Abstract
Multiple tyrosine kinase inhibitors (TKIs) are available for managing patients with chronic myeloid leukemia. Although most patients have a favorable outcome with their initial therapy, whether imatinib or a second-generation TKI was used, some will require subsequent use of one or more different TKIs. Such sequencing might be indicated in a reactive way (ie, for patients who have experienced resistance or intolerance to their initial therapy) or in a proactive way (ie, for patients with a somewhat favorable outcome who have not reached an “optimal” outcome). Sequencing of TKIs has become standard practice, and the proper use of sequenced TKIs is likely to optimize outcomes and resource utilization.
Learning Objectives
Assess the different scenarios in which TKI sequencing can be used in patients with chronic myeloid leukemia
Critically review the outcomes associated with sequencing of TKI in patients with chronic myeloid leukemia
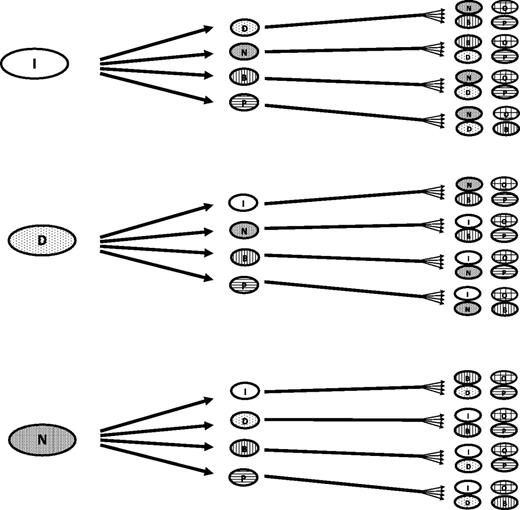
Tyrosine kinase inhibitors (TKIs) became standard therapy for patients with chronic myeloid leukemia (CML) not long after the first patient received STI571 (later known as imatinib mesylate) on a phase I clinical trial.1 The initial approved indication was for patients with resistance to or intolerance of interferon alpha–based therapy, the standard at the time. By 2003, imatinib was approved as frontline therapy for CML,2 and since then, nearly all patients with access to TKIs have received them as initial therapy. Shortly thereafter, second-generation agents, including dasatinib, nilotinib, and bosutinib, with increased potency, different structures that allowed them to overcome most mutations leading to resistance to TKIs, and different toxicity profiles, were introduced and eventually received regulatory approval. Later, ponatinib, a drug with activity against T315I and all mutations tested, proved effective in overcoming resistance in most patients, even those who had received 3 or more prior TKIs. All TKIs were eventually compared with imatinib in the first-line setting, and they generally demonstrated higher response rates, with deeper and faster responses which, in the case of dasatinib and nilotinib, were sufficient to lead to their approval as initial therapy for patients with chronic phase CML (CML-CP). This rapid development has resulted in the availability of multiple TKIs, and along with this, the sequential use of various TKIs in patients with CML-CP (Figure 1).
Possible treatment sequencing approaches in CML. At least 48 unique sequences can be identified. Not all options might be available in all parts of the world or they may have different indications. Stem cell transplant, investigational options, combination therapy, and others are not included for simplicity; including these options increases the number of sequence options available. B, bosutinib; D, dasatinib; I, imatinib; N, nilotinib; O, omacetaxine; P, ponatinib.
Possible treatment sequencing approaches in CML. At least 48 unique sequences can be identified. Not all options might be available in all parts of the world or they may have different indications. Stem cell transplant, investigational options, combination therapy, and others are not included for simplicity; including these options increases the number of sequence options available. B, bosutinib; D, dasatinib; I, imatinib; N, nilotinib; O, omacetaxine; P, ponatinib.
The use of sequential TKI therapy, regardless of the choice of initial therapy, is perhaps more common than is usually appreciated. Despite the professed efficacy of initial therapy with TKIs, at least one-third of all patients initially treated in clinical trials with any TKI for newly diagnosed CML, whether imatinib or a second-generation TKI, have received at least one additional TKI. After 5 years of follow-up in the DASISION trial (in the final report), 39% of patients initially treated with dasatinib and 37% of those treated with imatinib are no longer receiving their initial therapy.3 Similarly, the 5-year follow-up of the ENESTnd study showed that 40% of patients treated with nilotinib 300 mg twice per day (the standard dose for first-line therapy), 38% of those treated with 400 mg twice per day, and 50% of those treated with imatinib had discontinued therapy.4 With a shorter follow-up (24 months), the BELA trial reported that 37% and 29% of patients treated with bosutinib (not currently approved as first-line therapy) or imatinib, respectively, discontinued therapy.5 Studies with longer follow-up suggest that fewer patients discontinue therapy beyond the first 3 to 5 years. After 10 to 12 years of follow-up, 41% of patients treated with standard-dose (400 mg) imatinib and 43% of patients treated with high-dose (800 mg) imatinib had discontinued therapy.6
Unfortunately, the contribution of sequential therapy to the overall outcome of patients has received less attention than would be expected considering the frequency of its use. Most of the literature reports on the results of intervention with a given TKI for as long as it is used. Studies are frequently terminated prematurely (eg, IRIS after 8 years, DASISION after 5 years). The contribution of prior and subsequent therapy is infrequently considered in assessing long-term outcome. Thus, the effect of sequential therapy is incompletely understood and frequently inferred only indirectly. The value of such an approach can have a significant impact on different end points of interest for patients with CML-CP. Furthermore, sequential TKI therapy can be used reactively or proactively. In the reactive approach, a TKI switch is used when a patient experiences therapy failure, whether because of resistance or intolerance. In the proactive approach, the switch is implemented to improve a response or tolerability that is perhaps acceptable but not what could be considered optimal.
Long-term survival end points
The ultimate end point of cancer therapy is overall survival (OS). The effect of sequential therapy for CML-CP on survival has not been specifically reported but can be extrapolated with some accuracy on the basis of the published reports. Across multiple studies, despite the high rates of treatment discontinuation summarized above, the OS has remained excellent. In the DASISION study, 5-year OS was 91% in patients treated with dasatinib and 90% in those treated with imatinib.3 Similarly, in the ENESTnd study, the 5-year survival rate was 94% to 96% for patients treated with nilotinib and 92% for those treated with imatinib.4 With longer follow-up, 10-year survival rates of more than 80% with imatinib therapy have been reported.6 The reasons for change in therapy vary. Generally, in both the DASISION and ENESTnd studies, the most common reason for change from imatinib was related to efficacy (ie, suboptimal response, treatment failure, or disease progression), whereas the most common reason for change from a second-generation TKI was the presence of adverse events.3,4 Regardless, these excellent rates of survival are at least in part due to the efficacy of subsequent therapy with TKIs, which are now routinely used in most patients.
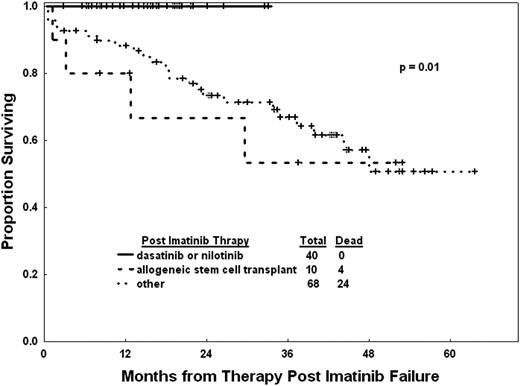
Before the wide availability of subsequent TKIs, patients with resistance or intolerance to imatinib had a projected 3-year survival rate of 72%.7 However, the value of subsequent use of TKIs was evident, even in these early stages of somewhat limited availability of second-generation TKIs. Patients in this category who received subsequent therapy with TKIs had a clear survival benefit compared with those treated with other modalities (Figure 2).7 Another way to assess this is by measuring the conditional survival for patients after each year of therapy. Among 483 patients treated with TKIs as initial therapy and observed over a median of 99 months, the conditional survival probabilities of failure-free survival (FFS) decline slightly over the years whereas the conditional survival probabilities remained at nearly 100% regardless of response to therapy.8 FFS is affected by discontinuation of therapy for any reason, but the use of subsequent therapy in these instances results in a minimal impact on OS. As a result, the life expectancy of patients with CML today is very similar to that of the general population.9
Outcome of patients for whom imatinib has failed according to subsequent therapy.7
Outcome of patients for whom imatinib has failed according to subsequent therapy.7
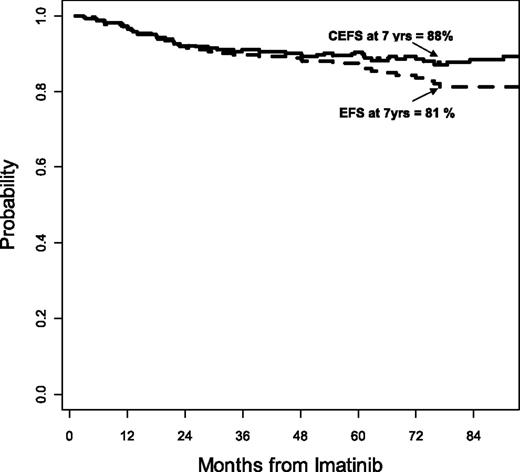
The favorable impact of sequential TKI therapy on event-free survival (EFS) is more difficult to assess because events are typically considered irreversible in the reports from most clinical trials. This means that in all CML TKI trials, EFS is reported only for the intervention in question. The standard approach is to consider that when a patient develops an event, which in CML is typically considered as the loss of a major cytogenetic response (MCyR), loss of hematologic response, transformation to accelerated or blast phase, or death, the event is irreversible regardless of subsequent interventions. Although this is universally true for death, and to some extent for transformation, particularly to blast phase, other events can be reversed when effective therapies are available. Such is the case in CML when 2 important conditions are met that permit reversibility of the event: first, the event should not lead inevitably (or at least not in high proportion) to death; second, there should be effective therapy that allows the possibility of rescuing a significant number of patients from such events and returning them to a state of adequate response. In CML, loss of a response (or for that matter, failing to achieve an optimal response as defined by the European LeukemiaNet [ELN]) is not usually associated with an immediate threat of death. The long-term outcome might be somewhat inferior, but most of the risks come after several months or even years. And the availability of multiple TKIs allows reversibility of many of these events. The stem cell transplant (SCT) literature suggests that interventions such as donor lymphocyte infusions upon relapse can reverse many instances of relapse and ultimately result in a long-term favorable outcome despite the initial relapse, which has led to the term “current EFS.” Calculating current EFS with medical intervention, one can measure the effect of subsequent intervention with TKIs to rescue patients after relapse. For patients receiving imatinib as initial therapy for CML, the EFS rate was reported to be 81% by the standard definition. When accounting for the effect of subsequent treatment with TKIs, the rate improved to 88% (Figure 3).10 Even this is likely an underrepresentation of the favorable effect of sequential TKI therapy because the report by Al-Kali et al was written early in the days of multiple TKIs, when drugs such as ponatinib were not yet available, and patients who received any other intervention between TKIs were considered to have irreversible events. Still, this analysis emphasizes the value of sequential TKI therapy in maintaining a favorable long-term outcome for patients.
Current EFS for patients receiving imatinib as initial therapy for CML-CP.10 CEFS, current event-free survival.
Current EFS for patients receiving imatinib as initial therapy for CML-CP.10 CEFS, current event-free survival.
Improving Molecular Response
Increasingly, a major goal of TKI therapy has been to improve molecular response (MR). This is pursued with the aim of decreasing the probability of transformation to accelerated or blast phase, improving long-term survival end points, and improving the probability of elective treatment discontinuation. Several studies have documented short-term improvement in MR upon changes in therapy. In the extension portion of the ENESTnd trial, for example, patients treated with imatinib or with nilotinib 300 mg twice per day could switch to nilotinib 400 mg twice per day if their treatment failed or if they had a suboptimal response. Of the 35 patients initially treated with imatinib who entered this extension study, 15 (58%) of 26 patients without complete CyR (CCyR) achieved this response. In addition, 11 (32%) of the 34 patients who entered the extension phase of the trial without major MR (MMR) achieved this response, including 2 of 8 who switched while in CCyR but who had no MMR.11
The LASOR study investigated switching to nilotinib vs increasing the dose of imatinib for patients with suboptimal response to therapy according to the ELN 2009 criteria (this subset of patients is classified as having treatment failure in the 2013 version of the ELN recommendations). That study included patients with no CyR at ≥3 to <6 months (ie, Philadelphia chromosome–positive [Ph+], >95%), no partial CyR at ≥6 to <12 months (ie, Ph+, 36% to 95%), or no CCyR at ≥12 to <18 months (ie, Ph+, 1% to 35%). The primary end point was achievement of CCyR 6 months after the start of study therapy. This end point was achieved by 50% of patients who switched to nilotinib and 42% of those who increased their dose of imatinib. The crossover design of the study allowed further exploration of the effects of sequential TKIs. Responses at 6 months excluding crossover, were 50% with nilotinib and 36% with a higher dose of imatinib, and additional responses occurred in 6% of the patients after crossover from imatinib to nilotinib. By 24 months after enrollment in the study, the CCyR rate by intention to treat was 51% in the nilotinib arm and 61% in the imatinib arm; however, most of the responses in the group initially assigned to imatinib dose escalation occurred after crossover to nilotinib. In addition, MMR at 12 months was achieved by 37% and 25%, respectively, on an intention-to-treat analysis, but 38% of the responses in the patients assigned to imatinib occurred after crossover. This suggests that the sequential use of imatinib followed by nilotinib resulted in a further increase in response.12
A more proactive approach to using sequential therapy is represented by the TIDEL II trial in which patients initially treated with imatinib 600 mg/day who did not achieve predetermined response targets (BCR-ABL1 ≤10% according to the International Scale [IS] at 3 months, ≤1% IS at 6 months, or ≤0.1% IS at 12 months) were offered a dose increase (cohort 1) or a switch to nilotinib (cohort 2). Of the 54 patients who switched to nilotinib, 39% achieved an MMR at 24 months. Importantly, this prospective sequential use of TKIs led to a cumulative rate of 34% MR 4.5-log (MR4.5) at 24 months, with 49 (69%) of the 71 patients who reached this milestone achieving it with imatinib alone.13 Interestingly, the rate of MR4.5 was 25% at 24 months with nilotinib 300 mg twice per day in the ENESTnd study14 and 17% with dasatinib in the DASISION study.15 Comparisons across different trials have many drawbacks, but analysis of these data suggests that this pre-emptive sequential use of TKIs might be a valid alternative to the use of second-generation TKIs from the start.
Aiming for deeper MR
One other approach in which pre-emptive use of sequential TKIs can be considered is for patients who, although they had an adequate response (eg, CCyR or MMR), had not reached an MR4.5 after a length of time on treatment considered adequate to expect such a response. The rationale for pursuing this strategy can be debated. In terms of achieving an improved OS or even EFS, the life expectancy of patients who achieve a CCyR is already nearly identical to that of the general population; thus, there is little or no gain in achieving deeper responses.16 Furthermore, as mentioned earlier, the conditional OS or EFS for someone who has remained alive and free of events for 2 to 3 years (ie, the median time to achieve MR4.5) is nearly 100% for each subsequent year.8 Thus, this strategy offers little benefit in this regard. However, discontinuation of elective treatment is currently considered only for patients who have achieved a sustained deeper MR defined as at least MR4.5 (although the most robust experience from the STIM trial used a >5-log reduction in transcript levels, and some more recent studies are exploring discontinuation with MR4). The ENESTcmr study pursued precisely this approach by enrolling patients with persistent minimal residual disease but with CCyR while on therapy with imatinib for at least 2 years. Patients were then randomly assigned to change to nilotinib 400 mg twice per day or continue with imatinib at the same dose they were receiving up to that time.17 The cumulative incidence of confirmed MR4.5 after 12 months was 12.5% with nilotinib and 5.8% with imatinib; the cumulative incidence increased to 22.1% and 8.7%, respectively, by 24 months. After crossover, which was allowed for patients receiving imatinib after 2 years from study entry, a further increase in the rate of MR4.5 was observed. By 4 years, 53.8% of patients on the nilotinib arm and 44.7% of patients on the imatinib arm had achieved MR4.5.18 Whether a strategy such as this or a strategy in which second-generation TKIs are used from the start are equivalent in achieving deep MRs is not known. However, some considerations can be made on the basis of available data. The projected cumulative rate of MR4.5 by 5 years with dasatinib is 42% in the DASISION study and 52% to 54% with nilotinib in the ENESTnd study; rates in both studies are 31% to 33% with imatinib. A large majority (>80%) of patients entering the ENESTcmr study had received imatinib for more than 3 years. The 2-year improvement in the rate of MR4.5 starting at year 3 was 20% (13% to 33%) in the DASISION trial and 16% (15% to 31%) in the ENESTnd trial.4 The magnitude of change is not fully comparable because the selection of patients was different, from diagnosis in the later studies to selected patients at a specific time point in the ENESTcmr study. But these results suggest that using a strategy of changing therapy only for patients who have not met the desired goal by a given time (eg, 3 years from start of therapy) could produce results similar to those achieved when all patients begin with second-generation TKIs from the start. Furthermore, in the TIDEL II trial, with 73 (35%) of 210 patients switching from imatinib to nilotinib (54 patients were unable to achieve efficacy end points, 19 because of intolerance), the cumulative rate of MR4.5 at 2 years was 34%,13 which seems to be somewhat similar to (and perhaps even better than) the 19% to 25% reported with dasatinib (DASISION) or nilotinib (ENESTnd) at the same time point. To be eligible for treatment discontinuation, however, patients should reach that level of response and also be able to maintain it for at least 2 years. According to the ENESTnd study, ∼75% of patients who achieve MR4.5 have maintained such a response, regardless of whether they achieved it with imatinib or nilotinib. This translates into 41% to 44% of all patients treated with nilotinib and 26% of those treated with imatinib.19 Considering the high rate of cumulative MR4.5 (44.7%) by 4 years in the ENESTcmr study with imatinib, it is reasonable to assume that, in the end, starting with imatinib therapy and changing therapy only for those who do not achieve the desired goal may result in a similar proportion of patients becoming eligible for treatment discontinuation.
Sequencing and safety
The safety of TKIs plays an important role in the long-term care of patients with CML-CP. Treatment interruptions and dose reductions are important considerations for the management of adverse events and should be considered good approaches, frequently successful, for addressing safety and quality of life. Still, sequencing of TKIs for safety reasons may be necessary in certain circumstances. One scenario is the need to change therapy because of intolerance. Several studies have shown that second-line TKI therapy is effective in this setting, with response rates generally higher than when sequencing is used because of resistance to initial therapy. This is true regardless of the initial treatment strategy. Importantly, the probability of cross-intolerance across different TKIs is generally low.20-22 This is particularly true for nonhematologic adverse events which occur infrequently, particularly at a grade 3 or 4 level, and rarely lead to treatment discontinuation for the same adverse event. In contrast, hematologic adverse events, particularly thrombocytopenia, are more likely to lead to cross-intolerance. Thus, change of therapy to an alternative TKI for intolerance to a prior TKI is common practice. Unfortunately, the definition of intolerance is vague, and changes are frequently made for adverse events that might be manageable.
An alternative scenario is to change therapy for low-grade, chronic adverse events. In the ENRICH trial, 52 patients experiencing grade 1 or 2 nonhematologic adverse events persisting for >2 months or that recurred >3 times while on treatment with imatinib were switched to nilotinib. Most adverse events resolved and some improved, with 58% of patients reporting improvement in all adverse events by the end of cycle 12; no patients reported overall worsening. Still, 98% of patients reported new or worsening adverse events while receiving nilotinib with fatigue, rash, and headache being the most common. Eight patients (15%) discontinued nilotinib because of adverse events.23 Thus, although changes in therapy may improve some symptoms, considerations for sequencing in such instances must be made cautiously because some symptoms may improve in exchange for the appearance of new ones.
This leads to a third safety consideration regarding elective sequencing—the possible trade-offs. Whenever an intervention is planned, the risks and benefits have to be weighted. When a patient has developed clear resistance, a change of therapy is justified in most instances. When aiming for more subtle benefits, this may not always be the case. For example, when applying TKI sequencing in an attempt to improve MR, careful consideration of the risk-benefit balance is required. As previously discussed, the ENESTcmr trial resulted in a general improvement in transcript levels, with a cumulative rate of confirmed undetectable BCR-ABL1 (defined as undetectable BCR-ABL1 by quantitative real-time polymerase chain reaction [qRT-PCR] with a sample sensitivity of at least 4.5-logs below the standardized baseline, expressed on the IS, and confirmed in the next qRT-PCR sample with a sensitivity of at least 4-logs) by 2 years of 22% with nilotinib (vs 9% with imatinib). The long-term benefits of this improvement have not yet been reported (eg, EFS, OS, or successful treatment discontinuation). However, 49% of patients experienced grade 3 to 4 adverse events with nilotinib (vs 22% with imatinib), with 12% and 3% discontinuing therapy for adverse events, respectively. Ischemic heart disease was observed in 4 patients (3 of whom were receiving nilotinib, with 1 being a fatality; 1 patient was receiving imatinib), and peripheral arterial disease was observed in 3 patients (all receiving nilotinib).17 Thus, careful weighting of risks and benefits is required before a decision is made for sequencing in these circumstances.
Cost-effectiveness
In an ideal world, medical decisions would not be influenced by economic forces. However, the high costs of health care and, to some extent, the often limited value offered by expensive new medicines has led to an increased focus on cost-effectiveness and value of care. With the availability of various treatment options for initial and subsequent therapy of CML, recent analyses have evaluated the cost-effectiveness of various strategies. These reports emphasize not only unvalued units (eg, response, survival years gained), but by quality-adjusted life-years (QALYs), and the incremental cost:utility ratios (ie, cost per QALY). In 1 report from Austria, initial therapy with imatinib with chemotherapy or SCT instead of second-line TKIs after imatinib failure was proposed as the most cost-effective strategy. Using first-line nilotinib resulted in an incremental cost:utility ratio of €121 400/QALY.24 If generic imatinib is considered instead of the brand name version, and considering a price for the generic version that is 52% that of the brand name version, the difference is €402 500/QALY relative to the next effective strategy not using imatinib as first-line therapy (nilotinib followed by dasatinib followed by SCT), and €656 400 if the price of generic imatinib is 10% of the current price for the brand name version. In a similar analysis from the United Kingdom, imatinib followed by nilotinib was found to be the most cost-effective strategy with an incremental cost:utility ratio of £192 000/QALY.25 A US-based analysis compared the use of generic imatinib at approximately 58% that of the brand name to physician’s choice. For this purpose, physician’s choice assumed an approximately equal distribution of patients among the three options (one-third of the patients each), and the use of 1 second-line TKI in all cases of resistance. The analysis showed an incremental cost-effectiveness ratio for the imatinib-first strategy of US$883 730.26 One important element that should be added to these analyses is the potential for treatment discontinuation. As mentioned above, eligibility for this strategy is augmented by approximately 15% by the initial use of nilotinib compared with imatinib. Although these models have limitations, they do force us to think about the cost-effectiveness of sequential therapy and proper use of resources.
A proposal
The majority of patients treated with imatinib have a favorable outcome. There are many other reasons why initial therapy with imatinib could be desirable for most patients, including wider availability throughout the world, lower cost with the growing availability of regulated generics, and a toxicity profile that includes fewer of the most serious adverse events such as arteriothrombotic events (perhaps in exchange for more of the less serious adverse events such as muscle cramps and peripheral edema). Thus, it makes sense to consider that most patients could be initially treated with imatinib. Some exceptions could be patients with high-risk features such as high-risk Sokal scores, or the few with p190, or perhaps those with high-risk features determined by emerging biomarkers.27,28 Proactive sequencing can then be planned by using strict criteria to optimize results. In this regard, it is important to set the goals for each patient from the start of therapy because goals may vary from patient to patient. For some patients, achieving a CCyR or an MMR may satisfy their needs, whereas others may want to aim for the best opportunity for successful treatment discontinuation. On this basis, proactive sequencing to optimize response may be considered when it is perceived that the desired outcome is considerably less likely to occur. Although BCR-ABL:ABL ratios of <10% at 3 months have been identified as such a threshold,29 most patients with higher values this early (60% to 80%)4,30,31 will still have a favorable EFS, and many will improve with continuation of therapy.32 Thus, until data become available from prospective trials showing that change in therapy at 3 months meaningfully changes long-term outcome for a significant number of patients compared with continuation of unchanged therapy, or change at a later time point (eg, at 6 or 12 months), changing to a second-generation drug at 3 months for patients lagging behind scheduled efficacy end points (eg, BCR-ABL1 >10% IS) remains investigational. However, these patients should be monitored closely. It is important to underscore that the goal is to improve long-term outcomes and not only short-term PCR trends, and studies demonstrating that these early interventions change long-term outcomes are lacking. Using sequencing therapy to improve on low-grade adverse events or in an attempt to deepen the MR in someone with an already good response should be discussed thoroughly with the patient considering real benefits and potential risks.
Conclusion
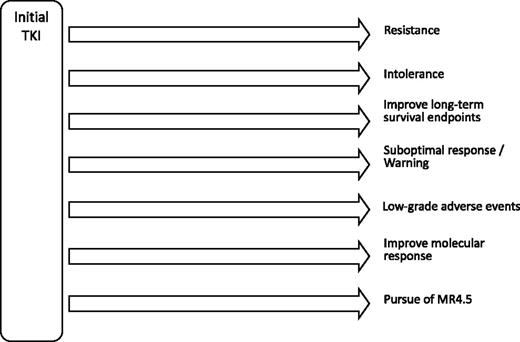
The increased availability of TKIs offers multiple approaches for treatment. When used wisely, most patients can have an optimal outcome. This means that most patients, even when treated with imatinib, may not need sequencing TKI therapy. Others can take advantage of adequate changes in therapy when appropriate to optimize outcome and tolerability (Figure 4). A sine qua non for this approach to work in the best interest of the patient is identification of treatment goals appropriate for each patient, proper monitoring at routine intervals of every 3 to 6 months, and adequate identification and management of adverse events.
Possible scenarios for TKI sequencing in CML. Data for most scenarios except resistance and intolerance, are mostly preliminary, uncontrolled, or lacking.
Possible scenarios for TKI sequencing in CML. Data for most scenarios except resistance and intolerance, are mostly preliminary, uncontrolled, or lacking.
Correspondence
Jorge Cortes, MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: jcortes@mdanderson.org.
References
Competing Interests
Conflict-of-interest disclosure: J.C. has received research funding from Novartis, Pfizer, TEVA Pharmaceuticals Industries, Arog Pharmaceuticals, Astellas Pharma, Ambit Biosciences, Bristol-Myers Squibb, and ARIAD Pharmaceuticals and has consulted for Novartis, Pfizer, Bristol-Myers Squibb, and ARIAD Pharmaceuticals. H.K. declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.