Abstract
Heavy menstrual bleeding (HMB) is frequently reported by adolescents. The role of the hematologist is threefold in evaluating such patients: (1) perform a clinical and laboratory evaluation for an underlying bleeding disorder on the basis of the degree of clinical suspicion, (2) identify and manage any concomitant iron deficiency, and (3) provide input to the referring provider regarding the management of HMB, particularly for patients with identified hemostatic defects. Several clues in the menstrual history should raise suspicion for an underlying bleeding disorder, such as menses lasting >7 days, menstrual flow which soaks >5 sanitary products per day or requires product change during the night, passage of large blood clots, or failure to respond to conventional therapies. A detailed personal and family history of other bleeding symptoms should also be obtained. Iron deficiency with and without anemia is commonly found in young women with HMB. Therefore, it is important to obtain measures of hemoglobin and ferritin levels when evaluating these patients. Iron supplementation is often a key component of management in the adolescent with heavy menses and is still needed in those who have received packed red cell transfusions as a result of severe anemia. Strategies for decreasing menstrual blood flow are similar for adults and adolescents with heavy menses, with combined hormonal contraceptives recommended as first-line therapy. However, there are adolescent-specific considerations for many of these agents, and they must be incorporated into shared decision-making when selecting the most appropriate treatment.
Learning Objectives
Summarize the clinical features of an adolescent with HMB that should increase suspicion for an inherited bleeding disorder and recognize that anovulatory bleeding does not rule out an underlying hemostatic defect
Screen for and manage iron deficiency in the adolescent with heavy menses and be able to contrast the benefits and limitations of the most commonly used management strategies to reduce menstrual blood loss
Introduction
Heavy menstrual bleeding (HMB) is frequently reported by adolescents. In a school-based survey of ∼1000 female adolescents in Sweden, 37% reported HMB.1 In an analysis of insurance claims data from more than 200 000 females age 10 to 17 years in the United States, 27% had an outpatient diagnostic code consistent with HMB at least once during the 3-year study period.2 Women with HMB have significantly lower perceived general health and poorer quality of life in terms of the ability to fully participate in school, work, and athletic and social activities.3,4 In the United States alone, hundreds of adolescents are hospitalized as a result of HMB and severe anemia at children’s hospitals each year, with 5% of these patients requiring admission to intensive care units.5
The American College of Obstetricians and Gynecologists defines HMB as bleeding lasting for >7 days and/or the loss of >80 mL of blood per menstrual cycle.6 The International Federation of Gynecology and Obstetrics provides a broader definition of HMB: they identify the condition as excessive menstrual blood loss that interferes with a woman’s physical, emotional, social, and material quality of life.7
Compared with HMB in older women, HMB in adolescents is typically a result of nonstructural causes, most commonly anovulatory bleeding because of immaturity of the hypothalamic-pituitary-ovarian axis.8 Endocrinologic causes such as polycystic ovarian syndrome and thyroid disease need to be considered, as do pregnancy and sexually transmitted infections. Underlying hemostatic defects are another common etiology of HMB. The reported frequency of bleeding disorders in adolescents with HMB ranges from 8% to 62%, but the true frequency remains unknown because of the lack of prospective studies with objective menstrual flow assessment, standardized hemostatic testing, and standardized laboratory definitions of von Willebrand disease and platelet function defects.9
Typically, when an adolescent with HMB is referred to a hematologist, it is because the referring provider suspects the possibility of an underlying bleeding disorder. The role of the hematologist is threefold in the evaluation of such patients: (1) perform a clinical and laboratory evaluation for an underlying bleeding disorder on the basis of the degree of clinical suspicion, (2) identify and manage any concomitant iron deficiency, and (3) provide input to the referring provider regarding the management of HMB, particularly for patients with identified hemostatic defects. This review will cover these 3 aspects of the evaluation and management of adolescents with HMB who present to a hematologist.
Evaluating the adolescent with HMB for an underlying bleeding disorder
Clinical history
The evaluation of patients presenting with easy bruising or bleeding remains a challenge for the practicing hematologist, because mild bleeding events are common in patients with and without bleeding disorders. In a study that obtained comprehensive bleeding histories from 500 healthy adults, 25% reported epistaxis, 18% reported excessive bleeding after tooth extraction, and 47% of females reported HMB.10 Thus, the hematologist is charged with discerning whether a reported bleeding symptom is clinically significant. In 2008, the National Hemophilia Foundation initiated Project Red Flag, which aimed to increase public awareness of bleeding disorders in women. They highlighted the following red flags in a patient’s menstrual history that would suggest a history of excessive bleeding: menses lasting >7 days, menstrual flow that requires double protection with sanitary products, flow that soaks >5 sanitary products per day, flow that requires changing sanitary products during the night, the sensation of gushing, or the passage of blood clots greater than 1 inch (2.5 cm) in diameter.11 In 2009, a panel of international experts published a consensus statement on the diagnosis and management of bleeding disorders in women.12 The expert panel agreed upon the following associations as being indicative of HMB: soaking through a pad or tampon within 1 hour, soaking through bedclothes, low ferritin or anemia, and a pictorial blood assessment chart (PBAC) score >100. The panel also recommended that an underlying bleeding disorder should be considered if any of the following indicators were present: HMB since menarche, family history of bleeding disorder, failure to respond to conventional HMB management strategies, or personal history of other bleeding symptoms (Table 1).
Signs and symptoms that suggest an underlying bleeding disorder
| With the acceptance that menorrhagia is generally defined as >80 mL of blood loss per menstrual cycle, the following associations were agreed on as being indicative of menorrhagia |
| Soaking through a pad or tampon within 1 h |
| Soaking through bedclothes |
| Below normal ferritin |
| Anemia |
| Pictorial blood assessment chart score >100 |
| An underlying bleeding disorder should be considered if any of the following are present: |
| Menorrhagia since menarche |
| Family history of a bleeding disorder |
| Personal history of 1, but usually several, of the following symptoms: |
| Epistaxis (generally bilateral epistaxis, >10 min duration, once in the last year possibly necessitating packing or cautery) |
| Notable bruising without injury (and >2 cm in diameter) |
| Minor wound bleeding (ie, from trivial cuts lasting for >5 min) |
| Bleeding of oral cavity or gastrointestinal tract without an obvious anatomic lesion |
| Prolonged or excessive bleeding after dental extraction |
| Unexpected postsurgical bleeding |
| Hemorrhage from ovarian cysts or corpus luteum, possibly with accompanying pain during ovulation (termed mittleschmerz) |
| Hemorrhage that required blood transfusion |
| Postpartum hemorrhage (PPH), especially delayed PPH (after 24 h; no response to conventional management of menorrhagia) |
| With the acceptance that menorrhagia is generally defined as >80 mL of blood loss per menstrual cycle, the following associations were agreed on as being indicative of menorrhagia |
| Soaking through a pad or tampon within 1 h |
| Soaking through bedclothes |
| Below normal ferritin |
| Anemia |
| Pictorial blood assessment chart score >100 |
| An underlying bleeding disorder should be considered if any of the following are present: |
| Menorrhagia since menarche |
| Family history of a bleeding disorder |
| Personal history of 1, but usually several, of the following symptoms: |
| Epistaxis (generally bilateral epistaxis, >10 min duration, once in the last year possibly necessitating packing or cautery) |
| Notable bruising without injury (and >2 cm in diameter) |
| Minor wound bleeding (ie, from trivial cuts lasting for >5 min) |
| Bleeding of oral cavity or gastrointestinal tract without an obvious anatomic lesion |
| Prolonged or excessive bleeding after dental extraction |
| Unexpected postsurgical bleeding |
| Hemorrhage from ovarian cysts or corpus luteum, possibly with accompanying pain during ovulation (termed mittleschmerz) |
| Hemorrhage that required blood transfusion |
| Postpartum hemorrhage (PPH), especially delayed PPH (after 24 h; no response to conventional management of menorrhagia) |
Content modified from James et al.12
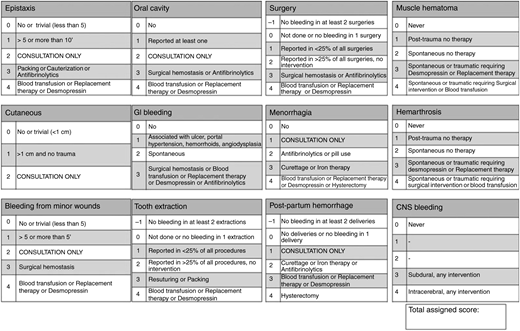
Given the subjective nature of bleeding symptoms, there has also been increasing interest among researchers and clinicians to more precisely and objectively quantify bleeding. Over the past decade, a variety of bleeding assessment tools have been validated, most of them based on the original score published by investigators in Vicenza, Italy, and they have consistently demonstrated high specificity and negative predictive value for the diagnosis of von Willebrand disease.13 The Vicenza-based tools collect information on 12 different bleeding symptoms and score them from 0 to 4 on the basis of the frequency or severity of the symptom or the intensity of intervention required (Figure 1). Given the increasing number of published bleeding assessment tools and the lack of a consensus on which to use, the International Society on Thrombosis and Haemostasis (ISTH) published the ISTH Bleeding Assessment Tool (ISTH-BAT) in 2010, a Vicenza-based tool that aims to provide a standardized method of reporting bleeding symptoms in both adults and children.14 The ISTH-BAT can be found at https://c.ymcdn.com/sites/www.isth.org/resource/resmgr/ssc/isth-ssc_bleeding_assessment.pdf. Abnormal ISTH-BAT scores have been identified for children (≥2), adult males (>3), and adult females (>5).15
Condensed version of the Bleeding Assessment Tool. Reprinted from Tosetto et al75 with permission from John Wiley & Sons.
Condensed version of the Bleeding Assessment Tool. Reprinted from Tosetto et al75 with permission from John Wiley & Sons.
In the Vicenza-based tools, the severity of menstrual bleeding is based on the treatment provided (eg, hormonal contraceptives, iron therapy, blood transfusion, hysterectomy). One challenge of using these tools to evaluate women with HMB is that, given high rates of hormonal contraceptive use in women with and without bleeding disorders and the frequency of iron deficiency in women with HMB, women referred to a hematologist for heavy menses are likely to have similar severity scores. In addition, adolescent females make up a gray area in the field of bleeding assessment tools and have not been well studied. Therefore, the abnormal bleeding score for an adolescent female (many of whom have experienced menarche but have not typically experienced childbirth or the same number of surgical challenges as an adult) remains unknown but likely falls somewhere between 2 and 5.
In the past several years, self-administered bleeding assessment tools have been validated in both adult and pediatric populations, which increases the accessibility of these tools for busy clinical practices and for the lay population. In one effort to increase awareness of undiagnosed bleeding disorders, an informational Web site (http://letstalkperiod.ca) allows the user to complete an online self-BAT.16 The result is displayed to the user along with a recommendation to seek medical attention if the score is abnormal.
Philipp and colleagues17,18 have developed and tested a brief screening tool specifically for women with heavy menses to identify those at increased risk of an underlying bleeding disorder. The tool consists of 8 questions that include warning signs for excessive menstrual blood loss (duration ≥7 days, sensation of gushing, soaking a sanitary product in ≤2 hours), personal history of anemia, family history of bleeding disorders, and personal history of bleeding after surgical challenges or childbirth. Among adult women with a PBAC score of >100 presenting to a hematology clinic, the sensitivity of the tool for an underlying bleeding disorder was 89%. However, age differences have been noted in the sensitivity of the Philipp tool, with patients age 13 to 19 years having the lowest sensitivity (62%) in the original investigation. Additional adolescent-specific studies, particularly in primary care settings, are needed to determine the utility of this tool for this patient population.
As mentioned earlier, the PBAC, which estimates the number of menstrual products used and the degree of soiling, is the most commonly used tool in clinical practice to attempt to measure menstrual blood loss.19 Patients are asked to complete the chart during their menstrual period, and points are tallied for each sanitary product used. For example, 1 point is scored for each lightly stained product, 5 points for each moderately soiled product, and 20 points if the product is completely saturated.
Episodes of flooding and passage of blood clots are also recorded. Studies have demonstrated that a score of 150 or 185 on the PBAC correlates with 80 mL of menstrual blood loss as measured by the alkaline hematin test.20-22 In a retrospective study of adult women (n = 199) referred to a coagulation center for HMB, a PBAC score of >100 demonstrated 91% sensitivity for identifying patients with a coagulation disorder, 100% specificity, 100% positive predictive value, and 85.5% negative predictive value.23 The PBAC has not been well studied in adolescents with heavy menses, but early evidence demonstrates a correlation between PBAC scores and self-identification among adolescents as having heavy, normal, or light menses.24
Anovulatory bleeding as a result of an immature hypothalamic-pituitary-ovarian axis is a common cause of HMB in adolescents. Anovulatory bleeding is suggested by a history of irregular menstrual cycles occurring in the first 1 to 2 years after menarche, typically characterized by 2 or more months without bleeding followed by bleeding which can range from spotting, to heavy menses, to acute hemorrhage.25 An absence of menses-associated symptoms such as breast tenderness, moodiness, and/or dysmenorrhea may also suggest anovulation. However, it is important to remember that HMB can be multifactorial, and that women with underlying bleeding disorders also experience anovulatory bleeding. In a study of 115 women age 13 to 53 years presenting for evaluation of HMB, adolescents and perimenopausal women were just as likely to have an underlying hemostatic defect as women age 20 to 44 years.26 Therefore, even if the menstrual history is consistent with anovulatory bleeding, young women who develop severe anemia, do not respond to first-line therapies, or have personal or family history of easy bruising or bleeding still require laboratory evaluation for a bleeding disorder.
The most common bleeding disorders identified in adolescents with HMB are platelet function defects and von Willebrand disease.9 Other conditions that have been identified include hemophilia carrier states, other clotting factor deficiencies, fibrinolytic disorders, and bleeding tendency as a result of generalized joint hypermobility. Rarely, adolescents with immune thrombocytopenia purpura may present with HMB as their primary symptom.
Laboratory evaluation
The laboratory evaluation for an underlying bleeding disorder in a woman with HMB does not differ from the laboratory assessment in any patient presenting with unusual bruising or bleeding and is the aspect of the evaluation with which hematologists are likely most comfortable. However, it is worth mentioning a few caveats regarding testing in the setting of acute menorrhagia. von Willebrand factor levels will increase in the setting of stress, inflammation, and illness,27 and in our clinical experience, they are often found to be quite elevated when measured in adolescents hospitalized for severe HMB. Platelet function analyzer (PFA-100) closure times and platelet aggregation are delayed in the setting of anemia (hemoglobin <10 g/dL), so they should not be measured until the anemia has been corrected.28
High-dose estrogen therapy also elevates von Willebrand factor levels, but the influence of low-dose (standard dose) estrogen is less clear. The majority of studies in healthy women who use low-dose combined hormonal contraceptives have shown no significant increase in von Willebrand factor levels; however, there have been no studies in women with low von Willebrand disease or low von Willebrand factor levels at baseline.29 For patients started on a taper of combined hormonal contraceptives or a high-dose pill, our clinical practice is to delay testing for von Willebrand disease until the patient has been tapered down to standard low-dose (30-35 μg estrogen) for ∼3 months. To avoid continued or recurrent HMB, treatment with combined hormonal contraceptives should not be delayed or withheld to complete testing for von Willebrand disease.30 The PFA-100 was developed as a screening tool for von Willebrand disease and may be helpful in identifying this condition in women who need to remain on high-dose estrogen because results are not affected by estrogen therapy. Even though the PFA-100 is sensitive in identifying severe platelet function defects such as Glanzmann thrombasthenia and Bernard-Soulier syndrome, it is less effective at detecting milder, nonspecific platelet function defects. Therefore, full platelet aggregation studies should be performed in any patient for whom there is high clinical suspicion for an underlying bleeding disorder.
It is not infrequent for hematologists to care for patients who clearly have a bleeding tendency (Table 1) but for whom available hemostatic testing does not reveal a diagnosable condition.31 These bleeders of unknown cause may still benefit from continued follow-up in hematology and management similar to patients with defined bleeding disorders.32 For such patients, it is also important to consider a bleeding tendency that may result from a benign joint hypermobility syndrome and to assess for hypermobility during the physical examination.33,34 Joint hypermobility is more common in females, and patients with joint hypermobility syndromes (Ehlers-Danlos syndrome being the most common) may bleed because of increased capillary fragility, alterations in collagen protein involvement in platelet function, or changes in the interaction between exposed collagen in endothelial walls and platelet receptors or von Willebrand factor. Prolonged menses, irregular menses, and dysmenorrhea are all commonly reported by women with Ehlers-Danlos syndrome.35 Although many of these patients will have prolonged bleeding times,36 results from hemostatic tests performed in the standard manner are typically normal.37
Identifying and managing iron deficiency in the adolescent with HMB
Low serum ferritin is predictive of excessive menstrual blood loss of >80 mL.38 As a result, iron deficiency with and without anemia is commonly found in young women with HMB, with a prevalence of 51% in our own patient registry of young women with HMB.39 Because iron is an essential component of hemoglobin, myoglobin, and several metabolic enzymes, symptoms of iron deficiency may be caused by impairment of metabolic functions in tissues other than the bone marrow. The most common symptom of iron deficiency is fatigue, and fatigue severity scores in young women with HMB have been shown to be significantly higher than those in healthy female controls.40 Iron deficiency without anemia has also been related to increased muscle fatigue and decreased verbal learning and memory in adolescents.41-43 Therefore, it is important to assess not only hemoglobin level but also ferritin level when evaluating an adolescent with HMB. Definitions of iron deficiency vary, but serum ferritin <15 μg/L is a commonly used cutoff.44,45
The initial management of the adolescent with iron deficiency anemia depends on the severity of the anemia and symptom burden, and dosing recommendations can vary considerably. Recent studies in adult women (iron deficient but without anemia) have identified that lower and less frequent dosing of oral iron maximizes the fractional amount of iron absorbed per dose, and that alternate day dosing may even be preferred.46,47 Efficacy of low-dose (3 mg/kg once per day) oral iron regimens has also been demonstrated in young children with nutritional iron deficiency anemia.48 Although adolescent-specific studies are lacking, a once-per-day regimen of either 65 or 130 mg elemental iron, depending on the severity of the anemia, often aids in compliance for adolescents. Once-per-day dosing, particularly when iron is taken in the evening with food, can minimize gastrointestinal intolerance. For patients experiencing substantial fatigue, headaches, or exercise intolerance, we prefer intravenous iron to achieve a faster response. Transfusion of red blood cells would be administered in addition to iron supplementation in patients with hemodynamic changes (tachycardia, orthostatic hypotension) or shortness of breath with routine activities (such as climbing the stairs at home). Our practice is to continue iron supplementation until the anemia resolves and then continue for an additional 3 months to replace iron stores.
Managing HMB in the adolescent patient
The hematologist should be familiar with management strategies for both acute and chronic heavy menses. In the acute setting, a patient is typically hospitalized to receive a packed red cell transfusion for severe anemia and achieve cessation of bleeding. Although management decisions may be driven by the admitting hospitalist, adolescent specialist, or gynecologist, the hematologist may be asked to weigh in on the benefit of additive antifibrinolytics or may be asked to advise on the appropriate management for a patient with a suspected or confirmed bleeding disorder. In the outpatient setting, hematologists often play a leading role in HMB management for patients with inherited bleeding disorders. A growing number of multidisciplinary clinics have been established in which hematologists work side-by-side with gynecologists or adolescent medicine specialists to provide a comprehensive medical home for women seeking evaluation and management of HMB.49
The first-line therapy for acute HMB is oral estrogen-containing or progestin-only hormones, with intravenous dosing used if needed. Typically, high doses are initiated, and once bleeding is controlled, the dose is tapered over several weeks to a lower daily maintenance therapy.8 Occasionally, antifibrinolytic medications are added to the regimen and, in rarer instances, used alone to help manage acute HMB. In an analysis of more than 1100 admissions for acute HMB at children’s hospitals in the United States, 50% of patients received monotherapy with a hormone, most commonly a combination oral contraceptive.5 Thirty percent required 2 forms of hormonal therapy, 8% used both hormonal and antifibrinolytic therapy, and only 1% received antifibrinolytics alone. Practice patterns may vary by geography, with oral progestin-only strategies potentially more commonly used in areas outside the United States. Surgical interventions are reserved for the unusual case of persistent heavy bleeding despite aggressive medical management and can include endometrial balloon tamponade or dilation and curettage.50 As stated above, most patients admitted for HMB also have anemia and require iron replacement. It is also important to initiate iron therapy for patients who receive a red cell transfusion, because iron within transfused red cells is not sufficient for replenishing storage iron. Close outpatient follow-up for these young women is key to ensuring continued control of menstrual bleeding and complete correction of iron deficiency anemia.
In the outpatient setting, a variety of therapies are available for HMB, although in some cases, adolescent patients need special consideration (Table 2). Although first-line therapies are typically similar for women with and without inherited bleeding disorders, additional disease-specific interventions are available.51 For women with von Willebrand disease, guidelines from the National Heart, Lung, and Blood Institute state that the first choice of therapy for HMB should be combined oral contraceptives, with the levonorgestrel intrauterine device as second choice.52 Nasal desmopressin (DDAVP), antifibrinolytics, and von Willebrand factor concentrate are alternative options. Nasal desmopressin and antifibrinolytics can also be used in women with platelet function defects, factor VIII deficiency, and bleeding tendency as a result of generalized joint hypermobility. Factor VIII or factor IX replacement may be necessary in women with mild hemophilia or symptomatic carriers who do not respond to first-line therapies. Likewise, recombinant factor VIIa can be considered in women with Glanzmann thrombasthenia and congenital factor VII deficiency who do not respond to initial therapies.53
Benefits and commonly seen limitations of available therapeutic strategies for HMB in adolescents
| Therapeutic strategy . | Benefits . | Limitations . |
|---|---|---|
| Combined oral contraceptives | Easy to titrate dose and cycling; reduces menstrual irregularity, dysmenorrhea, and acne | Daily compliance is particularly challenging for adolescents; nausea; cannot be used by patients with thrombosis, thrombophilia, migraine with aura |
| Transdermal contraception | Requires only a once-per-week patch change; reduces menstrual irregularity, dysmenorrhea, and acne | Adolescents may have increased concern with visibility in locker rooms and during swimming; cannot be used by patients with thrombosis, thrombophilia, migraine with aura |
| Vaginal ring | Discrete; requires removal and insertion only once per month; reduces menstrual irregularity, dysmenorrhea, and acne | Adolescents may have discomfort with idea of vaginal insertion; cannot be used by patients with thrombosis, thrombophilia, migraine with aura |
| Levonorgestrel-containing intrauterine system | Discrete; effective for 3 to 5 years, depending on brand; many patients have a large reduction or complete cessation of menstrual bleeding; most effective for contraception; excellent safety profile for patients with increased risk of thrombosis | Requires pelvic examination for insertion; patients often have irregular bleeding for 4 to 6 months after insertion; higher upfront costs |
| Progestin-only pills (mini-pills) | Safe for women with estrogen contraindications | Requirement for strict adherence in timing of daily dose difficult for adolescent schedules; breakthrough bleeding a common cause for discontinuation |
| Norethindrone | Many patients experience complete cessation of menstrual bleeding | Weight gain and moodiness are common adverse effects, especially at higher doses (7.5 mg or 10 mg per day) |
| Depot medroxyprogesterone acetate | Safe for women with estrogen contraindications; discrete; requires injection only once every 12 weeks; many patients have substantial reduction in menstrual bleeding | Requires intramuscular injection; reinjection must be timely; risk of decreased bone density; although weight gain >2 kg not supported by available evidence, some patients do experience weight gain, and there is fear regarding this adverse effect in the adolescent community |
| Progestin-only implantable | Discrete; lasts for 3 years; highly effective contraceptive; safe for women with estrogen contraindications | Requires minor procedure for insertion; higher upfront costs; breakthrough bleeding a common cause for discontinuation |
| Tranexamic acid | Option for families who do not want to use a hormonal strategy; taken only during menses; minimal adverse effect profile | Does not provide cycle regulation; not indicated for dysmenorrhea; 3-times-per-day dosing is challenging for adolescents |
| Nasal desmopressin | Option for families who do not want to use a hormonal strategy; taken only during menses | Does not provide cycle regulation; not indicated for dysmenorrhea; because of tachyphylaxis, can be used for only 2 to 3 days during menses; facial flushing and headaches are commonly reported adverse effects |
| Therapeutic strategy . | Benefits . | Limitations . |
|---|---|---|
| Combined oral contraceptives | Easy to titrate dose and cycling; reduces menstrual irregularity, dysmenorrhea, and acne | Daily compliance is particularly challenging for adolescents; nausea; cannot be used by patients with thrombosis, thrombophilia, migraine with aura |
| Transdermal contraception | Requires only a once-per-week patch change; reduces menstrual irregularity, dysmenorrhea, and acne | Adolescents may have increased concern with visibility in locker rooms and during swimming; cannot be used by patients with thrombosis, thrombophilia, migraine with aura |
| Vaginal ring | Discrete; requires removal and insertion only once per month; reduces menstrual irregularity, dysmenorrhea, and acne | Adolescents may have discomfort with idea of vaginal insertion; cannot be used by patients with thrombosis, thrombophilia, migraine with aura |
| Levonorgestrel-containing intrauterine system | Discrete; effective for 3 to 5 years, depending on brand; many patients have a large reduction or complete cessation of menstrual bleeding; most effective for contraception; excellent safety profile for patients with increased risk of thrombosis | Requires pelvic examination for insertion; patients often have irregular bleeding for 4 to 6 months after insertion; higher upfront costs |
| Progestin-only pills (mini-pills) | Safe for women with estrogen contraindications | Requirement for strict adherence in timing of daily dose difficult for adolescent schedules; breakthrough bleeding a common cause for discontinuation |
| Norethindrone | Many patients experience complete cessation of menstrual bleeding | Weight gain and moodiness are common adverse effects, especially at higher doses (7.5 mg or 10 mg per day) |
| Depot medroxyprogesterone acetate | Safe for women with estrogen contraindications; discrete; requires injection only once every 12 weeks; many patients have substantial reduction in menstrual bleeding | Requires intramuscular injection; reinjection must be timely; risk of decreased bone density; although weight gain >2 kg not supported by available evidence, some patients do experience weight gain, and there is fear regarding this adverse effect in the adolescent community |
| Progestin-only implantable | Discrete; lasts for 3 years; highly effective contraceptive; safe for women with estrogen contraindications | Requires minor procedure for insertion; higher upfront costs; breakthrough bleeding a common cause for discontinuation |
| Tranexamic acid | Option for families who do not want to use a hormonal strategy; taken only during menses; minimal adverse effect profile | Does not provide cycle regulation; not indicated for dysmenorrhea; 3-times-per-day dosing is challenging for adolescents |
| Nasal desmopressin | Option for families who do not want to use a hormonal strategy; taken only during menses | Does not provide cycle regulation; not indicated for dysmenorrhea; because of tachyphylaxis, can be used for only 2 to 3 days during menses; facial flushing and headaches are commonly reported adverse effects |
The management of HMB in adolescents presents unique challenges, because the etiology is often multifactorial. In addition, patients or parents may be reluctant or unwilling to use the hormonal agents recommended as first-line therapies. There are few randomized studies of HMB treatment in women of any age, with particularly limited research in adolescents.54,55
Combination hormonal contraceptives
Combination hormonal contraceptives, which contain both estrogen and progestin, are available in oral, transdermal, and vaginal ring formulations. These agents reduce menstrual loss by inducing changes that thin the endometrium. Additional benefits of these agents include regulation of the menstrual cycle, reduction of dysmenorrhea, and improvement in acne. In published reports from 2 multidisciplinary hematology and gynecology clinics, combined oral contraceptives were the most commonly used therapy in their adolescent patients (55% and 70%, respectively).56,57 These agents also offer the option of extended cycling, in which patients skip the placebo week and take active pills only. Although breakthrough bleeding is a possible adverse effect of extended cycling, this strategy may be particularly helpful for those presenting with severe HMB until the anemia has resolved. We recommend the use of a standard monophasic low-dose pill containing 30 to 35 μg of estrogen. In a Cochrane review58 of monophasic vs triphasic oral contraceptives, several trials reported less breakthrough bleeding in those who used triphasics vs monophasics. Although studies have not been performed in adolescents comparing monophasic to triphasic contraceptives, or ultra-low-dose to low-dose estrogen, in our clinical experience, we find that breakthrough bleeding seems more common in patients originally started on triphasics or formulations with ≤20 μg of estrogen and often resolves when the patient is switched to monophasics with 30 to 35 μg of estrogen.
Time must be allotted for detailed patient and family counseling when prescribing hormonal contraception, particularly in younger adolescents. This may be the first daily medication for an otherwise healthy young woman, so tips on achieving good adherence will be necessary. Other family questions and concerns we see frequently in clinical practice include long-term effects of hormonal contraception use on fertility and risk of breast cancer, or concerns about the social implications of taking a birth control pill. Discussing the frequency of HMB in adolescents, even though it may be a topic not discussed among peers, and the many young women who successfully use these medications to improve their symptoms can often help reassure teens and parents.
Levonorgestrel intrauterine system
Randomized clinical trials in adult women consistently demonstrate that the levonorgestrel intrauterine system (LNG-IUS) has superior efficacy to oral medications in the treatment of HMB.59 A systematic review of data from participants younger than age 25 years revealed that adolescents and young women have high (74%) 12-month continuation rates for intrauterine devices.60 Breakthrough bleeding in the first 6 months of use is fairly typical and can be more troublesome in women with heavy menses. In our practice, we have found the use of once-per-day norethindrone (starting dose, 5 mg) to be helpful in managing patients who experience particularly heavy breakthrough bleeding after insertion of an LNG-IUS.
The short-term and long-term efficacy of the LNG-IUS has also been reported in a small cohort of adults with inherited bleeding disorders in the United Kingdom and in several adolescents.61,62 In one small study from Canada of LNG-IUS use in women (n = 20) with bleeding disorders, 50% had the LNG-IUS removed because of poor patient satisfaction, malposition, or expulsion.63 Studies are still needed to document expulsion rates and patterns of menstrual bleeding in young nulliparous women with these devices. Physician-patient-parent discussions regarding this device also require substantial time, because patients and parents often have misperceptions that these devices negatively affect fertility, cannot be placed in nulliparous women, cannot be removed once placed, can only be placed in women who have had children, or perhaps are even limited to those who have completed their planned child bearing.
Other progestin-only agents
It is not unusual for a hematologist to be asked to consult on HMB management for an adolescent with a history of thrombosis or thrombophilia, or a positive family history of the same. In other cases, the adolescent may not be eligible for estrogen-containing therapies because of a history of migraine with aura. Several progestin-only options are available, including progestin-only pills (the mini-pill), depot medroxyprogesterone acetate, and the progestin implant. Benefits and limitations of each are reviewed in Table 2. The LNG-IUS described above is another progestin-only option, and it has an excellent safety record with regard to thrombotic risk.64,65 Another type of progestin pill, norethindrone, has also been used for acute HMB and maintenance management of HMB.66 In our practice, we have found norethindrone (starting dose, 5 mg, increasing to 7.5 mg or 10 mg as needed) to be quite helpful in achieving complete cessation of menses in patients with inadequate response to combined hormonal contraceptives.
Nonhormonal agents
Desmopressin and antifibrinolytics are alternative treatments for young women who do not want to use hormonal agents or who do not fully respond to them. However, neither agent provides regulation of the menstrual cycle, which can be problematic in adolescents with inherited bleeding disorders and anovulatory bleeding because of hormonal immaturity. Although desmopressin has been relatively well studied in adult women with a variety of mild inherited bleeding disorders, there are limited data in adolescents. In a study of 22 adolescents with von Willebrand disease, intranasal desmopressin improved PBAC scores in 77% of patients.67 Desmopressin has also been used to treat HMB in adolescents with platelet function defects68 and can be used in women with bleeding tendencies that result from joint hypermobility syndromes.36 The main limitation of desmopressin in this setting is the effect of tachyphylaxis, because the efficacy of desmopressin depends on endothelial stores of von Willebrand factor. Therefore, use of desmopressin is limited to the first 2 to 3 days of heavy flow of the menstrual cycle.69 For our small population of patients who use desmopressin as single-agent therapy for HMB, we recommend desmopressin on days 1, 2, or 4 of the menstrual cycle or days 2, 3, and 5, depending on the whether the patient tends to start their menses with spotting or with heavy flow. Our patients typically prefer to use desmopressin at bedtime to reduce the impact of adverse effects.
The antifibrinolytic agent tranexamic acid has long been used for the treatment of HMB.70 Doses are typically 1000 mg, and reported regimens include dosing 2, 3, or 4 times per day. In the United States, tranexamic acid is available in 650-mg tablets and dosing is typically 1300 mg 3 times per day. In a crossover study of intranasal desmopressin and tranexamic acid in adult women with a variety of bleeding disorders, both agents reduced menstrual blood flow and improved quality of life.71 For both outcomes, however, tranexamic acid was found to be more effective than desmopressin. In a pilot study comparing the efficacy of tranexamic acid with combined oral contraceptives in adolescents with HMB, both agents seemed equally efficacious in decreasing menstrual blood loss and improving quality of life, but only oral contraceptives decreased the number of days of menstrual flow.72 In a report of 42 adolescents with HMB and inherited bleeding disorders, tranexamic acid (1 g 4 times per day during menses) was the most commonly prescribed therapy and was used in 98% of patients.56 In about one-third of these patients, tranexamic acid alone adequately controlled bleeding.
Although the great majority of patients will respond to 1 of the interventions described here, second-line therapies for women with severe refractory HMB and inherited bleeding disorders include von Willebrand factor replacement, factor VIII concentrates, recombinant factor VIIa, and recombinant interleukin-11, depending on the specific underlying bleeding disorder.53,73,74 In a survey of 1321 women with von Willebrand disease seen at hemophilia treatment centers in the United States, von Willebrand factor replacement was used as third-line therapy in 13 women (1.6%).73 These same authors performed a literature review of 88 additional women with von Willebrand disease and reported that in all 101 women, a reduction in menstrual blood loss was reported with a dose of 33 to 100 U/kg. Most women administered factor products on days 1 to 6 of the menstrual cycle.
Summary
HMB is frequently reported by adolescents, and the evaluation often includes referral to a hematologist for consideration of an underlying bleeding disorder. A history of menses lasting >7 days, menstrual flow which soaks >5 sanitary products per day or requires product change during the night, passage of large blood clots, failure to respond to conventional therapies, or a personal or family history of easy bruising or bleeding should all raise suspicion for an underlying bleeding disorder. Iron deficiency with and without anemia is commonly found in young women with HMB, so hemoglobin and ferritin levels should be assessed in these patients. Strategies for decreasing menstrual blood flow are similar between adults and adolescents with heavy menses, with combined hormonal contraceptives recommended as first-line therapy. However, there are adolescent-specific considerations for many of these therapies, and they must be incorporated into shared decision-making when selecting the most appropriate treatment.
Correspondence
Sarah H. O’Brien, The Research Institute at Nationwide Children’s Hospital, 700 Children’s Dr, Columbus, OH 43205; e-mail: sarah.obrien@nationwidechildrens.org.
References
Author notes
This article was selected by the Blood and Hematology 2018 American Society of Hematology Education Program editors for concurrent submission to Blood and Hematology 2018. It is reprinted from Blood 2018, Volume 132.
Competing Interests
Conflict of Interest: S.H.O. received research funding from the Children’s Oncology Group for a study funded by Bristol-Myers Squibb and has served on advisory committees for Pfizer, CSL Behring, and Shire within the past 2 years.
Off-label drug use: This article describes off-label drug use of nasal desmopressin in patients with platelet function defects or joint hypermobility.