Mycosis fungoides (MF) is the most common variant of cutaneous T-cell lymphomas (CTCLs) among the EORTC-ISCL classification.1,2 The majority of MF patients present with early-stage disease or limited patches and or plaques (IA-IIA) and their skin lesions can be well controlled using skin-directed therapies. Systemic treatments are added for patients with refractory plaques or tumors, nodal, or blood involvement and chemotherapy is avoided except in the most advanced states.3 Early stage MF is characterized by the degree of skin involvement by patches or plaques (T-stage). T1 is involvement of <10% of the body surface.T2 is >10% of the body involvement by patches (a) or plaques (b). Advanced stage MF includes patients with tumors (T3−), erythroderma (T4) with or without Sézary syndrome (SS), as well as patients with blood, nodal, bone marrow, or visceral disease. Sézary syndrome is defined as erythroderma >80% and leukemic blood involvement (B2) >1000 circulating atypical lymphocytes. Bone morrow or blood involvement is included in Stage IVA1, nodal is IVA2, whereas stage IVB includes involvement of visceral organs such as lung, liver, brain, or other.4,5
The annual incidence of CTCL in the US was estimated to be 6.4 cases per million between 1993 and 2002 or 7.7 cases per million persons from 2001 to 2005.6
Because CTCLs are rare, there is limited evidence supporting one treatment over another. Overall response rates have been predominantly used to assess response in phase II trials, although a global response tool has recently been introduced that should allow comparison.7 Overall survival has been related to T stage in several retrospective analyses performed at the larger CTCL centers with similar findings.8-10
Treatment by stage
The first randomized trial conducted by Bunn et al at the National Institutes of Health concluded that chemotherapy was not superior to sequential conservative therapies with respect to overall survival.11 The pathophysiology of CTCL includes the accumulation of atypical memory T cells in epidermis and dermis where they are susceptible to skin-directed treatment for patients presenting with patch/plaque T1,T2 MF.12 Although skin-directed therapies alone (topical steroids or retinoids, nitrogen mustard, or phototherapy) will often control or clear patients with early disease, systemic biologic response modifiers (interferon alpha or gamma, retinoids) are added to treat refractory early-stage patients or for advanced stage patients with extensive skin involvement, including Sézary syndrome. Targeted therapies and small molecule are gaining favor as ways to debulk tumors and blood compartments as an alternative for chemotherapy approaches.3,13
Chemotherapy is usually reserved for treatment of advanced stage patients with disseminated bulky nodes, disseminated tumors, or visceral disease. Because chemotherapy does not cure CTCL patients and induces immunosuppression, monotherapy with high response rates are preferred to combination therapies in these highly immunocompromised advanced stage patients.3,14,15 In a recent study of 140 advanced MF/SS patients, those receiving biologic response modifiers, histone deacetylase inhibitors or targeted therapies had a superior overall survival of 2.5 years in contrast to 9 months in patients treated with chemotherapy.16 Non-ablative allogenetic transplantation following electron beam radiation can induce complete responses in selected advanced stage patients, especially those with SS, but there are risks including mortality from infection, recurrent disease after transplantation, and chronic graft-versus-host disease.17 Thus, the goal of therapy in early-stage patients is to put the disease into durable remission. In advanced stage patients, the goals are to reduce skin involvement and palliate the symptoms without inducing immunosuppression and disease progression.
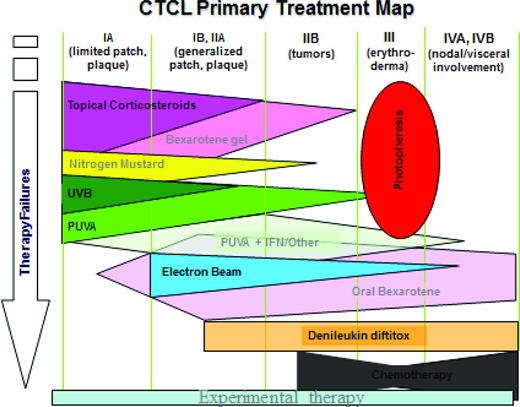
In our recent analysis of 1243 prospectively followed patients MF/SS, their median overall survival was 24 years.18 In contrast, 140 advanced MF/SS patients from this cohort had a median survival of only 2.47 years.16 The overall survival of patients with SS increased from 2.5 years from historical data19 to 5.4 years in patients with SS, unless their SS count exceeded10 000 cells/μL.4 There was a significant decrease in overall survival in patients with plaques in addition to patches, even if only <10% of the body was involved (T1B) and especially if skin involvement was >10% (T2B). Thus, the treatment of early-stage MF patients with plaques should be augmented with systemic biologic response modifiers: oral retinoids (bexarotene, acitretin) and/or interferons (alpha and/or gamma), if MF patients are refractory to the skin-directed therapies alone. A treatment algorithm by stage is shown in Figure 1.
Skin-directed topical therapies
Topical agents used to treat early-stage MF and are important adjuvant therapy to control skin lesions in patients with advanced disease.3,12,20 In MF, atypical T cells are skin homing resident memory T lymphocytes, and skin-directed therapies can produce long-term remissions that can last for many years. Most of the agents available for use topically induce the apoptosis of T cells and retinoids may also alter epidermal differentiation removing growth factor support from the malignant cells. There is currently no evidence that aggressive upfront aggressive chemotherapy is able to cure MF or that it impacts overall survival.21 Hence, sequential skin directed therapy for early-stage MF patients is recommended in the NCCN guidelines,22 and has been extensively reviewed in detail.23 First-line skin-directed therapy should include removing staphylococcus aureus, topical emollients, topical steroids, or retinoids for hypertropic areas. Staph decolonization, emollients, and topical steroids are critical for managing SS or erythrodermic patients who have high colonization rates.24 Topical nitrogen mustard is chemotherapy that can be applied to lesions or the whole skin.25,26 Second-line skin-directed therapy includes phototherapy (UVB or PUVA) or local radiation.23
Imiquimod and resiquimod are novel topical immune response modifiers belonging to the imidazoquinolone family of drugs.27 They are toll agonists that when used topically or injected into lesions or tumors may have systemic effects. Imiquimod induces synthesis and release of the cytokines interferon (IFN)-α, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-12 that activate the adaptive immune response toward the TH-1 or cell-mediated pathway while inhibiting the TH-2 pathway.
Local and total skin electron beam therapy
Total body skin electron beam (TBSEB) is perhaps the most effective of all skin-directed therapies. It is often used locally in patients with skin-limited disease, especially resistant plaques and tumors. Combined international data of 1165 patients receiving TBSEB therapy showed complete response rates of close to 70%.28-31 Complete response rates are highest in patients with T1-limited disease where use of early, low-dose radiation to a solitary lesion may lead to a cure. Although given an already favorable prognosis of IA patients with multiple lesions, TBSEB is usually reserved for patients with greater skin involvement, especially for extensive plaques or for palliation of SS or prior to non-ablative allogeneic stem cell transplant.
Five-year disease-free survival was shown to be 40%-60% for stage IA, 25% for stage IB, 15% for stage IIA, 2%-20% for stage IIB, and 10%-25% for stage III, patients.28 Disease control may be achieved using lower dosing of 2 Gy 4× administered for single refractory lesions or using low-dose 12 Gy total body administration. Lower doses are effective and provide opportunity for multiple doses to be given without undue toxicity.32
Patients beyond stage IA who achieve a complete response are prone to relapse. TBSEB is effective in patients with stage IIB tumor disease; however, complete responses may be short-lived because of relapse with new tumors. The use of adjuvant nitrogen mustard after TBSEB remission can increase disease-free survival from approximately 15 to 55% at 5 years in patients with T2 stage disease.33 Patients with T4 stage erythrodermic MF may also have prolonged remissions when TBSEB is combined with extracorporeal photochemotherapy (ECP) for disease palliation.34
Side effects of TSEB include erythema, swelling, exfoliation, tenderness, blister formation, alopecia, anhidrosis, and nail loss.35 Effects are usually transient although hair and nail thinning may persist and skin aging is common. There is also an increased risk of developing non-melanoma skin cancers in patients who receive adjuvant PUVA or topical nitrogen mustard. Because of the side effect profile of TBSEB, studies were done to determine the efficacy of low-dose TBSEB (12 Gy) which has the advantage of shorter time course and potential for repeated use and reduced side effects.32
Systemic biologic response modifiers
Biologic response modifiers, including the interferons (IFN-α and -γ) and RXR and RAR retinoids alone or with phototherapy, are widely used first-line systemic therapies in MF patients who have extensive skin involvement that is refractory to skin-directed therapies. In particular, oral retinoids and interferon are commonly combined with phototherapy for patch/plaque MF or with extracorporeal photopheresis in patients with SS. Retinoids induce T-cell apoptosis, modulate antigen presenting cells, and favorably modulate epidermal differentiation.36 MF advances from a Th1 immune response to an excess of tumor cells making Th2 cytokines, eosinophilia, atopy, and increasing immunosuppression.37 Biologic response modifiers are very effective positive modulators of the Th1 immune response and counteract Th2 cytokines produced by malignant clones. The most effective cytokine is alpha interferon that increases gamma interferon and tumor necrosis factor (TNF) alpha production, generating a cytotoxic CD8+ T-cell response toward the CD4+ tumor cells. Interleukin 12, induced by gamma interferon, also induces a CD8+ T-cell response but may have more side effects than interferons.38,39
Retinoids and rexinoids
Oral retinoids are often the first systemic biologic agents selected for managing CTCL patients whose skin involvement is >10% (T2).40 Retinoids modulate pathways involved in inflammation, cellular differentiation, apoptosis, and sebaceous gland differentiation.41 They normalize epidermal differentiation and counteract the atrophy of corticosteroids. The RAR retinoids: (isotretinoin and etretinate replaced by acitretin) have been used to treat patients with MF/SS since the 1980s,40,42 but there is little data comparing one retinoid to another.40 The first RXR receptor selective retinoid or “rexinoid,” bexarotene, was approved in 1999 for skin manifestations of CTCL and is a common first-line systemic therapy.40,43-45
Retinoids are vitamin A derivatives that modulate proliferation and differentiation of both keratinocytes and lymphocytes. Retinoids are the ligands for RAR or RXR receptors that belong to the large super-family of steroid hormone receptors.46 This family includes glucocorticoids, thyroid hormone, and vitamin D3 receptors functioning as DNA-binding proteins. Ligand- receptor dimers bind to specific retinoid response elements in the DNA of promoters creating nuclear transcription complexes that modulate gene transcription.
The cumulative data for RAR retinoids show an ORR of 50% that is similar to the response rates reported for the RXR selective oral bexarotene.42,47-49 Oral administration and lack of immune suppression are advantages treatment with bexarotene or acitretin affords compared with chemotherapy alternatives. In vitro, bexarotene induced apoptosis of CTCL cell lines but required high doses suggesting other effects may be important.36 Richardson et al reported that bexarotene alters adhesion molecules that govern T-cell trafficking resulting in a shift of T cells from the skin to the periphery.50
Two multicenter phase II multicenter trials, 1 in early-stage disease, and the other in advanced-stage patients, led to FDA approval of oral bexarotene based on overall response rates.44,45 In advanced stage MF patients, the pivotal trial's starting dose was 650 mg/m2 per day, was reduced to 500 mg/m2 per day, and then to an optimal dose of 300 mg/m2 per day based on response and dose-limiting toxicity. The response rates for advanced-stage disease patients were 55% at ≥300 mg/m2 per day and 45% at 300 mg/m2 per day.45 Although highest doses of bexarotene were associated with higher response rates, they also were associated with higher triglyceride levels and risk of pancreatitis.45 Dose-limiting toxicity, hyperlipidemia with pancreatitis, occurred in a few patients at a dose of >300 mg/m2/day. The response rates for early-stage MF were 67% at doses >300 mg/m2 per day versus 54% at the suggested dose of 300 mg/m2 per day.155
Lack of response at the lowest dose arm of 6 mg/m2 was the rational for using 300 mg/m2/d as the optimal dose for response and tolerability. At the optimal dose, overall response was 48% in the combined group of early and late stage MF patients.44,45 However, in practice, oral bexarotene is often started at a dose of 2-3 tablets and increased gradually to 300 mg/m2, the recommended dose.51,52 A dose ranging study comparing 150-300 mg/m2 was conducted but has not been published.
We evaluated 70 MF/SS patients (stage IA-IVB) treated with oral bexarotene as a monotherapy or used in combination with other agents. We confirmed a response rate of 48% for bexarotene monotherapy and achieved a response rate of 90% when bexarotene was combined with 2 lipid lowering agents (fenofibrate and atorvostatin) and the free T4 was corrected.9 Bexarotene may also reduce tumor and lymph node burden and has demonstrated efficacy in treating some patients with large-cell transformation and erythrodermic MF.9,53 Combinations of bexarotene or acitretin with PUVA, interferon, photopheresis, and denileukin diftitox may lead to higher overall response rates of 90%.9
Retinoids are also often used in combination with PUVA,47 photopheresis,54,55 or interferon.56-58 We reported long- term disease control and complete responses with isotretinoin in combination with interferon alpha, which were used as the first arm of a combination approach56,57 Although response rates are similar with retinoids plus PUVA versus PUVA alone, the benefits of combination therapy include a lower PUVA dose for clearing skin lesions and a longer remission on retinoid maintenance.47 There has been no demonstrated improvement in the response rates of chemotherapy plus retinoids versus chemotherapy alone.42
All retinoids are teratogenic and should never be used in women who are pregnant or contemplating pregnancy. Side effects of RAR retinoids (isotretinoin) include dryness, alopecia, arthritis, hepatitis, and bone spurs, and are less well tolerated than the side effects of bexarotene.40,59 Etretinate which is stored in fat has a long half-life, has the potential to cause hepatitis, and has been replaced with acitretin for treatment of psoriasis. It is also used to treat CTCL patients without the benefit of clinical trials.
The most common adverse effects reported with bexarotene in the registration trials are dose-dependent and include hypertriglyceridemia (82%), hypercholesterolemia (30%), central hypothyroidism (29%), headache (20%), asthenia (16%), pruritus (13%), and leukopenia (11%).45 Because systemic bexarotene induces hypertriglyceridemia and central hypothyroidism with suppressed TSH mRNA,60 fenofibrate and levo-thyroxine are also administered with bexarotene to normalize the fasting triglyceride and free thyroxine levels. Hypertriglyceridemia can be prevented or reduced in severity by including lipid-lowering agents and levothyroxine replacement, initiated with treatment. We prefer fenofibrate at 145 mg/d with addition of a statin if needed. Low-fat diet and omega 3 fatty acids are also helpful. Gemfibrozil should not be used to control bexarotene-induced hypertriglyceridemia as it is associated with higher bexarotene levels, increased hypertriglyceridemia, and increased risk for pancreatitis.45
Free thyroxine and triglyceride levels should be monitored frequently initially until they stabilize. Levothyroxine can be administered with dose escalation until the free T4 has normalized. We generally give 25 mcg of levothyroxine for each 75 mg capsule of bexarotene, increasing the levothyroxine by 25 mcg for each additional bexarotene tablet given. Normalization of thyroid function occurs as early as 8 days after cessation of therapy,60 and patients should be weaned off levothyroxine when bexarotene is discontinued. Bexarotene binds to RXR receptors and they can bind to peroxisome proliferator-activated receptors (PPARs), increasing insulin sensitivity leading to hypoglycemia in diabetic patients on insulin. Thus, glucose levels should be monitored carefully, especially in diabetic patients. Following a response to bexarotene, we slowly taper the dose rather than stopping therapy to prevent relapses.
Interferons
Interferons comprise type I interferons-α, -β, and type 2 -γ. Interferons are polypeptides produced by eukaryotic cells with antiviral activity.61 Interferons have anti-proliferative, cytotoxic, and immunomodulating actions in CTCL. In particular, interferon alpha can inhibit Th2 cytokines, such as IL-4, IL5, and IL10, reducing eosinophilia. Commercial recombinant interferon alpha formulations include 2 made by Roche, INF-α2a (Roferon-A) and a pegylated (Pegays), and the most widely used, IFN-α2b (Intron A) made by Schering.
Imiquimod and resiquimod are also novel topical immune response modifiers belonging to the imidazoquinolone family of drugs.27 They are toll agonists that when used topically or injected into lesions or tumors may have systemic effects. Imiquimod induces synthesis and release of the cytokines interferon (IFN)-α, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-12 that activate the adaptive immune response toward the TH-1 or cell-mediated pathway while inhibiting the TH-2 pathway.
Although there is a definite relationship between dose and efficacy with interferons, there are dose limiting side effects that must be considered. As a single agent, interferon alpha has a partial response rate of 50% with a 20% complete responses rate.61 Bunn et al reported a 45% response rate in patients receiving at least 3 months of IFN-α2a at 50 million units 3 times per week.62 To examine the effect of dose, a phase II trial compared the doses of IFN-α2a at 3 versus 36 million units in 22 stage IA - IVA stage CTCL patients.63 The response rates were 38% with low-dose and 79% with high-dose interferon, although dose reductions were required. Olsen et al has shown that interferon alpha has similar response rates in early-stage MF/SS patients (73%) to late stage patients (60% response).63
Whenever possible, patients should continue to receive interferon until they achieve a complete response lasting for at least 3 months duration before it is gradually tapered.63 Patients with large cell transformation and tumors are less likely to respond, with exceptions.61 Intra-tumoral injection of interferon (1-2 Mu 3 times per week for 4 weeks) may be an effective route of administration.61 Administration of interferon-α (Intron-A) is usually initiated at a low dose of 1-5 million units subcutaneously daily or 3 times a week. If there is no response at 3 months, the dose may be gradually increased to 6-9 million units as tolerated.61,63 The maximum tolerated dose is 18 million units daily.63 Because of predictable and dose-related effects61 including fatigue, myalgias, fever, hepatitis, neutropenia, depression, most patients receive 3-5 million units daily or 3 times per week. Elderly patients generally do not tolerate interferon even at low doses. Some of the B-symptoms can be ameliorated if interferon is given at bedtime with non-steroidal anti-inflammatory agents or acetaminophen.63 Leukopenia, thrombocytopenia, hepatitis, mental status changes, erectile dysfunction, fatigue, diarrhea, and anorexia are dose-related side effects and improve with a 50% reduction in dose. IFN-α is associated with a 6% incidence of thyroid dysfunction; hypothyroidism is more common than hyperthyroidism.64 Myelosuppression and elevated liver enzymes are common but usually not clinically significant.61 Patients may become tolerant but side effects which interfere with quality-of-life generally require a break in therapy followed by a dose reduction.
Another side effect is the development of neutralizing antibodies which are influenced by the dose, regimen, duration of therapy and route of administration. Sub-cutaneous and intra-muscular administration over a long period of time is correlated with higher levels of antibodies.65 IFN-α2b may have a lower incidence of antibodies than IFN-α2a.65 Interferon alpha conjugated to propylene glycol (Peg) approved for melanoma is administered weekly at a dose of 180 μg subcutaneous injection.
Two forms are available, 40 kDa PEG-IFN-alpha 2b and PEG-IFN-alpha 2a, with similar pharmacokinetics and side effect profile in normal controls.66
IFN-α plus photopheresis is commonly administered as front line combined immunomodulary therapy for patients with SS or erythrodermic-CTCL with B1 blood involvement.63,67,68 Low-dose bexarotene (150 mg/m2) can also be added if no response is noted. The synergistic combination of PUVA plus IFN-α is also widely used for extensive or refractory or relapsed stage IB (T2b). In a study of 39 patients with all stages of MF and SS, 36 of 39 patients achieved a complete response (62%) or partial response (28%) with median duration of 28 months on a combined regimen of IFN-α and PUVA.69 The overall response and response duration are superior with a combination of PUVA and IFN, to either treatment alone.70,71 However, patients may develop anti-interferon antibodies that may induce tolerance and resistance to response from drug. Combined therapy may suppress anti-interferon antibody formation.65 In a study of 24 MF patients treated with combination of PUVA and IFN alpha, none developed antibodies.72 Interferon-alpha and PUVA are usually initiated concurrently, each given 3 times per week. The frequency of PUVA administration can be tapered gradually if the patients' skin lesions clear.
Surprisingly, in advanced MF patients the combination of IFN-α plus oral retinoid had a complete response rate similar to either therapy alone.73-75 Bexarotene plus IFN-α induced a response rate of 39%76 with 95% confidence interval [CI] 17%-64%, compared with the response rate of bexarotene monotherapy in advanced or early-stage MF patients (45%-54%).44,45 The reason for the low response rate may be the small number of patients and prior therapies in the combination therapy trial. A case series of only 12 patients with refractory late-stage MF reported an 83% complete response rate with interferon alpha and oral etretinoin.77 Etretinoin is no longer available because of its long half-life, and it has been replaced with acitretin which has not been as extensively studied in clinical trials. Disease stage is predictive of response to IFN-α therapy as a single agent, with more complete responses in patients with stage I (62.5% complete remission) compared with stage III-IV disease (16.5% complete remission).78 Duration of disease is also a predictor of response. Patients who have had the disease longer may perform less well on IFN-α and other therapies in general.63,65
IFN-α has also been used in combination with systemic chemotherapy. A large randomized phase I trial examined interferon α2b combined with either low-dose methotrexate versus interferon combined with all trans retinoic acid in 373 refractory/resistant CTCL patients who had a complete response rate of 80% in both arms. The interferon was given to 108 patients for 5 years without requiring dose reductions. Progression-free survival at 5 years was 60% or 62% in the IFN/MTX versus IFN/retinoid group.79 Methotrexate can act as a DNA-demethylating agent, enhancing sensitivity to Fas-mediated apoptosis by demethylating the promotor.80
Interferon may be beneficial when combined with fludarabine or vinblastine compared to either chemotherapy alone.81,82 The response to IFN-α plus pentostatin (overall response rate of 41%)83 was similar to response with pentostatin alone (overall response rate for CTCL ranging from 14% to 60%, ≥35% for majority of trials).84-89
IFN-γ and -β have not demonstrated superiority to IFN-α and are less well studied. In a phase II study of 16 patients who received intramuscular recombinant IFN-γ, 31% of patients demonstrated partial responses and no complete clinical remissions.90 There is 1 case of a patient achieving complete response after intravenous administration of IFN-γ (14-16 MU/wk) for 22 weeks.91 Rook has reported responses to addition of interferon gamma in SS patients who have failed to improve with interferon alpha and photopheresis.92-94 Interferon-β has been studied the least, and preliminary data shows poor efficacy in the treatment of CTCL.95
Adverse effects of IFN-α and -γ include flu-like symptoms consisting of fever, chills, myalgias, and malaise which may be prevented or reduced by premedication with acetaminophen or nonsteroidal anti-inflammatory agents.65 Leukopenia, thrombocytopenia, hepatitis, mental status changes, fatigue, diarrhea, and anorexia are dose-related side effects. IFN-α is associated with a 6% incidence of thyroid dysfunction; hypothyroidism is more common than hyperthyroidism.64
Interleukin-12
Defects in IL-12 production may play a role in the cytokine profile shift from Th1 to Th2 type that accompanies disease progression.37,96 IL-12 plays a significant role in the activation and differentiation of cytotoxic T lymphocytes.97 Recombinant 1L-12 normalizes interferon alpha production, enhances cell-mediated cytotoxicity, and augments natural killer cell cytotoxicity when added to peripheral blood mononuclear cells from advanced CTCL patients.39 Three clinical trials with recombinant human IL-12 have shown its potency in treating CTCL patients, however, the agent is not currently available. IL-12 administered subcutaneously was associated with a 50% response rate.98,99 A multicenter study of 23 MF patients treated with subcutaneous IL-12 at a dose of 300 ng/kg reported a partial response rate of 43%. Adverse effects are similar to interferons.38
HDAC inhibitors: vorinostat and romidepsin
Histone deacetylases (HDACs) are enzymes that remove acetyl groups from core histone proteins.100 Core histone proteins control access of transcription factors to DNA promoter binding regions and therefore modulate gene transcription. HDAC inhibitors are small molecules that bind to and block the deacetylation mediated by HDACs. Pan-HDACs appear to be effective in CTCL and have been investigated as anti-neoplastic agents in clinical trials. HDAC- inhibitors repress deacetylation of tumor suppressor genes and cell cycle regulatory genes, leading to the arrest of neoplastic cell growth and apoptosis.101 HDAC inhibitors (HDACi's) are anti-angiogenesis agents mediated by decreasing VEGF expression.102,103 HDAC enzymes are divided into families (class I-IV) based on homology to yeast HDAC proteins.104,105
HDAC inhibitors have demonstrated additive or synergistic effects with anthracyclines, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), and all-trans retinoic acid.101,102,106-108 They are efficient radiation modifying agents and may be used as clinical radiation sensitizers/protectors.109 However, an unpublished, multicenter study combining low-dose electron beam radiation with vorinostat or no vorinostat did not show improved efficacy or duration of response in the patients receiving vorinostat plus radiation (Kim Y et al. SID Abst 2014).
Vorinostat (Zolinza, Merck), formerly Suberoylanilide Hydroxamic Acid (SAHA), was FDA approved for the treatment of relapsed or refractory CTCL in October, 2006. Vorinostat is an orally bioavailable inhibitor of class I and II HDACs.101,103 In addition to vorinostat, HDAC inhibitors romidepsin (depsipeptide, FK-228), belinostat (PXD101), and LAQ824/LBH589 (panobinostat) have demonstrated therapeutic benefit as monotherapy in CTCL.110 Vorinostat has also demonstrated anti-neoplastic effects in leukemia, lymphoma, and solid tumor models in vivo.102,108,111,112 Preclinical studies showed that vorinostat causes accumulation of acetylated histones in treated patients' tumors and blood cells and induces apoptosis in a broad range of cancer cell lines including Sézary cells.113 Vorinostat induces tumor cell apoptosis at concentrations to which normal cells are relatively resistant.114 Romidepsin also induces apoptosis of CTCL cells in vitro.115 Both vorinostat and romidepsin down-modulate expression of the Th2 cytokine IL-10 which is overexpressed in tumor cells.116
Kelly et al at Memorial Sloan Kettering performed a dose-escalation study of 37 patients with advanced cancer given vorinostat/SAHA by 2 hour intravenous infusions.107 The starting dose of 75 mg/m2/d was escalated to <900 mg/m2/d with no dose-limiting toxicities. Vorinostat was also administered for 5 days every 1-3 weeks for solid tumor patients (n = 17) and 5 days for 3 weeks for patients with hematologic malignancies (n = 12). The maximum-tolerated dose (MTD) in hematologic malignancies was 300 mg/m2/d ×5 days for 3 weeks and median duration of therapy was 6.4 weeks (range, 1.6-40 weeks).107 In 73 patients with hematologic or solid tumors treated with oral vorinostat, the maximum tolerated dose was 400 mg/d, 200 mg twice daily for continuous daily dosing, and 300 mg twice daily for 3 consecutive days per week. Thrombocytopenia was reported in 87% of the patients with hematologic malignancies compared with 44% of the patients with solid tumors.
In 35 patients with hematologic malignancies treated in phase I with either intravenous (n = 12) or oral vorinostat (n = 23) at continuous doses in the range of 400-600 mg/day or 200-400 mg twice daily, vorinostat demonstrated activity in Hodgkin's disease, diffuse B-cell lymphomas and CTCL (1 MF patient with a >4 month PR).117 Observed dose-limiting toxicities were anorexia, dehydration, diarrhea, fatigue, neutropenia, and thrombocytopenia.
In a phase II dose-ranging trial, 33 patients with refractory or relapsed CTCL (stage IA-IVB) were treated with oral vorinostat in 1 of 3 sequential dosing cohorts.101 Four of the 33 patients participated in 2 different dosing cohorts. Patients had a median of 5 prior therapies (range, 1-15), 85% had advanced stage (≥IIB) CTCL, and one-third had SS. Twenty-four percent of patients achieved a documented PR, defined by ≥50% decrease in severity-weighted assessment tool (mSWAT) score, and one-third had pruritus relief, stable disease, or both. Responses were seen in a broad spectrum of patients: early-stage refractory MF, tumor stage with large-cell transformation, and in nodal and/or blood involvement. The median duration of response was 15.1 weeks (range, 9.4-19.4 weeks) overall, which was lowest (9.4 weeks) in the intermittent dosing group of 300 mg twice daily 3 days of 7 and highest (16.1 weeks) in the group treated with 400 mg/day. Grade 3/4 thrombocytopenia was most common (42%) in the cohort treated with 300 mg twice daily continuously for 14 days and was less common (8%) in the other 2 cohorts. The most common toxicities were fatigue, diarrhea, altered taste, nausea, and dehydration. Overall, the 400 mg/d dose provided the most favorable risk-benefit profile, and was selected for the pivotal registration trial conducted by Olsen et al.118
In a phase IIB multicenter trial, oral vorinostat at 400 mg/d was administered to 74 patients with stage IB-IVA MF/SS.118,119 The overall response was 29.7%, 32% patients had pruritus relief, and 1 patient with facial tumors had a near complete long-lasting response. Median time to progression (TTP) in all patients was 4.9 months. Eleven percent of patients had related serious adverse events, 11 patients required dose modifications, and there were 3 deaths in the study including 1 patient with hypertension and valvular heart disease. The most common drug-related adverse events were gastrointestinal symptoms [diarrhea (49%), nausea (43%), anorexia (26%), dysgeusia, dry mouth, vomiting, constipation, and anorexia] or fatigue (46%), thrombocytopenia, weight decrease, alopecia, muscle spasms, increase in creatinine, anemia, and chills.118 Caution is indicated in patients with a history of deep-vein thrombosis or on warfarin therapy due to reported adverse events of pulmonary embolism and thrombocytopenia. ECG changes including ST-T wave changes and QT prolongation were observed but were clinically insignificant.118
Romidepsin (depsipeptide) is a cyclic pan-HDAC-inhibitor that was approved by the FDA in November 2009 for treatment of patients with CTCL who have received at least 1 prior systemic therapy.120 Approval was based on 2 phase II studies, including the following multicenter international study.121 The overall response rate was 38% with 5 complete responses and median duration of response of 15 months. More recently, depsipeptide (romidepsin) also received approval for peripheral T-cell lymphoma based on a response rate of 38% and median duration of response of 8.9 months (2-74 months).122,123 Romidepsin is administered intravenously at 14 mg/m2 over 4 hours, 3 of 4 weeks. It has a slightly higher overall response rate in MF of 34%, which is slightly higher than vorinostat's. The adverse events are similar to other Pan- HDAC-inhibitors. Vorinostat as an oral agent tends to cause mostly gastrointestinal symptoms: change in taste, nausea, vomiting, and diarrhea, whereas patients on romidepsin often complain of fatigue. Because of the potential for HDAC inhibitors to prolong the QT-interval, checking an EKG at baseline is recommended. Both histone deacetylase inhibitors were associated with decrease in pruritus scores in patients who had baseline pruritus.
Photopheresis and combined immunomodulatory therapy
Extracorporeal photochemotherapy (ECP; photopheresis) combines phototherapy with leukophoresis and is based on the DNA damaging effect of light combined with photoactivated 8-methoxypsoralen (8-MOP) on pathogenic T-lymphocytes.124-126 Psoralens are furocumarins, a group of chemicals which strongly absorb UV light maximally in the UV-A range.127 The most extensively used therapeutic psoralen, 8-MOPP, intercalates between DNA base pairs. Upon exposure to UV-A radiation, covalent crosslinking of DNA occurs resulting in proliferative arrest of treated cells. The combination of photosensitizing agent 8-MOP and mononuclear cells collected by apheresis are irradiated by ultraviolet light A (UV-A) ex vivo and reinfused into the patient. The mechanism(s) of action of ECP have been studied extensively. Multiple mechanisms are thought to generate an immune response or vaccination response against tumor cell antigens with the generation of cytotoxic CD8 cells. Treatment effects include photo destruction of cells, induction of T-cell apoptosis, monocyte activation, and maturation, stimulation of cytokines, and stimulation of cell-mediated immune response with changes in immune reactivity of the patient.92,128-132 In an animal model, ECP reverses graft-versus-host disease (GVHD) by inducing donor regulatory T cells.133 CTCL patients, especially those with erythrodermic MF and Sézary syndrome, have been treated with ECP for >20 years using 4 FDA-approved photopheresis devices for CTCL.134 ECP has been effective in SS,135 GVHD,134 solid organ transplantation rejection, and is being investigated as therapy for multiple autoimmune diseases. Whether the mechanism of action is the same in different conditions is unknown but the ratio of monocytoid to dendritic cells appears to be different in SS versus GVHD.136
In 1987, Edelson et al published the first multicenter trial suggesting the benefits of ECP in CTCL.137 Twenty-seven of 37 patients (73%) had >25% improvement, with an average 64% decrease in cutaneous involvement after 22 weeks. Additionally, 88% of lymphocytes in the treated cell concentrate were not viable after treatment. Reinfusion of the damaged cells led to a reduction in the CD4+/CD8+ ratio. Long-term follow-up demonstrated that erythrodermic patients treated with ECP had prolonged survival (median 60 months) compared with historic control groups (median 30 months).137
Studies involving ECP as a monotherapy have shown partial response rates from 20% to 88% and complete response rates of 13% to 33% of patients.138-152 Studies demonstrating that ECP clinically improves and prolongs survival in patients with erythrodermic and advanced-stage CTCL support the use of ECP as first-line for stages III and IV patients.126,144,153,159 There is controversy regarding the benefit of ECP in SS in prolonging survival.160 Fraser-Andrews et al found no significant difference in overall survival of 29 patients with SS who had received ECP (median 39 months) compared with 15 patients who did not receive ECP (median 22 and 27.5 months).160 Opponents argued that the study was limited by a small study population, patients were inadequately treated, ECP patients were heavily pretreated, and the ECP-treated patients may have had worse disease.153,154 We have studied overall survival in a cohort of 124 erythrodermic CTCL patients who were treated with ECP combined with immunotherapy (interferon alpha and/or bexarotene). Their median overall survival was 5.4 versus 2.5 years previously reported a decade before.161 Leukemic SS patients whose count exceeded 10 000 SS cells/μL had a 2.5 year overall survival, which was also reported in a British study.162 Prospective studies are needed to confirm the importance of ECP alone versus ECP combined with immunomodular therapy in patients with SS.
Factors in patients with CTCL that are associated with a more favorable therapeutic include the presence of CD8+ T cells,140,141,163-168 an absence of bulky lymphadenopathy or visceral involvement, lower numbers of Sézary cells (10%-20% of mononuclear cells), limited leukemia (WBC <20 000 per mm3), short duration of disease (<2 years), normal numbers of cytotoxic T cells and normal natural killer cell activity, early response to treatment (within 5 months of treatment), and plaque stage <10%-15% of the skin surface.140,141,163-168 ECP should always be given prior to chemotherapy as it requires an intact immune response.
The role of ECP in early-stage MF patients remains to be established but some dramatic clinical responses have occurred. In a recent review by Miller et al, 124 early-stage patients treated with ECP or ECP plus adjuvant therapy from 1987-2007 were identified in 16 different reports.54,126,138,142-145,151-156,159,168-175 Response rates for early-stage patients varied from 33%-88%. Most of these reports had insufficient patient numbers to enable adequate statistical analysis within each cohort. We recently treated 19 early-stage MF patients with photopheresis with favorable and durable responses noted.176 Large-scale randomized prospective studies are needed to establish if ECP is beneficial in this patient population.
To improve response rates to ECP, interferon (IFN) and/or systemic retinoids have been added as a combined immuno-modality regimen.68 Oral bexarotene is the most commonly used retinoid for combined immunomodulatory therapy. Although the optimal dose is 300 mg/m2, as a monotherapy, lower doses 75-225 mg are generally used with photophoresis to avoid lipemic plasma. Patients who were initially on ECP monotherapy experienced higher response rates when systemic retinoids were added to their regimen.54 The addition of IFN α-2b, the first reported therapy used with ECP, may also have a synergistic effect with ECP.170,177 The dose of IFN alpha used with ECP is also lower than when used as monotherapy: 1-5 million units subcutaneously 3 times weekly and the dose can be increased as tolerated. Anemia from the ECP, interferon, and bexarotene is often present after prolonged therapy. Zackheim criticized studies comparing ECP as a monotherapy versus ECP combined with interferon because other studies have documented that IFN α-2b maybe be as good or better when used as monotherapy.178 Prospective, randomized studies are lacking to confirm these observations.
Maintenance with ECP following total skin electron beam therapy (TBSEB) may improve overall survival.179 Wilson et al evaluated patients who achieved a PR or CR to TBSEB who subsequently were treated with either adjuvant chemotherapy (doxorubicin/cyclophosphamide) or ECP. At 3 years, the group treated with ECP had improved overall survival which approached statistical significance (p < 0.06). They also evaluated erythrodermic (T4) patients treated with TBSEB and concurrent ECP or TBSEB only.34 Patients with CR had a disease-free survival (DFS) of 63%. Within this group, DFS was 49% for patients who received TBSEB alone versus 81% for patients who had received TBSEB and ECP.
There has been 1 report of a higher response rate in 44 patients (17 SS and 26 MF) stage IIB-IV) treated with ECP preceded byfludarabine monophosphate (FAMP at 25 mg/m2 5 days per month) versus fludarabine monotherapy. At a median follow-up of 4.2 years, 63% of the FAMP-ECP patients responded versus 24% of the monotherapy patients (p = 0.021).180 Time to progression was 13 in the combination group versus 7 months in FAMP alone. Other nucleoside analogues including deoxycoformycin, 2-chlorodeoxyadenosine, and forodesine (purine nucleoside phosphorylase inhibitor) are active as monotherapy, especially in SS patients.180,181
Targeted therapies
New targets for the treatment of CTCL include 2 types of agents: those which directly target the clonal tumor cells based on surface markers and those which modulate immunomodulatory cytokines favoring differentiation toward Th1 cells. Targeted therapy to the malignant clone is preferable to preserve the immune system of patients.
Denileukin diftitox
Patients who fail interferon and oral bexarotene or who have tumors or nodal disease (stage IIB to IV MF) are good candidates to receive denileukin diftitox (ONTAK).182 This is a recombinant IL-2-diphtheria toxin fusion protein targeted to the IL-2 receptor expressed on T cells and it does not cause myelosuppression. Denileukin diftitox was approved by the FDA in 1998 for the treatment of cutaneous manifestations of relapsed CTCL at dose levels of 9 or 18 μg/kg. A phase III trial of denileukin diftitox in 73 patients with refractory CTCL who had received ≥3 prior therapies demonstrated a 30% ORR, a 10% complete response rate, and a median duration of response of 6.9 months from time of first dose.183 Denileukin diftitox is quite effective in patients with stage IIB tumor disease, with a response rate of 50%, and offers an attractive tumor burden debulking agent without causing neutropenia.183,184 Higher response rates of 60% and fewer acute symptoms were seen in patients with the highest levels of CD25 expression in lesional skin biopsies using the fixed tissue assay.185 The need for CD25 expression for denileukin diftitox to work is controversial and did not show up in a recent larger study.186
In a phase I study, denileukin diftitox was combined with bexarotene to increase expression of CD25 levels.187 Fourteen patients with relapsed or refractory CTCL were treated with escalating doses of bexarotene (75-300 mg/d) and denileukin diftitox (18 mcg/kg/d ×3 days every 21 days) had an overall response of 67% (4 complete responses, 4 partial responses).187
The results of a multicenter phase III double-blind randomized trial of denileukin diftitox, recently published, showed significant response rates at both 9 and 18 mcg/kg dose levels compared with placebo controls in patients who had received ≤3 prior therapies.188 Unfortunately, because of manufacturing issues, denileukin diftitoxin has been unavailable since fall 2011. A more stable version of denileukin diftotox, called E7777, has been evaluated in part 1 of a phase I/II dose escalation trial (ASH Abstract 2014).
The side effects of denileukin diftitox include constitutional symptoms, hypersensitivity rash, and transient elevation of hepatic transaminases, thyroiditis with subsequent hypothyroidism, and vision changes.183,189 Capillary leak syndrome, defined as edema, hypoalbuminemia, and hypotension, may occur in 20%-30% of individuals and is maximal at approximately day 10. It can be severe in some patients secondary to pulmonary edema but is generally self-limited. Premedication with systemic corticosteroids has been shown to decrease the frequency of acute hypersensitivity reactions but does not prevent capillary leak syndrome.190 Administering 500 cc of saline after each denileukin diftitox infusion may decrease the frequency of capillary leak syndrome but may also lead to increased peripheral edema.191 It is important to carefully monitor the patient's weight before, during, and after therapy and administer low doses of furosemide.
Targeted monoclonal antibodies
Monoclonal antibodies targeting key activation determinants expressed on T lymphocytes have shown clinical efficacy in preliminary studies in CTCL. An antibody targeted to the malignant T cell specifically would be extremely useful for CTCLs administered alone or in combination with other agents.
Alemtuzumab
Alemtuzumab (Campath-H1, Genzyme) is a humanized immunoglobulin that targets CD52, expressed on most T and B lymphocytes, and NK cells. A response rate of 50%-70% has been reported in CTCL patients treated with alemtuzumab by Lundin et al,192 however, prolonged depression of T, B, and NK cells is reported. Alemtuzumab has been associated with prolonged immunosuppression leading to reactivation of cytomegalovirus and other opportunistic infections,193 and general infectious prophylaxis is recommended. Alternative dosing schedules with lower doses and subcutaneous administration are being investigated. Querfeld et al reported favorable responses when intravenous alemtuzumab was followed with lower-dose subcutaneous antibody.194 Bernengo et al195 reported a 86% response rate in 12 of 14 refractory Sézary syndrome patients including 3 complete responses. Low doses were administered by subcutaneous injections on alternate days. Four patients received 3 mg alemtuzumab on day 1, 10 mg on day 3, and then 15 mg on alternate days. The remainder received 3 mg then 10 mg on alternate days until the SS count came down to <1000 cells/μL. Infections were noted at 28% of patients who received the higher 15 mg dose. Recovery of normal NK cells was seen after the first cycle.195
SGN-30 (anti-CD30 monoclonal antibody) and SGN-35–conjugated brentuximab vedotin
SGN-30 is a chimeric anti-CD30 monoclonal antibody targeting cells expressing CD30, the tumor necrosis factor-receptor family member 8 and Kiel-1 antigen. CD30 is expressed on Reed-Sternberg cells of Hodgkin's disease, in cutaneous anaplastic large cell lymphoma (ALCL), and in type C lymphomatoid papulosis lesions.196 CD30 is also expressed frequently at various levels on lesions of MF especially during transformation to large cell lymphoma.197 CD30 also may be induced by viral infections as an activation marker.
A 20% objective response rate in patients with systemic nodal CD30+ refractory ALCL was observed in patients treated with the naked CD30 antibody alone.198,199 In a phase II multicenter trial of patients with one or more primary cutaneous CD30+ lymphoproliferative disorder [primary cutaneous ALCL (PC-ALCL), lymphomatoid papulosis, or CD30+ MF], clinical responses were seen in 87% of patients with ALCL, CD30+-transformed MF, and lymphomatoid papulosis.198 Based on this study and high response rates seen in systemic relapsed ALCL, 2 investigator-initiated phase II trials of the tubulin inhibitor-conjugated MMAE to CD30 antibody brentuximab vedotin were conducted in patients with CD30+ CTCL. Patients in both studies received 1.8 mg/kg every 3 weeks up to 16 doses with global response as the primary end-point. At MD Anderson 48 patients with CD30+ MF or CD30+ lymphoproliferative disorders (ALCL or lymphomatoid papulosis) had an overall response rate of 73% with a complete response rate of 35%. There was a 100% response in patients with LyP or primary cutaneous ALCL. MF patients had a response rate of 54% irrespective of CD30 expression level.200 A second trial conducted at Stanford enrolled 30 MF patients with variable levels of CD30 expression, examined by immunohistochemistry and image processing.201 The global response rate was 70% (21 of 30 patients) and was lowest in patients with low expression (<5%).
The most frequent side effects in both studies were peripheral neuropathy, drug hypersensitivity rash, nausea, and fatigue. Grade 1-2 neuropathy was reported in 65% of patients and was ongoing in 55% after stopping the drug.200 Neuropathy may be associated with lower tumor burden (LyP) and number of courses given. Neuropathy is preventable; the drug should be stopped when patients develop grade 2 symptoms. Gabapentin may be helpful for the symptoms. Based on the success of the investigator-initiated trials, there is a large multicenter randomized trial comparing BV to dealer's choice of methotrexate or bexarotene therapy.
Mogamulizumab: defucosylated anti-CCR4
Malignant T cells in CTCL and PTCL patients express the chemokine receptor CCR4, which is increased with progression of MF.202 This synthetic defucosylated antibody has been approved in Japan for patients with adult T-cell lymphoma.203 The antibody versus vorinostat is currently in a multicenter phase III randomized clinical registration trial. A phase I/II trial of 38 MF/SS patients found no dose-limiting toxicity at 0.1, 0.3, and 1 mg/kg infused every week for 4 doses and then every other week until progression.204 The overall global response rate was 36.8% in all patients, 28.6% in MF patients, and 47.1% in SS patients. The reduction of circulating SS cells was rapid with 11 CRs in blood, and prolonged complete global responses were seen. Side effects in 20%-30% of patients included nausea, chills, headache, rash, and infusion reactions. Of interest, treatment was associated with reduction of CD4+CCR4+ FoxP3+ T-regulatory cells and increased NK and CD8+ T cells, suggesting a beneficial dual mode of action.205
Lenalidomide and velcaide
A smallphase II exploratory multicenter trial examined the activity of lenalidomide in CTCL patients.206 Although initial responses were seen in SS patients at the oral dose of 25 mg daily, lower doses with escalation were not as effective and the overall response was only 19%. Other myeloma therapy including protease inhibitor velcaide has anecdotal evidence for responses in CTCL patients.
Single-agent chemotherapy
In CTCL patients with tumors, nodes, or visceral disease, single-agent or combined chemotherapy is often administered with expectation of inducing a partial remission. Single-agent chemotherapy can be effective but the duration of response may be short. Choice of therapy is based on stage, concomitant medical conditions, and prior treatments as each agent has a unique side effect and efficacy profile.
Methotrexate, pegylated liposomal doxorubicin (Doxil, Ortho Biotech Products), gemcitabine (Gemzar, Eli Lilly), and pentostatin (Nipent, SuperGen) have all been studied in small phase 2 studies of CTCL patients.
Praletrexate
Praletrexate, a folic acid inhibitor, has recently shown efficacy in both MF and in transformed MF in a phase I/II dose de-escalation trial.207 The optimal dose of 15 mg/kg was given 3 of 4 weeks with an overall response rate of 45%. Praletrexate has also been evaluated in combination with oral bexarotene 150 mg/m2 based on the initial preclinical activity with promising long-term responses.208 The main side effects of praletrexate are mouth ulcers, skin lesion necrosis, and myelosuppression mucositis may be prevented or treated by giving 25 mg of leukovorin after the dose and a B12 shot given every 2 months.
Gemcitabine
Gemcitabine hydrochloride (Gemzar, Eli Lilly), a nucleoside analogue of deoxycytidine that inhibits DNA synthesis, has shown activity against solid tumors as well as hematologic malignancies.209 In 1998, Zinzani et al first documented 1 CR and 4 PRs to gemcitabine (1200 mg/m2) in 8 patients with cutaneous peripheral T-cell lymphoma (PTCL) and 4 of 5 patients with MF.210 In 2001, Sallah et al reported an overall response rate of 60% with a median duration of response of 13.5-16.2 months in 10 patients treated with gemcitabine at 1200 mg/m2.211 The multicenter phase II clinical trial conducted by Zinzani et al included 44 patients (30 MF, 14 PTCLs) treated with gemcitabine (1200 mg/m2 administered for 3 of 4 weeks for 3 courses) and reported an ORR of 70.5% with a median duration of response of 15 months.212 Similar results were documented in the phase II study by Marchi et al of 32 patients (26 MF, 1 SS, 5 PTCLs) treated with gemcitabine (1200 mg/m2 once per week for 3 of 4 weeks for 6 courses) with an ORR of 75% and a median duration of response of 10 months (4-22 months).213
We have demonstrated that a lower dose of gemcitabine (1000 mg/m2 once per week for 3 week cycles) produced an ORR of 68% in 25 patients with advanced-stage and refractory MF.214 It was especially active in MF patients with cutaneous tumors. Gemcitabine can be used in combination with bexarotene maintenance therapy to manage the plaques and patches of mycosis fungoides.15 It can also be alternated with liposomal doxorubicin infusions to prolong the duration of chemotherapy.
Adverse effects of gemcitabine have most frequently involved bone marrow suppression (leukopenia, anemia), mild alopecia, generalized hyperpigmentation, and elevation of hepatic transaminase and creatinine levels.214 Three of 25 CTCL patients with SS developed hemolytic uremic syndrome in our study, although the overall incidence previously reported was only 0.6%.214 Rasburicase was used successfully to bring down the high uric acid levels. Sapacitabine, (also known as CYC682) a deoxycytidine analogue like gemcitabine, was studied in a phase I/II trial but was not active at the low doses tested.
Pentostatin
Pentostatin (2′-deoxycoformycin or dCF or Nipent, SuperGen) is a potent inhibitor of adenosine deaminase, is selectively toxic to lymphocytes.84,85,215 Griener et al first documented an ORR of 39% in 18 patients with stage I to IVB CTCL treated with 4 to 5 mg/m2 of intravenous pentostatin every 1 to 4 weeks. Two patients had CRs with a duration of response of 4 months to 6 years and 5 patients had PRs lasting for 1.5 to 6 months.85 Foss et al reported a 40% OR rate and 7% CR rate in 94 CTCL patients treated with pentostatin studied in multicenter phase II trials. The median time to progression ranged from 1.3 to 8.3 months.216 Kurzrock et al reported a 71% OR rate and 25% CR rate in 14 patients with SS and 6 patients with tumor-stage MF treated with pentostatin.217 In a phase II study combining pentostatin with intermittent high-dose interferon-α, Foss demonstrated median progression-free survival of responders of 13.1 months.83 Although duration of response was longer, response rates (ORR 41%) were similar to those seen with single-agent pentostatin.83 Toxicities include hematologic, renal insufficiency, nausea, and conjunctivitis.217 Pentostatin has also been associated with angina and myocardial infarction, heart failure, and acute arrhythmias in patients with predisposing conditions such as coronary artery disease, congestive heart failure, hypertension, and pulmonary metastases.218 It is now recommended to correct anemia by transfusion if warranted, optimize cardiac medications, control nausea and vomiting, correct hypercalcemia, reduce pentostatin dose for patients with impaired renal function, and monitor fluid balance to prevent fluid overload.218
Pegylated liposomal doxorubicin
Doxorubicin (Doxil, Ortho Biotech Products) is an anthracycline with antineoplastic effects in nodal lymphomas,219 solid tumors,219 myeloma,220 and acute leukemia.221 The pegylated liposomal form of doxorubicin allows for reduced toxicity, improved efficacy, and a longer half-life.222 Wollina et al first published the efficacy and safety of liposomal doxorubicin in 2000.219 Ten patients with MF (stage IB to IVA) were treated with liposomal doxorubicin at a dose of 20 mg/m2 with an OR rate of 80% and a high CR rate of 60%. Mean disease-free survival was 13.3 months.219 In a retrospective multicenter study evaluating 34 CTCL patients treated with various doses and schedules of liposomal doxorubicin (20-40 mg/m2 every 2-4 weeks), an OR rate of 88% was reported.223 Grade 3-4 toxicities included 3 patients with lymphopenia, 3 with anemia, and 1 with capillary leak syndrome. Side effects include nausea, vomiting, hand/foot syndrome, and myelosuppression. Cardiomyopathy is dose-dependent and not generally seen in cumulative doses <450-500 mg/m2.
We studied liposomal doxorubicin at 30 mg/m2 given every 3 weeks followed by bexarotene maintenance therapy in a small exploratory phase II trial.76 The response rate of 43% was lower than expected. Although patients with transformed MF tumors had dramatic responses, their tumors relapsed on bexarotene maintenance. One patient with blood and node and erythroderma (SS) has had a durable complete response.
Combination chemotherapy
Multiple studies have reported results of combination chemotherapies alone or combined with topical nitrogen mustard or total skin electron beam radiation. With combination chemotherapy, the response rates are high but duration of response may be short-lived.21,224-228
In 1974, Winkelmann et al first advocated the use of oral low-dose chlorambucil and prednisolone as a relatively non-toxic chemotherapeutic option for palliation of Sézary syndrome. In 21 patients treated with this regimen, the OR rate was 57% with 3 complete responders (14%).224 CVP (cyclophosphamide, vincristine, prednisone), CVPB (cyclophosphamide, vincristine, prednisone, and bleomycin), and CHOP (cyclophosphamide, adriamycin, vincristine, and prednisone) have also been effective in MF.225,226 In 1998, Hallahan et al treated patients with T3 stage MF with TSBEB and MOPP (methotrexate, vincristine, procarbazine, prednisone) or COPP (cyclophosphamide, vincristine, procarbazine, prednisone) and reported a 70% OR rate and 14 month duration of response.227 In the first randomized trial for MF, Kaye et al compared combined modality (TBSEB and chemotherapy) to more conservative topical sequential conservative therapy (mechlorethamine, PUVA, TBSEB, methotrexate) and found no difference in disease-free survival and overall survival with either modality.21 Zakem et al treated 10 patients with stage IIB-IVB MF with a combination chemotherapy program consisting of bleomycin and methotrexate weekly, doxorubicin every 3 weeks, and topical nitrogen mustard daily (BAM-M). The OR rate was 80% with 7 patients obtaining histologically documented complete remissions lasting 4-105+ months.228
Although CTCL is often transiently responsive to combined regimen chemotherapy, the effect on increased survival or ability to induce durable remissions is limited. To improve treatment efficacy and outcome in patients with MF/SS, we conducted a combined modality protocol using 3-4 consecutive phases of therapy, which was initiated in 1987.56 Between 1987 and 2001, 95 patients with early-stage (IA-IIA, n = 50) or late stage (IIB-IVB, n = 45) MF were treated initially with subcutaneous interferon-α2a (IFN-α) and oral isotretinoin (1 mg/kg) daily for 4 months, followed by total body skin electron beam at 36 Gy (TBSEB), and long-term maintenance therapy with topical nitrogen mustard and IFN-α. Patients with late-stage (IIB-IVB) disease also received 6 cycles of combination chemotherapy with cyclophosphamide, methotrexate, etoposide, and dexamethasone (CMED) before receiving electron beam radiation. Standard CMED was given as a 21 day cycle according to the following schedule: intravenous (IV) cyclophosphamide, 500 mg/m2 on day 1; IV methotrexate, 1 gm/m2 on day 3; IV etoposide, 100 mg/m2 daily on days 1 to 3; and oral dexamethasone, 40 mg daily for 5 days. Combined modality therapy yielded a response rate of 85% with a 60% complete response rate. Thirty-eight patients (76%) with early-stage disease and 18 of 45 (40%) patients with late-stage MF and SS achieved complete response. Nine (24%) patients with early-stage MF and 3 patients (17%) with late-stage MF have achieved sustained remissions lasting >5 years. Median disease-free survival (DFS) for early and late stages of disease was 62 and 7 months, respectively, with 5 year Kaplan-Meier estimated rates of 50% and 27%, respectively.56 The multiphase combined modality regimen is well tolerated and may yield higher response rates and disease-free survival than TBSEB therapy alone.56
Allogeneic stem cell transplant
Non-ablative, allogeneic hematopoietic stem cell transplant (HSCT) is now being considered for younger, healthy patients with advanced CTCL (≥IIB) who have advanced stage at presentation and fail to respond to first-line therapy.17 Patients need to have a related or unrelated matched donor and be physically and emotionally able to undergo the procedure. The existence of a graft versus T-cell lymphoma effect has been suggested in recent reports, particularly using non-myeloablative conditioning.229 Select patients have achieved long-term remissions and curative responses.230 The timing of HSCT is controversial and patients with rapidly progressing MF/SS often become ineligible for treatment. Tumor debulking with chemotherapy for nodal disease or with TBSEB for skin involvement must be successful prior to transplant. Allogeneic stem cell transplant has superior survival and event-free outcome over autologous HSCT in MF/SS. The procedure remains high-risk, thus early-stage patients with good prognosis are not candidates for this procedure. Although this is not standard procedure at all centers, we found that pretreatment with TBSEB reduces the rate of relapse, lengthens disease-free survival, and may reduce severity of acute GVHD.
Treating MF tumors 2015
Treatment should target the tumor and preserve the immune anti-tumor response.3,13 Our analysis of survival in advanced MF patients suggests that patients who receive radiation or biologic agents live 2.5 times longer than patients treated with chemotherapy.16 For solitary tumors local radiation is extremely effective and TBSEB can be lifesaving in patients with multiple tumors who are able to go to allogeneic transplant rapidly.17 For CD30+ tumors, brentuximab vedotin has high response rates with convenient administration every 3 weeks and with low toxicity.198 HDAC-inhibitors romidepsin, vorinostat, or belinostat have response rates of ∼30% and reduce tumor burden in some patients. Denileuken diftitox had a 37% response rate for tumors but is temporarily unavailable.118 Patients with multiple tumors failing the above therapies are treated with gemcitabine212,214 or liposomal doxyrubicin214 before use of combination chemotherapy.15 Praletrexate (15-20 mg/m2) alone or combined with bexarotene (150 mg/m2) is another alternative monotherapy.208
Treating SS patients 2015
Erythrodermic patients with abnormal circulating T cells (Sézary syndrome) are colonized with staphylococcus and need emolliants, topical and oral antibiotics, and topical, not systemic, steroids.24 Combination immunotherapy (photopheresis with low-dose interferon and/or bexarotene) should be used as first-line therapy with response rates of >50%.177,231 Photopheresis works best in patients with T-reg CD4+FoxP3+CD25- T-reg cells231 and corrects dendritic cell ratios.232 For patients with a matched donor, total body skin electron beam followed by non-ablative allogeneic stem cell transplant can produce durable complete responses in ∼50% of patients.17,230 SS patients respond to second- or third-line therapy with histone deacetylase inhibitors or to targeted antibodies. Mogamulizumb, targeting CCR4 has a 47% response rate in SS and has produced long durable complete responses without immunosuppression.233 Because of the potential for infection and immunosuppression, use of alemtuzimab (CD52 antibody) should be limited to 10 mg given subcutaneously on alternate days and stopped when the SS are <1000 cells/μL.195
Conclusions
Cutaneous T-cell lymphomas, of which mycosis fungoides and Sézary syndrome are the most commonly encountered, are currently not curable in most patients. Patients with early, skin-limited disease do extremely well on skin-directed therapies and should not be subjected to therapy that will decrease their immune competency and lead to disease progression. Novel targeted therapy and combination therapies are producing higher response rates with more durable remissions. The key to finding better treatments is to better understand the disease pathogenesis and heterogeneity at a molecular level. The best clinical results are achieved when skin care and skin-directed therapy are combined with effective biologic response modifiers or targeted therapy. New therapies under development are exciting prospects to improve treatment. Recent withdrawal or unavailability of active agents including denileukin diftitox and liposomal doxorubicin have decreased access to highly effective therapies. Finally, lack of randomized trials and rising expense of available approved drugs have added to the challenge of treating CTCL successfully.
Correspondence
Madeleine Duvic, Department of Dermatology, University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Ste 1453, Houston, TX 77027; e-mail: mduvic@mdanderson.org.
References
Competing Interests
Conflict-of-interest disclosure: The author is on the Board of Directors or an advisory committee for Seattle Genetics, Millennium Pharmaceuticals, Kyowa Hakko Kirin, and Celgene; has consulted for Oncology Meeting Innovations, MiRagen Therapeutics, Huya Bioscience Int'l, ClearView Healthcare Partners, Cell Medica, Celgene, Array Biopharma; and has been affiliated with the Speakers Bureau for Therakos.
Author notes
Off-label drug use: None disclosed.