Abstract
Von Willebrand factor (VWF) is a critical regulator of hemostatic processes, including collagen binding, platelet adhesion, and platelet aggregation. It also serves as a carrier protein to normalize plasma factor VIII synthesis, release, and survival. While VWF protein measurements by immunoassay are reasonably comparable between institutions, the measurement of VWF ristocetin cofactor activity (VWF:RCo) has significant variability. Other tests of VWF function, including collagen binding or platelet glycoprotein IIb-IIIa binding, are not universally available, yet these functional defects may cause major bleeding even with normal VWF antigen (VWF:Ag) and VWF:RCo assays. This results in both the overdiagnosis and underdiagnosis of VWD. Newer assays of VWF function (using recombinant glycoprotein Ib rather than whole platelets) have been developed that may improve interlaboratory variability. Some of these tests are not uniformly available and may not be licensed in the United States. Large longitudinal studies of VWF in von Willebrand disease (VWD) patients are not available. Patients are sometimes diagnosed with a single diagnostic VWF panel. Plasma VWF levels increase with age, but it is not clear if this results in less bleeding or whether different normal ranges should be used to identify age-related decreases in VWF. In order to quantitatively compare bleeding symptoms in VWD patients and normal individuals, recent studies in the European Union, Canada, United Kingdom, Holland, and the United States have used semiquantitative bleeding assessment tools (BATs). Even with careful centralized testing, including functional assays of VWF, addition of a BAT does not solve all of the problems with VWD diagnosis. No matter where the line is drawn for diagnosis of VWD, VWF is still a continuous variable. Thus, VWD can be a severe hemorrhagic disease requiring frequent treatment or a mild condition that may not be clinically relevant. As will be discussed by Dr. Goodeve in her presentation, genetics has helped us to diagnose type 2 functional variants of VWD but has not been helpful for the many patients who are at the interface of normal and low VWF and carry the possible diagnosis of type 1 VWD. The hematologist’s management of patients with reduced levels of VWF still requires both the art and science of clinical medicine.
Learning Objectives
To review a large VWD cohort comprised of previously diagnosed VWD and review the uncertainty regarding the fidelity of the diagnosis of VWD in the United States
To identify new tests of VWF functions that improve the diagnosis of VWD and its variants
To examine the variability in clinical VWF assays between centers and among patients of varying ethnicity and race
Introduction
Von Willebrand disease (VWD) is a common bleeding disorder caused by reduced von Willebrand factor (VWF) synthesis or synthesis of a functionally defective VWF protein. Studies of VWD vary widely in terms of the minimal laboratory or clinical attributes required for the appropriate diagnosis. The functions of VWF include (1) binding to exposed subendothelial collagens, (2) conformational unraveling in response to shear, (3) binding to platelet glycoprotein Ib (GPIb) to effect platelet adhesion, (4) binding to platelet GPIIb-IIIa complex, and (5) binding to circulating factor VIII. These functions can be measured by individual discrete tests, but such assays are not always universally available. Thus, what is diagnosed as VWD or variant VWD varies between centers.
When VWF sequencing became widely available, there was the expectation that genetics would help to standardize the diagnosis of VWD. Large clinical studies have been undertaken in the European Union (EU) through the MCMDM-1 VWD Study,1 the Canadian Type 1 VWD Study,2 the UK HCDO VWD Study,3 the Dutch WiN Study,4 and the NIH-funded Zimmerman Program for the Molecular and Clinical Biology of VWD (ZPMCB-VWD).5 The initial focus of these studies was directed toward the role of VWF gene mutation in type 1 VWD. This will be discussed in greater detail by Anne Goodeve. While the Canadian and EU studies focused on type 1 VWD, the ZPMCB-VWD program has also focused on the problems with the fidelity of the VWD diagnosis within the United States and will be discussed here in greater detail. In all of these studies, molecular analysis has been performed and demonstrates that VWF gene defects are not universally present in type 1 VWD. However, in type 2 VWD (functional variants of VWD) defects and the more severe forms of type 1 and type 1C VWD, nearly all subjects have causative genetic defects identified. Most of the discussion in this paper will focus on the studies done within the ZPMCB-VWD that includes patients with type 1, type 2 (2A, 2B, 2M, and 2N), and type 3 VWD and their corresponding family members.
Most clinicians agree that VWD is an inheritable bleeding disorder that is due to reduced VWF concentration or reduced VWF function and can be treated by therapeutics (eg, DDAVP) that endogenously increase the concentration of VWF in plasma or infused VWF concentrate that can be used to increase the concentration of functionally normal VWF. At every step in this definition, however, there are qualifications that affect the veracity of the clinical diagnosis and alter the communication between physicians and patients. This can also create ambiguity with insurance providers who challenge the number and type of diagnostic tests as well as the need for clinical therapeutic intervention.
Our goals are to (1) illustrate the utility of large population-based studies, (2) confirm the diagnosis of VWD in subjects followed for VWD at US hemophilia treatment centers, (3) characterize ethnic and racial variations in VWF clinical assays and their impact on the diagnosis of VWD, (4) evaluate new assays of VWF function to identify subjects that would be missed with standard VWF functional assays, and (5) evaluate the changes in VWF levels over time.
ZPMCB-VWD
The ZPMCB-VWD is a large program project grant cooperative study that is funded by the National Institutes of Health to study the molecular and clinical biology of VWD. The purpose of the study was to study the molecular causes of VWD and to examine the fidelity of the diagnosis of VWD in the United States by examining index cases and their family members. The clinical cohort is based in the United States, and subjects were recruited from >30 clinical centers. Rather than including only subjects with unequivocal VWD or just type 1 VWD, the ZPMCB-VWD study, by design, only required that the index case (n = 724) had been diagnosed as VWD and was registered at one of the collaborating 35 hemophilia treatment centers. Subjects with acquired VWD were excluded from enrollment. Family members (2178) with or without the diagnosis of VWD or bleeding symptoms were also enrolled and studied. Normal controls were recruited and selected based on several self-reported ethnicities or races to study the ethnic variability in VWF levels. More recently, 332 subjects have been restudied during clinical follow-up over a 2- to 4-year interval. Thus, we could assess the fidelity of the diagnosis of type 1, variant type 2, and type 3 VWD and correlate the current results with historical laboratory testing, identify genetic sequence variations, and quantify their bleeding history as assessed with various bleeding assessment tools (BATs). It is this population-based study that will be explored in greater depth in this paper. We will not discuss the molecular discoveries in the ZPMCB-VWD, since that aspect is being discussed by Dr. Goodeve in a subsequent paper.
Index cases and diagnosis of VWD
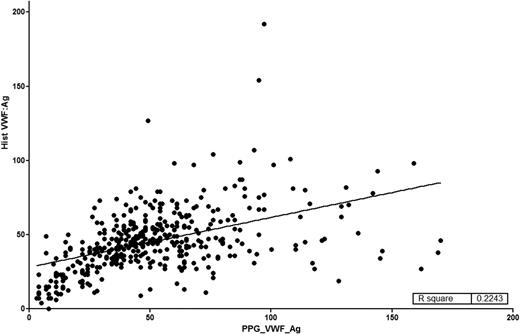
All of the index cases carried a diagnosis of VWD with an intent to treat based on this diagnosis. A summary of the studies done on the enrolled type 1 VWD subjects was recently published by Flood and coworkers.5 Primarily, index cases were diagnosed based on low VWF antigen (VWF:Ag) or VWF ristocetin cofactor activity (VWF:RCo) levels, but the majority of these historically diagnosed subjects had been identified by low VWF:RCo rather than low VWF:Ag. Even in the type 1 VWD subjects,5 the correlation between VWF:RCo and VWF:Ag was modest (r2 = 0.33). All of the index cases had central testing of their current VWF:Ag and VWF:RCo levels, and the correlation between VWF:RCo and VWF:Ag was strong (r2 = 0.92).5 For type 1 VWD subjects, as shown in Figure 1, there is relatively poor correlation of historical assays with current results (r2 = 0.22). Some of these differences can be attributed to the reproducibility and variability of the VWF:RCo performed in multiple clinical laboratories at the time of first diagnosis.
Comparison of historical VWF:Ag with VWF:Ag on entry into the ZPMCB-VWD. On enrollment into the ZPMCB-VWD, local centers entered the results (y-axis) of the diagnostic tests used to establish the diagnosis of VWD. Samples were drawn and sent to the central laboratory and the results of the VWF:Ag are entered on the a-axis. The correlation coefficient was r2 = 0.2243.
Comparison of historical VWF:Ag with VWF:Ag on entry into the ZPMCB-VWD. On enrollment into the ZPMCB-VWD, local centers entered the results (y-axis) of the diagnostic tests used to establish the diagnosis of VWD. Samples were drawn and sent to the central laboratory and the results of the VWF:Ag are entered on the a-axis. The correlation coefficient was r2 = 0.2243.
There are obvious problems with the fidelity of VWD diagnosis, including (1) the reliability of the testing that might have been done many decades ago, (2) historical diagnosis that may not have been substantiated by repeat or follow-up diagnostic testing, and (3) clinicians diagnosing VWD based only on a VWF level that is below the normal range, even in the absence of bleeding symptoms. Because the critical establishment of a normal range is usually a geometric normal range ± 2 SDs, 2.5% of the population is below the normal range and 2.5% is above the normal range. If that result is obtained in a patient with “bleeding symptoms,” that person is labeled as having type 1 VWD. Even if the patient’s bleeding symptoms are reduced by treatment, DDAVP is recognized to reduce bleeding symptoms in subjects without VWD who might have platelet function defects, uremia, or liver disease.6 Thus, establishing a cause and effect relationship between VWF and bleeding is not necessarily simple. This observation has been repeatedly emphasized by Evan Sadler.7-9
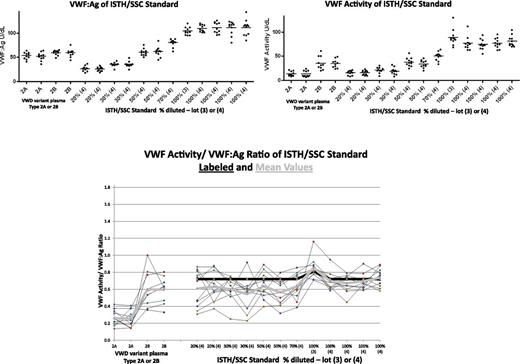
All laboratories use what are thought to be high-quality reference standards. However, sometimes the standards used in one institution for one assay (eg, VWF:Ag) may differ from those used for another (VWF:RCo), even though there are high-quality standards that could meet the needs of both assays and provide tighter correlation. We sent periodic blinded standards to our 11 participating laboratories over a 2-year period. Figure 2 demonstrates the results of these blinded studies of VWF:Ag, VWF activity, and the VWF activity/VWF:Ag ratio. Thus, even in strong academic clinical hemostasis laboratories, the difference in specific activity is quite striking. Studies are in progress to further understand the reason for this variability, but these results highlight the difficulties in obtaining accurate VWF levels.
Results of blinded quality control studied by 11 local participating laboratories. The International Society of Thrombosis and Haemostasis (ISTH)/Scientific Standardization Committee (SSC) standard plasma was aliquoted undiluted or diluted to 70%, 50%, 30%, or 20% using type 3 VWD plasma, and the type 2A and 2B plasmas were sent undiluted. All samples were blinded and sent to the 11 collaborating laboratories for assays of VWF:Ag and VWF activity (various methods) over a 2-year time period. The 2 top panels demonstrate the results of VWF:Ag and VWF activity from these laboratories. The bottom panel represents the specific activity of these results as calculated by VWF activity/VWF:Ag. On a 30% sample, the specific activity ranged between 0.22 and 0.91. There was also wide variation in the specific activity of type 2B VWD samples.
Results of blinded quality control studied by 11 local participating laboratories. The International Society of Thrombosis and Haemostasis (ISTH)/Scientific Standardization Committee (SSC) standard plasma was aliquoted undiluted or diluted to 70%, 50%, 30%, or 20% using type 3 VWD plasma, and the type 2A and 2B plasmas were sent undiluted. All samples were blinded and sent to the 11 collaborating laboratories for assays of VWF:Ag and VWF activity (various methods) over a 2-year time period. The 2 top panels demonstrate the results of VWF:Ag and VWF activity from these laboratories. The bottom panel represents the specific activity of these results as calculated by VWF activity/VWF:Ag. On a 30% sample, the specific activity ranged between 0.22 and 0.91. There was also wide variation in the specific activity of type 2B VWD samples.
In clinical practice, once the laboratory testing suggests VWD or low VWF, follow-up confirmatory testing is not routinely performed. Clinicians recognize that VWF is an acute-phase protein that increases under stress. Thus, if VWF levels are not reduced, repeat VWD testing is often performed to confirm the results. Testing is not routinely repeated if VWF levels are reduced, even though we use laboratory tests with coefficients of variation of 5% to 10%. This may result in the overdiagnosis of VWD, particularly in those with VWF levels near the lower end of the normal range. Our experience suggests that confirmatory testing should be performed to demonstrate low VWF levels. Some consideration is being given to using the diagnosis of type 1 VWD for those with an abnormal bleeding history and confirmed reduced plasma VWF levels (<30 IU/dL). A diagnosis of low VWF might be applied to those with VWF levels ≥30 IU/dL but below the normal range for VWF. However, there is a lack of concurrence with this recommendation, particularly because there is not an ICD-10 code for “low VWF,” and some insurance companies with not cover VWF replacement therapeutics unless there is a VWD diagnosis.
Levels of VWF in nonwhite normal controls, blood groups, and age groups
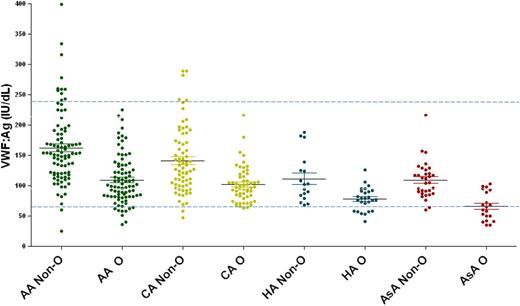
Levels of VWF have been shown to vary among different ethnic and racial groups. For example, African Americans have 20% higher VWF:Ag levels compared with whites.10-12 Interestingly, while African American plasma VWF:Ag levels are higher, their VWF:RCo levels are not. Their VWF:RCo levels are comparable to those found in whites. Flood and coworkers identified a VWF gene polymorphism (p.D1472H) present in two-thirds of African Americans and in one-sixth of whites that affects the binding of the ristocetin laboratory reagent to VWF. This results in the erroneous conclusion that there is a reduction in VWF’s specific activity, but there is no association of this observation with a clinical bleeding. Two groups have developed assays that measure binding of VWF to recombinant platelet GPIb containing gain-of-function mutations that induce GPIb binding to VWF without requiring ristocetin.10,11,13 Such assays are now termed VWF:GPIbM to demonstrate that they use “mutant” GPIb that spontaneously binds VWF without the addition of ristocetin. Using such assays demonstrates the VWF activity to VWF:Ag ratio is the same for whites and African Americans but does not explain the elevated levels of plasma VWF in African Americans. Furthermore, recent studies done on normal controls demonstrate variations in other ethnic and racial groups (Figure 3). Whether this increase in African Americans is caused by altered synthesis or altered clearance is not yet known. There are no data to suggest that different normal ranges should be applied to the diagnosis of VWD in these groups, but further work is required in this area.
VWF:Ag levels by ethnicity and blood type. Normal controls were enrolled in the ZPMCB-VWD and separated by self-reported race or ethnicity. The racial and ethnic groups are African American (AA), Caucasian American (CA), Hispanic American (HA), and Asian American (AsA) and are divided by blood type (O vs non-O). In all cohorts type O individuals had significantly lower VWF:Ag, with the most striking difference in AsA, with mean VWF:Ag of 66 in blood group O and 109 in non-O. A total of 33% of AsAs had VWF:Ag levels below the normal range (dashed line represents the upper and lower limits of VWF:Ag).
VWF:Ag levels by ethnicity and blood type. Normal controls were enrolled in the ZPMCB-VWD and separated by self-reported race or ethnicity. The racial and ethnic groups are African American (AA), Caucasian American (CA), Hispanic American (HA), and Asian American (AsA) and are divided by blood type (O vs non-O). In all cohorts type O individuals had significantly lower VWF:Ag, with the most striking difference in AsA, with mean VWF:Ag of 66 in blood group O and 109 in non-O. A total of 33% of AsAs had VWF:Ag levels below the normal range (dashed line represents the upper and lower limits of VWF:Ag).
It has been recognized for nearly 30 years that plasma VWF levels are affected by age and blood groups.14 Type O blood group individuals have VWF levels that are ∼20% lower than those of other blood groups, which appears to be the result of the ABO glycans carried on VWF that may alter VWF clearance.15,16 In the same study by Gill and coworkers14 VWF levels increased with age in blood donors between 18 and 70 years; VWF:Ag levels increased ∼1% to 1.2% per year. How we deal with the variations in normal ranges by age, race, and ethnicity awaits further study. Most clinicians treat patients based on bleeding symptoms and presence of reduced VWF protein or function, but as one ages and moves into the normal rage for VWF, are bleeding symptoms reduced or are higher VWF levels required to achieve hemostasis in the elderly?
Clinical assays to measure VWF functions
A large, carefully studied VWF cohort, such as the ZPMCB-VWD, provides study subjects to evaluate newer diagnostic functional assays and will be discussed in greater detail below.
VWF propeptide assay
During the early phases of our ZPMCB-VWD program, we demonstrated the utility of the VWF propeptide VWFpp (formerly called VW:AgII).17,18 This assay measures the plasma concentration of the VWFpp. The ratio of VWFpp to VWF:Ag (VWFpp/VWF:Ag) was shown by Haberichter and coworkers17-20 to detect VWDVicenza or similar variants that are now referred to collectively as type 1C VWD. Type 1C VWD individuals have markedly reduced VWF:Ag levels and an increase in the VWFpp/VWF:Ag ratio due to accelerated clearance of VWF, hence the designation of type 1C VWD. Because VWF and VWFpp are both formed on an equimolar basis from pro-VWF, the VWF is markedly reduced but the VWFpp half-life is normal.
VWFpp levels have been used by a number of investigators19,21-23 as a sensitive indicator of endothelial cell perturbation, such as with thrombotic thrombocytopenic purpura or hemolytic uremic syndrome. Acquired VWD can be caused by a number of conditions, including aortic stenosis24-26 or ventriculoseptal cardiac defects.27 In elderly patients, acquired VWD can be caused by an autoantibody to VWF that causes VWF to be rapidly cleared, but VWFpp is unaffected.28 In these subjects, an elevated VWFpp/VWF:Ag ratio can be diagnostic.
VWF collagen-binding assays
In the United States, measuring VWF binding to collagen is not widely performed by most clinical laboratories. When collagen binding is performed, the collagen used is usually type I or III (VWF:CB1 or VWF:CB3). This identifies genetic defects in the A3 domain of VWF that are clinically significant and are a cause of type 2M VWD. It appears, however, that the more common VWF collagen-binding defects affect the A1 domain of VWF and must be studied using types IV or VI collagen (VWF:CB4 or VWF:CB6). Interestingly, a common variant, p.R1399H, which is present in 1 allele of 2% of a North American study population,29 results in absent binding to types IV and VI collagen if it is present on both alleles or if the second allele is a VWF-null allele. In a population that has a 2% frequency, homozygous deficiency should be 1 in 10 000 individuals. Because VWF:Ag and VWF:RCo in such individuals are normal, VWF:CB4 or VWF:CB6 assays are not usually performed. However, these patients have clinically severe type 2M VWD and would clinically benefit from VWF replacement therapy for bleeding.30 Thus, the true frequency of VWD caused by isolated collagen binding mutations of VWF is not yet known but is being studied in the ZPMCB-VWD.31,32
VWF:GPIbM assay
As mentioned above, the VWF:RCo assay incorrectly identifies an “apparent” VWF platelet-binding defect in many African Americans or in other racial groups with the VWF p.D1472H polymorphism. Assays were developed in the ZPMCB-VWD13 and by Schneppenheim and coworkers33,34 that measured VWF binding to platelet GPIb mutant proteins (VWF:GPIbM). This circumvents the aberrant interaction in VWF:RCo assays in which ristocetin is used. There may be other sequence variations in VWF that can affect ristocetin binding; thus, a critical appraisal is needed in some cases of type 2M VWD or in type 2B VWD.
VWF:2b3aB assay
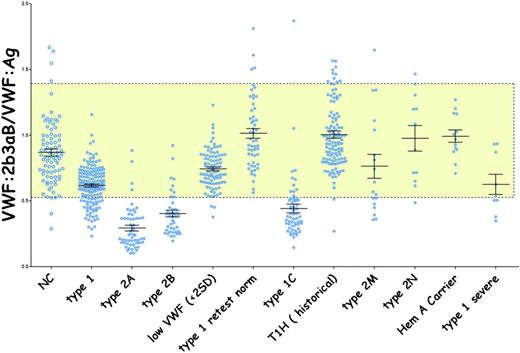
One of the known binding sites of VWF to platelets is platelet GPIIb-IIIa (integrin αIIb β3). Veyradier et al35 reported such a deficiency in a French patient. ZPMCB-VWD coworkers developed a different quantitative assay to measure this binding thereby permitting the study of our large cohort of VWD subjects. The αIIb β3 active head group was used in an enzyme-linked immunosorbent assay (ELISA) (VWF:2b3aB) to measure VWF binding to αIIb β3 in plasma samples from subjects enrolled in the ZPMCB-VWD. The results were presented at the 2016 annual meeting of the American Society of Hematology and are summarized in Figure 4. VWF binding to αIIb β3 was found to be dependent on VWF multimer size in a manner similar to that observed in the VWF:RCo and VWF:CB assays.
Quantitative VWF binding to platelet integrin αIIbβIII. The αIIbβIII head group has been crystallized by Zhu et al51 and is constitutively active. AP3 was used as a capture antibody for αIIbβIII. Heat-defibrinated standard or test plasma was added and washed with the bound VWF detected with a biotinylated monoclonal antibody to VWF. Binding was inhibited by 7E3. VWF binding to αIIbβIII was high-molecular-weight multimer dependent, similar to VWF binding to GPIb and collagen.
Quantitative VWF binding to platelet integrin αIIbβIII. The αIIbβIII head group has been crystallized by Zhu et al51 and is constitutively active. AP3 was used as a capture antibody for αIIbβIII. Heat-defibrinated standard or test plasma was added and washed with the bound VWF detected with a biotinylated monoclonal antibody to VWF. Binding was inhibited by 7E3. VWF binding to αIIbβIII was high-molecular-weight multimer dependent, similar to VWF binding to GPIb and collagen.
Rapid type 2 multifunction VWF assay
In order to correctly identify type 2 variant VWD, samples are sent to specialized referral laboratories where standards may differ from those used in the referring laboratory. While no single test will measures all functions of VWF, Roberts and coworkers36 developed a rapid ELISA that measured most of the functions of VWF within a single ELISA strip and thereby used a common assay platform in the same standard plasma. By linear discriminant analysis and comparison with a standardized control, the relative measurement of VWF functions could be determined through multiple comparisons with the large cohort of type 2 VWD subjects enrolled in the ZPMCB-VWD. The only function that currently cannot be measured in this multiwall ELISA is the binding of VWF to type IV or VI collagen, because a different plastic surface is used for these assays. In a single ELISA strip, comparison of VWF functions can identify types 1C, 2A, 2B, 2M, and 2N VWF and differentiate type 2M VWD due to platelet-binding defects from those do to a type 1 or 3 collagen-binding defect. This has the potential to have a standardized clinical laboratory assay that could be performed locally and would reliably classify patients with functionally abnormal VWF.
Follow-up testing of VWD patients
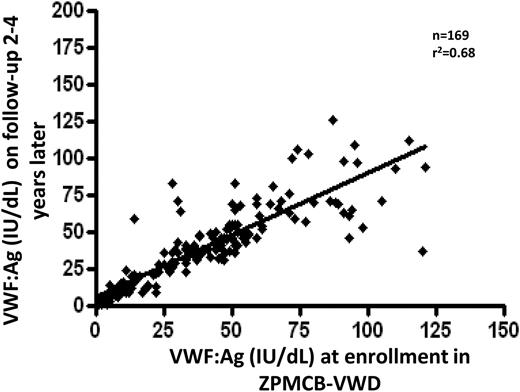
Although long-term longitudinal studies are needed, we have evaluated follow-up testing of samples done during a 2- to 4-year follow-up period. Figure 5 correlates the central laboratory VWF:Ag results at entry and when restudied at 2 to 4 years. This includes VWD patients as well as affected and unaffected family members. Friedman and coworkers studied 169 individuals, and the correlation coefficient for these results is r2 = 0.68 (K.D. Friedman and the ZPMCB-VWD principal investigators, personal communication).
Plasma VWF:Ag levels on follow-up testing compared with VWF:Ag on entry samples. VWF testing was done at the time of original enrollment in the ZPMCB-VWD. After several years, repeat testing was performed. Samples included index cases, family members, and some normal controls to evaluate the changes over time. The correlation coefficient was r2 = 0.68, with fairly comparable results in those whose VWF levels were <50 IU/dL.
Plasma VWF:Ag levels on follow-up testing compared with VWF:Ag on entry samples. VWF testing was done at the time of original enrollment in the ZPMCB-VWD. After several years, repeat testing was performed. Samples included index cases, family members, and some normal controls to evaluate the changes over time. The correlation coefficient was r2 = 0.68, with fairly comparable results in those whose VWF levels were <50 IU/dL.
Clinical studies on subjects in ZPMCB-VWD
BATs
Because VWD is a bleeding disorder, its diagnosis should require having clinical bleeding. In order to semiquantify the amount of clinical bleeding, Italian and European groups pioneered the development of a BAT and applied it to patients with VWD. Therefore, large VWD cohorts are useful in the development of bleeding scores. The Vicenza group initially developed a BAT for an international VWD study of individuals with the obligatory diagnosis of VWD (Vicenza BAT).37 This approach was subsequently modified for the EU MCMDM-1VWD study (MCMDM-1VWD BAT).38 All subjects enrolled in the ZPMCB-VWD have been evaluated by a BAT questionnaire that could use the questions to assess bleeding scores by several different BATs, including the Vicenza BAT, MCMDM-1VWD BAT, the Pediatric Bleeding Questionnaire,39 and the International Society of Thrombosis and Haemostasis, ISTH-BAT.40 Studies were undertaken in the ZPMCB-VWD to compare the relative sensitivity of the MCMDM-1VWD BAT, the Pediatric Bleeding Questionnaire, and the ISTH BAT, and the results were quite comparable. Christopherson and coworkers studied the impact of gender and age on the ISTH BAT (presented previously at American Society of Hematology and Hemostasis and Thrombosis Research Society41,42 ). In prepubescent children, no significant gender difference was seen in subjects with the diagnosis of VWD. While adolescent males and females had comparable bleeding scores, the types of bleeding symptoms varied. In adults, males reported more oral and surgical bleeding and women had primarily menorrhagia and coetaneous wound bleeding. Interestingly, although women’s bleeding scores continued to increase with age, this was not all due to menorrhagia or postpartum bleeding.
Both the Vicenza and the MCMDM-1VWD studies demonstrated a correlation between bleeding score and VWF levels in their VWD subjects. When the ZPMCB-VWD looked at their cohort, the correlation was not strong with either type 1 VWD or type 2 variant VWD subjects, but there was a difference in how the data were collected. The EU BATs were based on the bleeding histories at the time of initial diagnosis of VWD, while the ZPMCB-VWD studies were done at the time of enrollment. Because most patients were referred because of bleeding symptoms, the bleeding scores were usually abnormal. Furthermore, VWD was diagnosed, most patients were treated for symptoms or procedures, and this affected the bleeding score. Thus, bleeding scores were not correlated with the level of plasma VWF in either type 1 or type 2 index cases. However, in affected family members of both type 1 and type 2 VWD subjects, there was a correlation between VWF:Ag and bleeding score. This suggests that the BAT is most accurate at the time of diagnosis. A different bleeding assessment might be important to study interval bleeding severity following initial diagnosis, but such a tool has not yet been developed.
Summary
The definitive diagnosis of VWD continues to be problematic. When we are referred a patient with bleeding symptoms, we usually test for VWD. As Sadler7-9 has repeatedly pointed out, bleeding symptoms are common. Thus, it is not surprising that we get many borderline abnormal results for plasma levels of VWF. We therefore apply the diagnosis of VWD because there is both a bleeding history and a reduced VWF assay result. While it is reasonable that young children might not have sufficient hemostatic challenge to produce an abnormal bleeding score, large studies like the ZPMCB-VWD show that even adults with VWF levels <20% of normal may not have an abnormal BAT result. It is doubtful that we will ever have a test that gives a clear “yes” or a “no” to the diagnosis of VWD.
As we dissect the multifunctions of VWD, laboratory testing becomes more complex. New studies have demonstrated that many subjects with low VWF have this reduction due to accelerated clearance rather than reduced synthesis.43-47 It is only through the application of modern genomic exploration that these new pathways are being identified.48,49 Does it matter whether your VWF is low because of synthesis or clearance? It may, because subjects with type 1C VWD have low plasma levels but normal platelet levels of VWF. We need to ascertain who would benefit from being treated by increasing plasma VWF levels, infusing VWF concentrate to increase VWF levels, or providing a functionally normal VWF protein. The NHLBI VWD guidelines50 helped to emphasize many of the complexities around the diagnosis of VWD, yet these guidelines are now out of date. Several organizations are considering updating these guidelines.
We may never increase our precision in “correctly” diagnosing VWD because of the coefficient of variation of VWF laboratory testing and the clinical variability of bleeding symptoms. There are VWD patients that clearly require therapeutic increases in functional VWF and even some patients that need to be on long-term prophylaxis. However, there also are some patients with normal VWF function and borderline but normal levels of VWF that do not have VWD. It is the large group of patients in the middle with mild symptoms who may respond to VWF therapies that results in a diagnostic dilemma. Some have advocated terming these patients as having low VWF, but the confusion around this designation makes it difficult to communicate the clinical condition with patients. Insurance companies may challenge the need for follow-up or confirmatory testing or refuse coverage for VWF replacement without a diagnosis of VWD.
Large cooperative clinical studies such as the ZPMCB-VWD are still needed to correctly deal with mild bleeding disorders and the role VWF plays as a single etiology of the bleeding symptoms or as a modifying risk factor that still might require therapeutic VWF augmentation.
Correspondence
Robert R. Montgomery, Blood Research Institute, BloodCenter of Wisconsin, PO Box 2178, Milwaukee, WI 53201-2178; e-mail: bob.montgomery@bcw.edu.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.