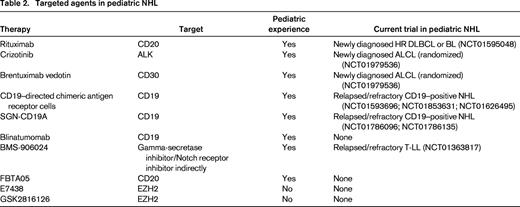
An additional obstacle that exists for improving NHL therapy for children is the small number of relapsed or refractory pediatric patients available to participate in early phase trials. This population is limited given the relative rarity of this entity and patients' lack of proximity to treatment centers that have developmental therapeutic trials available. Despite these difficulties, over the last decade, progress has been made both in identifying potential targets and utilizing novel therapies for pediatric NHL. Below, we highlight the experience with new, targeted treatments for pediatric NHL and discuss their role in newly diagnosed or relapsed/refractory patients (Table 2).
Targeted therapies for pediatric patients with newly diagnosed ALCL
Approximately 100 new cases of anaplastic large-cell lymphoma (ALCL) occur in patients <20 years of age in the United States every year.11 In multinational studies, there are overall good responses to initial chemotherapy, however, the 5-year EFS is repeatedly reported at ∼70%, regardless of staging or chemotherapy regimen (Table 1).12,13 After relapse, the success of salvage therapy has historically relied on HSCT in the pretargeted therapy era.5,6 Current therapy aims to build upon prior studies by utilizing agents with known efficacy (cyclophosphamide, dexamethasone, methotrexate, ifosfamide, etoposide, cytarabine, and doxorubicin), with the addition of targeted agents.
Two distinguishing features of pediatric ALCL lend themselves to novel therapies.
Brentuximab vedotin
ALCL tumor cells strongly express CD30 on their surface, making this receptor an attractive target for antibody therapy. Initial trials with an anti-CD30 monoclonal antibody showed tolerability, but only 17% response rate in patients with relapsed disease.14 Efforts to improve on this response resulted in the development of a novel agent, brentuximab vedotin (BV), which is a drug conjugate antibody that combines an anti-CD30 antibody with monomethyl auristatin E, a synthetic tubulin disruptor. The toxin is delivered to the surface of the CD30–positive cell and internalized which allows for the interruption of the microtubular network. BV was first approved by the Food and Drug Administration (FDA) in 2011 for: (1) patients with CD30+ Hodgkin lymphoma who had relapsed post-HSCT, based on a multicenter clinical trial (SG035-0003) with 102 patients; and (2) for patients with relapsed or refractory ALCL, based on a multicenter clinical trial (SG035-0004) with 58 patients. The complete response rate (CR) in patients with Hodgkin lymphoma was 32%, and in patients with ALCL was 57%.15 A phase II study that included pediatric and adult patients with relapsed or refractory ALCL showed that BV had an 86% overall response rate, with tumor reductions seen within 6 weeks of therapy initiation and mean duration of response of 12.6 months.16 A subsequent study in adult patients evaluated the use of BV in frontline therapy; either with, or followed by, traditional chemotherapy. BV, combined with chemotherapy, showed an objective response in 100% of patients and CR in 84%, compared with 85% and 62%, respectively, in patients who received BV followed by chemotherapy.17
Although fairly well tolerated, many patients experience low-grade neuropathy, with only rare events of high-grade neuropathy reported. Additionally, severe pulmonary toxicity has been reported when BV is combined with bleomycin, including 2 patients with fatal grade 5 toxicity that resulted in treatment guidelines to avoid the concomitant use of BV with bleomycin.18
Crizotinib
Approximately 25 years ago, the chromosomal rearrangement involving the anaplastic lymphoma kinase (ALK) gene was identified in the majority of ALCL in pediatric patients. ALK is a transmembrane receptor tyrosine kinase. Approximately 85% of these rearrangements are t(2;5)(p23;q35) leading to the expression of the fusion protein NPM-ALK, which is thought to play a role in lymphomagenesis via aberrant phosphorylation of intracellular substrates and activation of the RAS-ERK pathway.19,20 This is in contrast to the majority of ALCL in adult patients, in which most are ALK translocation negative and have a poorer prognosis. Crizotinib is an ALK, MET, and ROS1 inhibitor that was initially approved by the FDA in 2013 for use in ALK-positive non-small cell lung cancers.21 It has also been studied in patients with relapsed or refractory ALK-positive ALCL, although FDA approval currently exists only for lung cancer. Studies of crizotinib in adults showed a 2-year OS rate of 72% and 2-year progression-free survival of 63% in patients with ALCL who had persistent disease following conventional rescue therapy.22 Phase I studies in children found crizotinib to be well tolerated with an 88% CR rate in eight pediatric ALCL cases.23
Fatal toxicities in clinical trials included acute respiratory distress syndrome, arrhythmias, pneumonia, and pulmonary embolism, however, these were only observed in heavily pretreated, adult patients with lung cancer.21
Based on these promising results with targeted therapies for relapsed, refractory ALCL, the Children's Oncology Group (COG) has initiated a study to evaluate each of these in frontline therapy. In the COG ANHL12P1 protocol (NCT01979536), newly diagnosed patients will receive standard therapy. (Table 1) In addition, patients will be randomized to concurrently receive either BV or crizotinib. The study opened for accrual in 2013 and has exceeded expected recruitment. No efficacy data are available, and whether these targeted agents will actually improve the survival in this disease is still unknown. An earlier attempt to increase the EFS in newly diagnosed ALCL patients involved the incorporation of vinblastine. As a single agent, vinblastine had an 83% CR rate in relapsed ALCL but when added to the treatment regimens of newly diagnosed patients, it failed to improve the long-term EFS.12,24 ANHL12P1 constitutes a groundbreaking trial that tests the ability to combine novel, targeted therapies with multidrug regimens in newly diagnosed pediatric patients while simultaneously comparing the efficacy of these different treatments in a randomized platform.
Rituximab therapy in newly diagnosed pediatric NHL
The annual incidence of pediatric DLBCL and BL in the United States is 200 and 300 cases, respectively.11
A distinguishing feature of both DLBCL and BL is the expression of the protein CD20. CD20 is an ideal target for antibody therapy, in that it is a trans-membrane, nonsecreted protein found on all B cells except for early precursors in the bone marrow and plasma cells, and is not found on other off-target locations. Rituximab exerts its effect via several mechanisms, including antibody-dependent cell-mediated toxicity and complement activation, leading to cell lysis. It can also induce apoptosis and has been shown to inhibit secretion of IL-10 in some lymphoma cell lines, and inhibit B-cell receptor signaling in other lymphoma cell lines.25 Rituximab depletes all circulating CD20–positive cells within 48 hours, with recovery of B cells taking 6-9 months.
In 1998, the FDA approved rituximab for use in adult patients with DLBCL. A large European study of almost 400 adult patients with DLBCL showed improved EFS in patients receiving CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) therapy in conjunction with rituximab, versus CHOP therapy alone.26 In 2004, the COG implemented ANHL01P1, a pilot study evaluating the toxicity of adding rituximab to standard therapy for patients with newly diagnosed, pediatric BL and DLBCL. Patients were classified as group B, intermediate-risk, if lymphoma was above and below the diaphragm; or a primary intrathoracic tumor; or an extensive, unresectable abdominal tumor; or a paraspinal tumor; or in the bone marrow but <25% involvement. Group C, high-risk, patients included those with central nervous system disease or a bone marrow sample with >25% involvement of lymphoma. There were 45 group B patients treated, and the 3-year EFS was 93% with acceptable tolerability.27 The group C patients had a 3-year EFS of 90% in this trial.28 These survival rates are similar to the 4-year reported EFS in patients treated on FAB/LMB96, a similar backbone of chemotherapy without rituximab (Table 1).29,30 A multicenter European Phase II window study enrolled 136 pediatric patients with newly diagnosed B-cell NHL and administered 1 dose of rituximab prior to beginning standard chemotherapy. Disease improvement was noted in 41% of patients after a single dose of rituximab, but this did not translate into a decrease in the cumulative incidence of relapse.31 No study in pediatric patients with CD20–positive NHL has yet to demonstrate an improvement in EFS or OS with the addition of frontline rituximab therapy. The intergroup Phase III trial, ANHL1131 (NCT01595048), which was designed to test the efficacy of frontline rituximab therapy in pediatric patients with B-cell NHL opened in 2010, but no data are available to date. Given the histologic and molecular differences between adult and pediatric tumors, and inconclusive evidence from small pediatric trials, this larger scale pediatric trial is necessary to determine the significance of incorporating rituximab into the therapy for newly diagnosed pediatric patients with B-NHL.
Rituximab therapy is not without side effects. In safety studies, it was shown that B cell numbers and function typically return within 1 year of completion of rituximab therapy. However, case reports and single institution experiences have been published over the last several years that show up to 20% of patients who receive rituximab therapy will have prolonged hypogammaglobulinemia and impaired immune function, with some becoming dependent on intravenous immunoglobulin therapy due to persistent infections.32 Our preliminary studies show a heterogeneity in B-cell recovery after rituximab therapy and a higher-than-expected rate of infections and need for prolonged immunoglobulin therapy (Figure 1).33 Currently, several second- and third-generation anti-CD20 monoclonal antibodies are in development and undergoing trials in adult patients with NHL but with no pediatric experience. It is uncertain if they will have improved efficacy or fewer long-term effects.
Serum IgG levels after rituximab therapy for pediatric patients with NHL demonstrate heterogeneity in time to recovery, with hypogammaglobulinemia defined as <500 mg/dL. Twenty percent (4/20) remained hypogammaglobulinemic >24 months after ending therapy, with a paucity of switched memory B cells. All 4 patients have required IVIG therapy for persistent infections.
Serum IgG levels after rituximab therapy for pediatric patients with NHL demonstrate heterogeneity in time to recovery, with hypogammaglobulinemia defined as <500 mg/dL. Twenty percent (4/20) remained hypogammaglobulinemic >24 months after ending therapy, with a paucity of switched memory B cells. All 4 patients have required IVIG therapy for persistent infections.
Targeting CD19
CD19 became an attractive protein to target in attempts to treat B-cell malignancies because of its ubiquitous expression on B cells at most stages of maturation. CD19 is located on the surface of all B cells except plasma cells. The majority of BL, DLBCL, and B-lymphoblastic lymphoma (B-LL) will express CD19 on their surface.34 Many therapies directed against CD19 were first studied in the context of acute lymphoblastic leukemia (B-ALL) but indications are now being expanded to explore their role in B cell lymphomas. In addition, these therapies were first studied in adult patients and are now being moved into trials for children; however, extrapolating the initial experiences for pediatric patients may not be reliable based on biologic differences in these tumors in different age groups.10
SGN-CD19A
SGN-CD19A is an antibody-drug conjugate (ADC) similar to BV. It contains a humanized anti-CD19 monoclonal antibody that is joined to a tubulin inhibitor, monomethyl auristatin F (MMAF). Identical to the BV mechanism, the molecule is internalized upon binding to the target cell and subsequently the tubulin inhibitor is released and functions as a disruptor of cell-cycle division leading to cell death. A current open phase 1 trial is investigating the role of SGN-CD19A in B-ALL, B-LL and Burkitt lymphoma in adults and pediatric patients as young as 1 year of age (NCT01786096). Preliminary data was reported with the first 9 pediatric patients with no observable responses.35 An additional ongoing trial with adults and pediatric patients older than 11 years with B-NHL (NCT01786135) reported responses with SGN-CD19A with a 16% CR rate and 14% PR rate.36 A unique toxicity that has been observed in both trials is corneal complications described as superficial microcystic keratopathy. This toxicity has been managed with steroid eye drops.
Blinatumomab
Blinatumomab is a bispecific T-cell engager (BiTE) used in B-cell malignancies. BiTE therapies are a novel approach to immunotherapy where monoclonal antibodies to 2 separate proteins are joined together and force an interaction between a cytotoxic T cell, via CD3 binding, and cells that express the second protein; in the case of blinatumomab this is any cell that expresses CD19. This therapy relies on the patient's endogenous T cells to destroy the target B cells that express CD19 through cell lysis.37
The first reported clinical responses of blinatumomab were in adult NHL.38 There were 38 adult patients treated on this phase 1 trial, with the majority of patients having either follicular or mantle cell lymphoma and only 1 DLBCL. There were 4 CR and 7 partial responses (PRs) reported in these patients; the patient with DLBCL was not evaluated due to early cessation of therapy. Clinical experience with this drug and adult patients with NHL continued and a phase 2 trial studying blinatumomab in 21 evaluable adult patients with DLBCL recently reported a response rate of 43% including 5 CR and 4 PR.39
The majority of the clinical experience of blinatumomab has been in adult B-ALL with the most recent phase 2 studies including a combined total of 225 subjects. As a single agent, blinatumomab has demonstrated impressive responses in patients with refractory or relapsed B-ALL, including CR or complete response with incomplete hematologic recovery (CRh) ranging from 43% to 69% and attaining MRD negative state at rates ranging from 35% to 80%.40-42 Furthermore, these trials enrolled patients with various burdens of disease at start of treatment, ranging from minimal residual disease state to overt morphologic disease in the bone marrow. This observation is important when considering therapy for patients with lymphoma when the level of disease is likely high and relying on single-agent therapy risks failure. In fact, there appears to be a trend toward improved responses with decreasing levels of disease. A large phase 2 trial reported a CR and CRh of 29% in patients with greater than 50% blasts in the bone marrow at start of therapy compared with the remaining patients who had a 73% overall response.41 Additionally, a trial of patients with morphologically negative disease (<5% blasts in the bone marrow) but evidence of residual leukemia by molecular analysis demonstrated the best response of blinatumomab reported with 80% of patients achieving MRD negative status (<10−4).42 These findings may suggest utilizing this therapy with lower levels of disease.
Data are maturing regarding blinatumomab use in children. An international phase 1 pediatric trial was recently completed evaluating blinatumomab as a single agent in relapsed or refractory B-ALL. Disease burden was higher than most trials in adults with an eligibility criterion of at least 25% blasts in the bone marrow.43 CR and CRh were 32%, and 25% of patients achieved MRD negative bone marrows. The phase 2 portion of this study reported preliminary results of 31% CR with a 13% MRD negative rate, but the trial only recently completed and final results are pending.44
An important limitation in blinatumomab use is toxicity. In both adults and pediatric patients, common toxicities of leukemia therapy are observed, including decrease in peripheral blood cell counts and infections; however, unique side effects observed are cytokine release syndrome (CRS) and neurotoxicity ranging from mild confusion to overt encephalopathy. Steroid pretreatment and slow dose-escalation has diminished the incidence of CRS and neurotoxicity during blinatumomab therapy but these side effects remain complicating factors in using this drug. Although blinatumomab has not been studied specifically in pediatric NHL, responses in both pediatric B-ALL and adult DLBCL and B-ALL are encouraging and suggest pursing this treatment in pediatric subtypes of NHL with CD19 expression, such as DLBCL, BL, and B-LL.
CD19–specific CAR T cells
In addition to blinatumomab, chimeric antigen receptor T cells (CAR T cells) have also been developed to target CD19–expressing malignancies. The primary focus of CD19–specific CAR T cells has been relapsed/refractory B-ALL and CLL. CAR T-cell therapy utilizes gene transfer technology to generate tumor-specific T cells that bind the target antigen mandated by the antigen-binding domain of a monoclonal antibody fragment cloned into a construct that also includes a T cell-receptor-derived CD3 ζ signaling chain. In addition, most CARs in clinical use have additional costimulatory signal(s) incorporated into the vector to enhance the effector function of the CAR T cell.
A major difference between BiTE therapy and CAR T cells, is that BiTE therapy relies on endogenous T cells in the patient at the time of therapy, and their function may be limited if the patient is heavily pretreated. CAR T cells are generated from a patient but timing of collection could be earlier on in therapy if the patient is deemed high-risk, and therefore T cells may have preserved function when collected.
The first responses were reported in adult patients with CLL in 201145 ; however, since that time there have been reports of children with relapsed/refractory B-ALL treated with CD19–specific CAR T cells demonstrating extraordinary responses.46,47 In addition, adult patients with B-ALL have also had responses similar to those observed in pediatric patients.47,48 Recently, 2 groups have reported on activity in adult patients with DLBCL as well, either as a bridge to HSCT49 or as consolidation therapy following autologous stem cell transplant.50
Toxicities with CAR T-cell therapy are similar to those reported with blinatumomab with CRS and neurotoxicity being expected adverse events. Most occurrences have been reversible and the use of anti-IL6R therapy with tocilizumab has proven to be successful in moderating toxicity of both blinatumomab and CD19–specific CAR T cells.51
All 3 CD19–targeted therapies have had responses in patients with NHL, although only SGN-CD19A (NCT01786096, NCT01786135) and CD19–specific CAR T cells (NCT 01593696, NCT01853631, NCT01626495) have open trials for pediatric NHL cases. In the coming years, we expect to see additional clinical trials for all 3 of these therapies move forward for pediatric patients with CD19–positive NHL.
Novel therapies for NHL with limited or planned experience in pediatrics
Gamma secretase inhibitors
Novel therapies for refractory or relapsed T-cell malignancies are far fewer compared to B-cell malignancies. There have been attempts at targeting Notch receptors in T-ALL for years since the identification of activating mutations of Notch in this leukemia,52 however gastrointestinal toxicities and inability to provide adequate drug levels complicated the development of gamma secretase inhibitors (GSIs) which directly block Notch receptor activation through prevention of cleavage by gamma secretase. Notch receptors participate in transcription control of multiple pathways in normal hematopoiesis including self-renewal, differentiation, proliferation, and apoptosis.53 Although Notch receptor mutations are not unique to T-ell malignancies, it is an attractive target for either relapsed or refractory T-LL patients, where limited agents exist, or potentially for frontline therapy in the attempt at reducing toxicity from chemotherapy. Other forms of NHL may benefit from developing GSIs as well, including ALCL, based on preclinical data.54 Currently, a phase 1 trial with BMS-906024 (NCT01363817), a newer generation GSI, is actively accruing patients with both T-ALL and T-LL who are at least 12 years of age. Early responses have been reported in these patients with 1 leukemia patient achieving CR and 7 additional patients having at least 50% reduction in bone marrow blasts. Diarrhea was the most common toxicity reported, not surprising given previous experience with GSIs, but was tolerable with steroid treatment. To date, no pediatric NHL patient has been treated with BMS-906024.55
FBTA05
FBTA05 is a therapy from the trifunctional antibody class of molecules. FBTA05 is comprised of a monoclonal antibody to CD20 and CD3 allowing for interaction of T cells with an antigen on the surface of B-cell malignancies, similar to blinatumomab with CD19 antigen instead of CD20. However, FBTA05 also contains an Fc fragment that is capable of binding other immune accessory cells, thereby enhancing the destruction of the tumor cells and potentially providing long lasting T-cell responses against CD20 that exist even after cessation of therapy.56
FBTA05 was initially studied in adult patients with B-NHL post-HSCT in combination with donor leukocyte infusions.57 More recently a description of 10 pediatric patients with relapsed or refractory B-cell malignancies, including three patients with BL and three patients with DLBCL, were treated with FBTA05 on a compassionate use in a variety of treatment regimens. Although FBTA05 was only clearly shown to have a direct effect as a single agent in 1 patient with Burkitt leukemia, 2 patients with NHL (1 BL and 1 DLBCL) who received FBTA05 as consolidation therapy after already achieving CR are alive >2 years after first and second relapse, respectively. The role of FBTA05 in their durable remissions is unclear.58 Toxicities were similar to those described with other immunotherapies, including fevers and peripheral blood count suppression. Additionally, hepatic complications were observed in patients treated with concurrent therapies, including mercaptopurine and donor lymphocyte infusion, so direct correlation is difficult to ascertain. Treatment with FBTA05 has a similar limitation as blinatumomab, specifically, relying on the patient's endogenous immune system for the therapy to be successful; however, the evaluation of this molecule in a pediatric trial is warranted.
EZH2 inhibitors
Another potential strategy for treating pediatric NHL is inhibition of the enhancer of zest homolog2 (EZH2). This protein is a histone methyltransferase and has been shown to be mutated or up-regulated in a variety of tumors, including DLBCL, ALCL and T-LL.59,60 Given its role in epigenetic control of gene expression, inhibition of aberrant activity of EZH2 could provide a unique therapy that could complement standard chemotherapy. There are 2 small molecule EZH2 inhibitors in clinical trials for adults with relapsed or refractory NHL, E7438 (EPZ-6438; NCT01897571) and GSK-2816126 (NCT02082977). The adult trial with E7438 recently reported 2 partial responses in patients with DLBCL. Only 21 patients have been treated, so the toxicity profile is limited. Reported events thus far are peripheral blood count suppression, asthenia, pulmonary embolism and upper and lower gastrointestinal symptoms.61 A pediatric trial is in development for E7438, which will evaluate the safety of EZH2 inhibition in children (personal communication).
Conclusions
The success in treating newly diagnosed pediatric NHL has improved in the last 6 decades with the evolution of multiple chemotherapy regimens producing OS rates as high as 95% in subsets of patients. Unfortunately, not all subtypes of pediatric NHL have proven to be as easy to treat and novel approaches to therapy are warranted. This review highlighted some of the relevant therapies that are either accessible to children now or will be in the near future; however, there are a multitude of novel targeted agents that are being evaluated in adult patients with NHL that may prove even more successful and be pushed into pediatric trials sooner.
The current trial for newly diagnosed patients with ALCL is an example of revolutionizing clinical trials for children with malignancies that do not have excellent OS rates. Randomizing frontline therapy with 2 targeted agents, in addition to a standard backbone of chemotherapy, has not been attempted before and represents a new approach to bringing novel drugs to the forefront expeditiously. As these targeted therapies continue to be tested in clinical trials and the safety and efficacy becomes clearer, introduction of these drugs in more upfront settings will become a reality. Measuring clinical responses will need to improve as well to parallel the rapid integration of these treatments. Current characterization of responses in patients with NHL relies on radiographic imaging. As the knowledge of genomic alterations continues to grow in these diseases, the use of circulating cell-free tumor DNA as a tool for monitoring response to therapies will become useful, as was recently reported in adult patients with DLBCL.62 Treatments for NHL may start to mimic those for pediatric acute leukemias, where minimal residual disease monitoring enables the identification of those patients who will require either escalation or subtraction of therapy. Novel frontline trials in pediatric patients with NHL using the several potentially useful agents discussed in this review may provide improvement of EFS while diminishing toxicity from standard chemotherapy.
Correspondence
Rachel Kobos, Department of Pediatrics, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065; Phone: 212-639-8451; Fax: 212-717-3239; e-mail: kobosr@mskcc.org.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: Brentuximab for treatment of newly diagnosed pediatric ALCL Crizotinib for the treatment of newly diagnosed pediatric ALCL Rituximab for the treatment of newly diagnosed pediatric B-cell lymphoma Blinatumomab for the treatment of pediatric B-cell lymphoma.