Abstract
In 2020, for the great majority of patients with chronic phase chronic myeloid leukemia (CML), life expectancy is unaffected by a diagnosis of CML because of the unparalleled efficacy of ABL-targeted tyrosine kinase inhibitors (TKIs) in halting disease progression. A wealth of choices exist for first-line treatment selection, including the first-generation TKI imatinib and the second-generation TKIs bosutinib, dasatinib, and nilotinib. How I select first-line therapy between first-generation and second-generation TKIs is discussed in the context of patient-specific CML disease risk, therapy-related risks, and treatment goals. Although rare, identifying patients with CML at higher risk for disease progression or resistance is important and influences first-line TKI selection. I review the impact of first-generation vs second-generation TKI selection on treatment response and outcomes; the ability to achieve, as well as the timing of, treatment-free remission; and the impact of specific TKIs on longer-term health.
Learning Objectives
Identify disease-specific risk factors at chronic myeloid leukemia diagnosis that influence first-line tyrosine kinase inhibitor (TKI) selection
Delineate patient comorbidities that impact first-line TKI selection
Examine how first-line TKI selection impacts treatment-free remission
Introduction
Chronic myeloid leukemia (CML) while in chronic phase (CP) is driven by the constitutively active BCR-ABL tyrosine kinase resulting from the translocation t(9;22)(q34;q11). The ABL-targeted tyrosine kinase inhibitors (TKIs) imatinib, bosutinib, dasatinib, and nilotinib have transformed leukemia with poor overall survival (OS) into a disease in which life expectancy for most individuals is not impacted by CML, and many are now living with a CML diagnosis. I discuss how I select first-line TKI therapy for patients with CP CML, weighing CML risk factors, patient age, medical history, and treatment goals. Treatment-free remission (TFR), as discussed by Dr. Delphine Rea, is among the most important patient-described goals and is a strategy, if successful, that can limit health care costs. However, ∼80% of patients remain on TKI therapy in the longer term. How to promote safe management in patients with comorbidities, as discussed by Dr. Jorge Cortes, while aiming for TFR and optimal quality of life through appropriate TKI selection is a discussion between patients and health care providers that begins at diagnosis.
Clinical case 1
Patient 1 is a 47-year-old woman with no significant past medical history who presented with left upper quadrant abdominal pain, 8-pound weight loss, and fatigue. Her only medication is a daily multivitamin. She does not use tobacco or have a history of tobacco use, and she does not use alcohol. Her 10-year atherosclerotic cardiovascular disease (ASCVD) risk score is 0.8% (low; http://tools.acc.org/ASCVD-Risk-Estimator-Plus/#!/calculate/estimate/). Results of her evaluation are listed below:
Complete blood count: white blood cell count, 183 000/µL; 8% blasts; 9% basophils; 2% eosinophils; platelet count, 520 000/µL
Her spleen was palpable 9 cm below the costal margin.
Her peripheral blood BCR-ABL1 was 110%.
Bone marrow evaluation: hypercellularity (80%); 1+/3 focal areas of increased reticulin fibrosis; blasts 9%; chromosome banding analysis showing 46,XX, t(9;22)(q34;q11.2)[20]/46; no additional chromosome abnormalities (ACA) noted
Sokal score, 1.84, high risk (>1.2)
European Treatment and Outcome Study Long-Term Survival (ELTS) score (https://www.leukemia-net.org/content/leukemias/cml/project_info/index_eng.html), 2.2239, high risk (>2.2185)
Clinical case 2
Patient 2 is 61-year-old woman with a past medical history notable for hypertension and type 2 diabetes mellitus (T2DM) who presented with fatigue to her primary physician. Her medications include metformin and lisinopril. She has no tobacco or history of tobacco use and drinks one glass of wine per week. Her 10-year ASCVD risk score is 15.8% (intermediate). Results of her evaluation are listed below:
Complete blood count: white blood cell count, 28 900/µL; 1% blasts; 4% basophils; 2% eosinophils; platelet count, 602 000/µL
Her spleen was palpable 1 cm below the costal margin.
Her peripheral blood BCR-ABL1 was 44.9%.
Bone marrow evaluation: hypercellularity (100%); blasts 1%; chromosome banding analysis showing 46,XX, t(9;22)(q34;q11.2)[19]/46, XX[1]; no ACAs noted
Sokal score, 0.91, intermediate risk (0.8-1.2)
ELTS score, 1.2632, low risk (≤1.5680)
How do I identify risky patients at diagnosis?
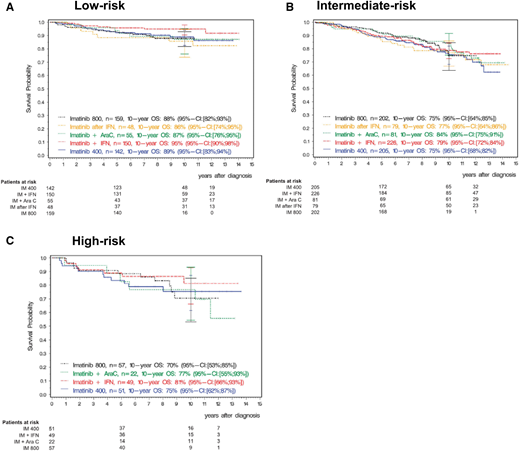
Ten-year updates of the imatinib first-line registration study IRIS1 demonstrate excellent longer-term OS: 91.1% vs 85.3% in patients with vs without major molecular response (MMR; BCR-ABL1, <0.1%) achieved at 12 months, respectively. Expected treatment responses are defined and reviewed in Tables 1 and 2. Although many patients with CP CML do well, identifying the small group of patients at risk for poor outcomes at diagnosis is highly clinically relevant. These patients require close monitoring to ensure that they achieve treatment milestones and rapid transition to alternative therapies when they do not. Clinical multivariate prognostic risk models (eg, Sokal, Euro/Hasford) remain valuable for risk stratification and identify patients at risk for poorer OS, progression-free survival (PFS), and event-free survival, as well as disease progression. In the IRIS study, in patients with high Sokal risk scores, estimated 10-year OS was inferior at 68.6% vs 80.3% (intermediate risk) and 89.9% (low risk), with similar observations made in the German CML Study IV (Figure 1).1,2 A newer risk model, the ELTS score, which, like the earlier Sokal score, includes the variables age, spleen size by manual palpation, platelet count, and blast percentage, was specifically derived in imatinib-treated patients to discriminate more accurately the risk for CML-related death.3 Reflecting good outcomes in older individuals, increasing age has a more limited negative impact on prognosis. Among 1120 out-of-study imatinib-treated patients used for validation, the 5-year cumulative incidence probability of dying of CML was 3% (95% confidence interval [CI], 2% to 5%) in low-risk patients (eg, patient 2), 4% (95% CI, 3% to 7%) in intermediate-risk patients, and 15% (95% CI, 10% to 22%) in high-risk patients (eg, patient 1).3 Retrospective analyses, including in “real-world” and second-generation TKI-treated patients, have validated the original observations, including in children and young adults.4,5
Chronic myeloid leukemia molecular responses defined
| Response . | Definition . |
|---|---|
| Early molecular response | BCR-ABL1 ≤10% (PB) |
| BCR-ABL1 < 1% | Molecular equivalent of CCyR |
| Major molecular response | BCR-ABL1 ≤0.1% (PB) (common trial endpoint) |
| Deeper molecular responses (MR) | BCR-ABL1 ≤0.01% (MR 4) (PB) |
| BCR-ABL1 ≤0.0032% (MR 4.5) (PB) | |
| Or undetectable BCR-ABL1 with specific detection of control gene* |
| Response . | Definition . |
|---|---|
| Early molecular response | BCR-ABL1 ≤10% (PB) |
| BCR-ABL1 < 1% | Molecular equivalent of CCyR |
| Major molecular response | BCR-ABL1 ≤0.1% (PB) (common trial endpoint) |
| Deeper molecular responses (MR) | BCR-ABL1 ≤0.01% (MR 4) (PB) |
| BCR-ABL1 ≤0.0032% (MR 4.5) (PB) | |
| Or undetectable BCR-ABL1 with specific detection of control gene* |
CCyR, complete cytogenetic response (no Philadelphia chromosome–positive metaphases by bone marrow examination); PB, peripheral blood.
Control genes include ABL, BCR, and GUSB. For example, specific control gene detection for MR4 requires a minimum of 10 000 ABL1 transcripts or 24 000 GUSB transcripts, and for MR4.5, it requires a minimum of 32 000 ABL1 transcripts or 77 000 GUSB transcripts.
European LeukemiaNet and National Comprehensive Cancer Network recommendations
| . | ELN optimal . | ELN warning . | ELN failure . | |
|---|---|---|---|---|
| Baseline | NA | High-risk ACA, high-risk ELTS score | NA | |
| 3 mo | ≤10% | >10% | >10% if confirmed within 1-3 mo | |
| 6 mo | ≤1% | >1%-10% | >10% | |
| 12 mo | ≤0.1% | >0.1%-1% | >1% | |
| Any time | ≤0.1% | >0.1%-1%, loss of ≤0.1% (MMR) | >1%, resistance mutations, high-risk ACA | |
| . | ELN optimal . | ELN warning . | ELN failure . | |
|---|---|---|---|---|
| Baseline | NA | High-risk ACA, high-risk ELTS score | NA | |
| 3 mo | ≤10% | >10% | >10% if confirmed within 1-3 mo | |
| 6 mo | ≤1% | >1%-10% | >10% | |
| 12 mo | ≤0.1% | >0.1%-1% | >1% | |
| Any time | ≤0.1% | >0.1%-1%, loss of ≤0.1% (MMR) | >1%, resistance mutations, high-risk ACA | |
| . | NCCN 3 months . | NCCN 6 months . | NCCN 12 months . |
|---|---|---|---|
| >10% | NCCN Possible TKI Resistance | NCCN TKI-resistant | NCCN TKI-resistant |
| >1% - 10% | NCCN TKI-sensitive | NCCN TKI-sensitive | NCCN Possible TKI Resistance |
| >0.1 - 1% | NCCN TKI-sensitive | NCCN TKI-sensitive | NCCN TKI sensitive* |
| ≤ 0.1% | NCCN TKI-sensitive | NCCN TKI-sensitive | NCCN TKI-sensitive |
| . | NCCN 3 months . | NCCN 6 months . | NCCN 12 months . |
|---|---|---|---|
| >10% | NCCN Possible TKI Resistance | NCCN TKI-resistant | NCCN TKI-resistant |
| >1% - 10% | NCCN TKI-sensitive | NCCN TKI-sensitive | NCCN Possible TKI Resistance |
| >0.1 - 1% | NCCN TKI-sensitive | NCCN TKI-sensitive | NCCN TKI sensitive* |
| ≤ 0.1% | NCCN TKI-sensitive | NCCN TKI-sensitive | NCCN TKI-sensitive |
ELN and NCCN milestone recommendations in 2020 are similar, although how each panel chooses to highlight timing of response and achievement of deeper molecular responses is slightly different. Absence of early molecular response at 6 months is considered failure by ELN recommendations and TKI resistant by NCCN recommendations. NCCN and ELN highlight achievement of <1% BCR-ABL1, which is associated with significant progression-free survival benefits. Not achieving this milestone denotes failure or TKI resistance. Both recognize that milestones such as major molecular response (MMR, BCR-ABL1 < 0.1%) must be achieved in patients aiming for treatment-free remission (TFR) and that there is a high likelihood of achieving a subsequent deep molecular response (MR4) for patients achieving MMR at 12 months. *Achievement of BCR-ABL1 >0.1-1% is associated with improved long-term overall survival even if MMR is not achieved. For ELN recommendations a change of treatment may be considered if MMR is not reached by 36 to 48 months, which may facilitate future achievement of TFR. NCCN recommendations also endorse shared decision-making with patients if MMR is not achieved. Assessment for ABL mutations are recommended for ELN warning/failure and NCCN possible TKI resistance/TKI resistance.
ACA, additional chromosome abnormalities in Philadelphia chromosome–positive cells; ELN, European LeukemiaNet; ELTS, European Treatment Outcome Study Long-Term Survival; MMR, major molecular response; NA, not applicable; NCCN, National Comprehensive Cancer Network; TKI, tyrosine kinase inhibitor.
Adapted with permission from the NCCN Guidelines® for Chronic Myeloid Leukemia V.1.2021. © 2021 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines and illustrations herein may not be reproduced in any form for any purpose without the express written permission of the NCCN. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Overall survival by Euro (Hasford) score with 10-year follow-up from the German CML Study IV. A total of 1551 patients with chronic phase CML were treated with imatinib 400 mg daily or 800 mg daily or with imatinib in combination with cytarabine or with interferon-α or with imatinib after interferon-α. Overall survival is shown by Euro risk score. (A) Low risk. (B) Intermediate risk. (C) High risk. Reprinted from Hehlmann et al2 with permission. AraC, cytarabine; IFN, interferon-α; IM, imatinib; OS, overall survival.
Overall survival by Euro (Hasford) score with 10-year follow-up from the German CML Study IV. A total of 1551 patients with chronic phase CML were treated with imatinib 400 mg daily or 800 mg daily or with imatinib in combination with cytarabine or with interferon-α or with imatinib after interferon-α. Overall survival is shown by Euro risk score. (A) Low risk. (B) Intermediate risk. (C) High risk. Reprinted from Hehlmann et al2 with permission. AraC, cytarabine; IFN, interferon-α; IM, imatinib; OS, overall survival.
Although this is rare, ∼5% of newly diagnosed patients harbor ACAs in Philadelphia (Ph) chromosome–positive cells, which technically also support a diagnosis of accelerated phase (AP) CML. Retrospective analyses from the German CML Study IV originally identified ACAs termed “major route” (trisomy 8, second Ph chromosome, isochromosome [17q], or trisomy 19) that were associated with poorer PFS and OS than in patients without ACAs or those with rarer ACAs (termed “minor route”).6,7 Another study identified only isochromosome (17q), −7/del7q, and 3q26.2 in association with poorer treatment responses and OS.8 Adding to the debate, a report of 603 patients treated with frontline therapy in various prospective clinical trials found no differences in cumulative complete cytogenetic response or MMR rates, PFS, or OS between patients with and those without ACAs.9 However, a caveat is that no patients in this study had isochromosome (17q), 3q26.2, or complex aberrant karyotypes associated with poorer outcomes in other series. In keeping with panel recommendations, I consider patients with trisomy 8, second Ph chromosome, trisomy 19, isochromosome (17q), −7/del7q, 11q23, 3q26.2 aberrations, or complex aberrant karyotypes as being at high risk, but I recognize that the first three abnormalities may be less worrisome.10,11
The p190 BCR-ABL protein isoform (e1a2 transcript), seen in acute lymphoblastic leukemia, is present in ∼1% to 2% of cases and is associated with inferior outcomes.12 Although recent updates are limited, I consider the p190 isoform to confer high risk. A difference in prognosis initially reported between BCR-ABL1 transcript types e13a2 and e14a2 (p210 protein isoform) has not been corroborated in more recent reviews.13,14 For other rare transcript variants, the impact on prognosis is unclear; these are not detected by quantitative polymerase chain reaction assays that measure only e1a2, e13a2, and e14a2 transcripts, which are commonly used at diagnosis, but they can be detected by qualitative BCR-ABL1 assays.
In other myeloid malignancies, next-generation sequencing (NGS) has identified specific myeloid mutations at diagnosis and examined clonal selection and new mutation development with therapy and disease progression. As recently reviewed, mutations in RUNX1, IKZF1, and ASXL1 are rarely detected in CP patients but are more common in AP and blast phase (BP) patients.15,16 A study of 100, predominantly CP, patients delineated patterns of mutation kinetics and new mutation acquisition.15 Some did not impact excellent TKI responses; however, treatment failure was common in the rare patients who acquired TP53, KMT2D, and TET2 mutations.
What are the benefits of first-line second-generation TKI use?
To date, when looking at all patients, no statistically significant improvement in OS or PFS has been reported for any second-generation TKI, used at recommended first-line doses, as compared with imatinib.17-19 However, first-line phase 3 randomized registration studies of dasatinib, nilotinib, and bosutinib vs imatinib (DASISION17 , ENESTnd18 , and BFORE19 , respectively) have observed that (1) patients receiving second-generation TKIs achieve more rapid molecular responses; (2) dasatinib- and nilotinib-treated patients develop fewer mutations conferring TKI resistance and achieve responses allowing TFR consideration more rapidly; and (3) although rare, nilotinib-treated patients have fewer progression events to AP or BP. Second-generation TKI use (summarized in Tables 3 and 4 and by risk score in Tables 5 and 6) resulted in higher probability of achieving early molecular response (EMR; BCR-ABL1, ≤10%), MMR, and deeper molecular responses (MR4 and MR4.5; BCR-ABL1, ≤0.01% and ≤0.0032%, respectively).17-19 Achieving EMR at 3 or 6 months is associated with improved OS and PFS, a benefit of ∼10% to 15%, regardless of TKI. Although fewer imatinib-treated patients achieve EMR at 3 months, data suggest that the absence of EMR at 6 months is the stronger indicator of poor PFS and OS.20,21 In addition, relevant to TFR, among 1442 evaluable patients treated with imatinib and with imatinib in combination in the German CML Study IV, the cumulative incidences of MR4 and MR4.5 were 68% and 53%, respectively, by 5 years and 81% and 72%, respectively, by 10 years.11
Molecular response with long-term follow-up
| Trial . | Study arms . | No. of patients . | Median follow-up . | EMR at 3 mo . | CCyR by 12 mo . | MMR 12 mo*,† . | MMR by 2 y . | MR4 by 2 y . | MR4.5 by 2 y . | MR4 by 5 y . | MR 4.5 by 5 y . | MR4 by 10 y . | MR4.5 by 10 y . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRIS‡ | Imatinib (400 mg) | 553 | 11 y | — | 69%* | 39%* | — | — | — | — | 40.2%§ | — | 63.2%§ |
| Interferon/cytarabine | 553 | — | — | — | — | — | — | — | — | ||||
| German CML Study IV | Imatinib (400 mg) arm (all) | 400 (1551) | 9.5 y | 68.5%|| | 67.5% | 36.7% | — | — | — | 65.7% | 49.4% | 81% | 67.2% |
| DASISION¶ | Dasatinib (100 mg) | 259 | 5 y | 84% | 83.0% | 46.0% | 64.0% | — | 17.0% | — | 42.0% | — | — |
| Imatinib (400 mg) | 260 | 64% | 72.0% | 28.0% | 46.0% | — | 8.0% | — | 33.0% | — | — | ||
| ENESTnd# | Nilotinib 300 mg twice daily | 282 | 5 y | 91% | 80.0% | 44%† | 71.0% | 39.0% | 25.0% | 65.6% | 53.5% | 73% | 64% |
| Nilotinib 400 mg twice daily | 281 | 89% | 78.0% | 43%† | 67.0% | 33.0% | 19.0% | 63.0% | 52.3% | — | — | ||
| Imatinib (400 mg) | 283 | 67% | 65.0% | 22%† | 44.0% | 18.0% | 9.0% | 41.7% | 31.4% | 56% | 44% | ||
| BFORE#,** | Bosutinib (400 mg) | 268 | 2 y | 75% | 77.2% | 47.2%† | 61.2% | 32.8% | 13.1% | — | — | — | — |
| Imatinib (400 mg) | 268 | 57% | 66.4% | 36.9%† | 50.7% | 25.7% | 10.8% | — | — | — | — |
| Trial . | Study arms . | No. of patients . | Median follow-up . | EMR at 3 mo . | CCyR by 12 mo . | MMR 12 mo*,† . | MMR by 2 y . | MR4 by 2 y . | MR4.5 by 2 y . | MR4 by 5 y . | MR 4.5 by 5 y . | MR4 by 10 y . | MR4.5 by 10 y . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRIS‡ | Imatinib (400 mg) | 553 | 11 y | — | 69%* | 39%* | — | — | — | — | 40.2%§ | — | 63.2%§ |
| Interferon/cytarabine | 553 | — | — | — | — | — | — | — | — | ||||
| German CML Study IV | Imatinib (400 mg) arm (all) | 400 (1551) | 9.5 y | 68.5%|| | 67.5% | 36.7% | — | — | — | 65.7% | 49.4% | 81% | 67.2% |
| DASISION¶ | Dasatinib (100 mg) | 259 | 5 y | 84% | 83.0% | 46.0% | 64.0% | — | 17.0% | — | 42.0% | — | — |
| Imatinib (400 mg) | 260 | 64% | 72.0% | 28.0% | 46.0% | — | 8.0% | — | 33.0% | — | — | ||
| ENESTnd# | Nilotinib 300 mg twice daily | 282 | 5 y | 91% | 80.0% | 44%† | 71.0% | 39.0% | 25.0% | 65.6% | 53.5% | 73% | 64% |
| Nilotinib 400 mg twice daily | 281 | 89% | 78.0% | 43%† | 67.0% | 33.0% | 19.0% | 63.0% | 52.3% | — | — | ||
| Imatinib (400 mg) | 283 | 67% | 65.0% | 22%† | 44.0% | 18.0% | 9.0% | 41.7% | 31.4% | 56% | 44% | ||
| BFORE#,** | Bosutinib (400 mg) | 268 | 2 y | 75% | 77.2% | 47.2%† | 61.2% | 32.8% | 13.1% | — | — | — | — |
| Imatinib (400 mg) | 268 | 57% | 66.4% | 36.9%† | 50.7% | 25.7% | 10.8% | — | — | — | — |
Data from 4 first-line phase 3 randomized registration studies (IRIS, DASISION, ENESTnd, and BFORE) and the first-line imatinib 400 mg daily arm of the German CML Study IV are shown. MRs at various time points are shown. These trials cannot be directly compared, because different methods of trial evaluation were used (eg, rates at a specific time point vs cumulative incidence estimates).
CCyR, complete cytogenetic response (no Philadelphia chromosome–positive metaphases by bone marrow examination); EMR, early molecular response; MMR, major molecular response; MR, molecular response.
Estimated rate.
†Rate at 12 months (ie, not cumulative).
‡The primary endpoint for IRIS was event-free survival (survival without transformation to accelerated phase/blast phase, loss of complete hematologic response, loss of major cytogenetic response, or increased white blood cell count); survival outcomes include 363 patients who crossed over to imatinib.
Rate at the specific time point (eg, at 5 years and at 10 years).
Includes all patients in all arms.
The primary endpoint for the DASISION study was confirmed complete cytogenetic response by 12 months.
The primary endpoint of the ENESTnd and BFORE studies was MMR rate at 12 months.
Twenty-four-month BFORE trial updates have been presented in abstract format.41
Disease progression, progression-free survival, and overall survival with long-term follow-up
| Trial . | Study arms . | No. of patients . | Median follow-up . | Disease progression, n (%) . | PFS . | OS . |
|---|---|---|---|---|---|---|
| IRIS* | Imatinib (400 mg) | 553 | 11 y | 38 (6.9%) | 92.1% | 83.3% |
| Interferon/cytarabine | 553 | 71 (12.8%) | — | 78.8% | ||
| German CML Study IV | Imatinib (400 mg) arm (all) | 400 (1551) | 9.5 y | 17 (4.2%) | 80.0% | 80.0% |
| DASISION | Dasatinib (100 mg) | 259 | 5 y | 12 (5%) | 85.0% | 91.0% |
| Imatinib (400 mg) | 260 | 19 (7%) | 86.0% | 90.0% | ||
| ENESTnd† | Nilotinib 300 mg twice daily | 282 | 5 y | 10 (4%) | 92.0% | 94.0% |
| Nilotinib 400 mg twice daily | 281 | 6 (2%) | 96.0% | 96.0% | ||
| Imatinib (400 mg) | 283 | 21 (7%) | 91.0% | 92.0% | ||
| BFORE‡ | Bosutinib (400 mg) | 268 | 2 y | 6 (2%) | — | 99.2% |
| Imatinib (400 mg) | 268 | 7 (3%) | — | 97.0% |
| Trial . | Study arms . | No. of patients . | Median follow-up . | Disease progression, n (%) . | PFS . | OS . |
|---|---|---|---|---|---|---|
| IRIS* | Imatinib (400 mg) | 553 | 11 y | 38 (6.9%) | 92.1% | 83.3% |
| Interferon/cytarabine | 553 | 71 (12.8%) | — | 78.8% | ||
| German CML Study IV | Imatinib (400 mg) arm (all) | 400 (1551) | 9.5 y | 17 (4.2%) | 80.0% | 80.0% |
| DASISION | Dasatinib (100 mg) | 259 | 5 y | 12 (5%) | 85.0% | 91.0% |
| Imatinib (400 mg) | 260 | 19 (7%) | 86.0% | 90.0% | ||
| ENESTnd† | Nilotinib 300 mg twice daily | 282 | 5 y | 10 (4%) | 92.0% | 94.0% |
| Nilotinib 400 mg twice daily | 281 | 6 (2%) | 96.0% | 96.0% | ||
| Imatinib (400 mg) | 283 | 21 (7%) | 91.0% | 92.0% | ||
| BFORE‡ | Bosutinib (400 mg) | 268 | 2 y | 6 (2%) | — | 99.2% |
| Imatinib (400 mg) | 268 | 7 (3%) | — | 97.0% |
Data from 4 first-line phase 3 randomized registration studies (IRIS, DASISION, ENESTnd, and BFORE) and the first-line imatinib 400 mg daily arm of the German CML Study IV are shown. PFS, OS, and disease progression (defined as progression to accelerated phase or blast phase) are shown.
OS, overall survival; PFS, progression-free survival. Adapted with permission from the NCCN GuidelinesⓇ for Chronic Myeloid Leukemia V.1.2021. © 2021 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines and illustrations herein may not be reproduced in any form for any purpose without the express written permission of the NCCN. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Survival outcomes include 363 patients who crossed over to imatinib.
†Progression to accelerated phase/blast phase during the study.
‡Twenty-four-month BFORE trial updates have been presented in abstract format.41
Molecular response rates by risk score
| Trial . | Study arms . | Low risk . | Intermediate risk . | High risk . | |||
|---|---|---|---|---|---|---|---|
| MMR . | MR4.5 . | MMR . | MR4.5 . | MMR . | MR4.5 . | ||
| DASISION | Dasatinib (100 mg) | 90% | 55% | 71% | 43% | 67% | 31% |
| Imatinib (400 mg) | 69% | 44% | 65% | 28% | 54% | 30% | |
| ENESTnd | Nilotinib 300 mg twice daily | — | 53% | — | 60% | — | 45% |
| Nilotinib 400 mg twice daily | — | 62% | — | 50% | — | 42% | |
| Imatinib (400 mg) | — | 38% | — | 33% | — | 23% | |
| BFORE | Bosutinib (400 mg) | 58% | — | 45% | — | 34% | — |
| Imatinib (400 mg) | 46% | — | 39% | — | 17% | — | |
| Trial . | Study arms . | Low risk . | Intermediate risk . | High risk . | |||
|---|---|---|---|---|---|---|---|
| MMR . | MR4.5 . | MMR . | MR4.5 . | MMR . | MR4.5 . | ||
| DASISION | Dasatinib (100 mg) | 90% | 55% | 71% | 43% | 67% | 31% |
| Imatinib (400 mg) | 69% | 44% | 65% | 28% | 54% | 30% | |
| ENESTnd | Nilotinib 300 mg twice daily | — | 53% | — | 60% | — | 45% |
| Nilotinib 400 mg twice daily | — | 62% | — | 50% | — | 42% | |
| Imatinib (400 mg) | — | 38% | — | 33% | — | 23% | |
| BFORE | Bosutinib (400 mg) | 58% | — | 45% | — | 34% | — |
| Imatinib (400 mg) | 46% | — | 39% | — | 17% | — | |
The MMR and MR4.5 rates of these trials cannot be directly compared, because different methods of trial evaluation and different time points were presented in published data. For DASISION, MMR and MR4.5 are reported at any time with 5-year follow-up by Hasford (Euro) score. For ENESTnd, MMR and MR4.5 are rates are reported by 5-year by Sokal score. For BFORE, MMR rates are reported at 12 months by Sokal score.
MMR, major molecular response; MR, molecular response.
Outcomes by risk score
| ENESTnd study arms . | Low risk . | Intermediate risk . | High risk . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Disease progression, n (%) . | PFS . | OS . | Disease progression, n (%) . | PFS . | OS . | Disease progression, n (%) . | PFS . | OS . | |
| Nilotinib 300 mg twice daily | 1 (1%) | 96.0% | 97.0% | 2 (2%) | 92.9% | 93.8% | 7 (9%) | 86.2% | 88.8% |
| Nilotinib 400 mg twice daily | 1 (1%) | 99.0% | 99.0% | 1 (1%) | 96.9% | 96.9% | 4 (5.1%) | 90.0% | 91.5% |
| Imatinib (400 mg) | 0 | 100.0% | 100.0% | 10 (9.9%) | 87.9% | 88.5% | 11 (14.1%) | 82.6% | 84.2% |
| ENESTnd study arms . | Low risk . | Intermediate risk . | High risk . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Disease progression, n (%) . | PFS . | OS . | Disease progression, n (%) . | PFS . | OS . | Disease progression, n (%) . | PFS . | OS . | |
| Nilotinib 300 mg twice daily | 1 (1%) | 96.0% | 97.0% | 2 (2%) | 92.9% | 93.8% | 7 (9%) | 86.2% | 88.8% |
| Nilotinib 400 mg twice daily | 1 (1%) | 99.0% | 99.0% | 1 (1%) | 96.9% | 96.9% | 4 (5.1%) | 90.0% | 91.5% |
| Imatinib (400 mg) | 0 | 100.0% | 100.0% | 10 (9.9%) | 87.9% | 88.5% | 11 (14.1%) | 82.6% | 84.2% |
Estimated 5-year PFS and OS and progression to accelerated phase or blast phase on study for ENESTnd are shown. The ENESTnd data show that disease progression occurs more frequently in high-risk patients and that for nilotinib-treated intermediate- and high-risk patients, the risk of progression was lower with nilotinib than with imatinib.
Adapted with permission from National Comprehensive Cancer Network10 . Guidelines® for Chronic Myeloid Leukemia V.1.2021. © 2021 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines and illustrations herein may not be reproduced in any form for any purpose without the express written permission of the NCCN. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
OS, overall survival; PFS, progression-free survival.
A clinically relevant question is whether sequencing a second-generation TKI after imatinib rather than starting a first-line second-generation TKI misses a critical treatment window. Across various studies of imatinib ∼25% to 30% of patients have been reported either not to achieve a response or to lose response. For many patients with appropriate monitoring, switching from imatinib to a second-generation TKI for resistance (Table 2) will result in good outcomes.22-25 In 2020, patients are more likely to switch therapy and to do so earlier in the treatment course, which may be beneficial. However, for some high-risk patients, it may matter. For me, “hitting” high-risk CML “hard” up front with a second-generation TKI is appealing because the longer the time the patient spends with higher levels of unopposed BCR-ABL1, the more likely it is that new ABL-independent genetic and molecular changes will appear.
Case 1
For patient 1, Sokal and ELTS risk scores were calculated as high risk; no other risk features (eg, ACA) were observed at diagnosis. The data supporting second-generation TKI use in high–risk score patients are most clearly delineated for nilotinib.18 Five-year OS was 88.8% in high-risk Sokal score patients treated with nilotinib at 300 mg twice daily vs 84.2% for imatinib-treated patients (Tables 5 and 6). In ENESTnd, rates of progression to AP or BP while in the study or during follow-up occurred in 10 of 282 patients (3.5%; nilotinib 300 mg twice daily) vs 6 of 281 patients (2.1%; nilotinib 400 mg twice daily) vs 21 of 283 (7.4%; imatinib).18 With 5-year follow-up for DASISION and looking specifically at deaths, 9 (34.6%) of 26 patients vs 17 (65.4%) of 26 patients in the dasatinib and imatinib treatment arms, respectively, died of CML-related causes.17 However, because OS does not differ substantially, it is possible that second-generation TKIs contribute to greater drug-related fatal complications, such as cardiovascular complications. For bosutinib, for which follow-up is shorter, no significant difference in progression events has yet been reported.19
Patient 1 had no significant comorbidities and was started on nilotinib 300 mg twice daily. EMR was not achieved by 6 months (BCR-ABL1, 20%). Three months later, during follow-up, circulating myeloid blasts were detected. No mutations in ABL were identified. Her bone marrow examination revealed 35% myeloid blasts, 14 of 20 cells were Ph+, and 13 of these cells had evidence of new monosomy 7. NGS revealed a mutation in the runt homology domain of RUNX1 (p.Arg107Cys; not germline). Although NGS assessment is increasingly available at some centers, there are no formal recommendations to select treatment on the basis of detection at diagnosis; however, emerging data may support mutations in RUNX1 are worrisome. Patient 1 received induction chemotherapy with ponatinib followed by a matched related donor allogeneic hematopoietic cell transplant. This is not a typical case but highlights a rare but very risky group of patients with no EMR receiving first-line second-generation TKIs who may benefit from an early switch to the powerful third-generation TKI ponatinib and early consideration for allogeneic hematopoietic cell transplant.
What are the risks of second-generation TKI use?
Each TKI has unique toxicities that, in combination with patient-specific medical history, inform selection. Despite early warnings regarding reduced cardiac ejection fraction for imatinib, no significant irreversible toxicities have been identified, and consequently its long-term safety profile is excellent. Early data support that responses to generic imatinib after imatinib mesylate (Gleevec) are stable or improving.26 Fewer data are available for patients starting generic imatinib as first-line treatment; however, it is reasonable to expect that generic drugs meeting standards of production quality and bioavailability will have similar efficacy, although potentially different side effects. For nilotinib, the most significant and potentially irreversible complications are cerebrovascular, cardiovascular, and peripheral arterial occlusive disease.27-30 Across retrospective studies, events have occurred in 10% to 20% of nilotinib-treated patients. A recent update to ENESTnd with ≥10 years of follow-up reported that among non–CML-related deaths (90% of all deaths), 7 (19%) of 37 patients died of cardiac or vascular disorders, and events continued over time.30 I avoid use of nilotinib in patients with diabetes mellitus, cardiovascular disease, metabolic syndrome, or hyperlipidemia. Unique dasatinib risks include pleural effusion (a risk that increases with age); mild platelet dysfunction that can result in bleeding; and, more rarely, pericardial effusion and pulmonary arterial hypertension (PAH). A recent retrospective review of a pooled population of 11 trials, DASISION, and 034/dose optimization studies (N = 2,712) demonstrated that the annual risk of pleural effusion is ∼5% to 15% and was 28% at 5 years for the first-line DASISION study.31 Diarrhea is the most common and annoying bosutinib-related toxicity, reported in ∼70% of patients, and bosutinib use can be problematic in patients with irritable bowel syndrome.19 However, grade 3 diarrhea is rarer (7.8%), and symptoms will often improve over time and respond to dose adjustments.32 Drug-induced liver injury has been reported with bosutinib but is rare, and risks for pleural effusion and cardiovascular events are low.
Case 2
Sokal score stratified patient 2 as being at intermediate risk, and her ELTS score classified her as low risk. Because of hypertension and T2DM, nilotinib was a less than ideal choice, and she started dasatinib at 100 mg daily. Her BCR-ABL1 at 3 months was 1.8% and by 15 months was undetectable and remained undetectable. Approximately 34 months after starting dasatinib, she presented with shortness of breath and cough. Her chest x-ray revealed a large right pleural effusion. The pleural fluid was exudative. Her echocardiogram demonstrated a normal ejection fraction (61%) and no wall motion abnormalities; however, a small circumferential pericardial effusion was noted, and her pulmonary artery systolic pressures (PASPs) were severely elevated at 76 to 81 mm Hg. The findings of right heart catheterization correlated with the echocardiogram findings. Dasatinib was stopped. After 3 years, her PASP returned to nearly normal at 38 mm Hg, an observation in keeping with a retrospective review of PAH cases in which improved or normalized PASPs were observed in 34 (94%) of 36 patients after dasatinib cessation.33,34 Patient 2 remains in TFR (4 years). Her excellent BCR-ABL1 response allowed early TFR, but she developed significant dasatinib-associated complications requiring years to improve. I avoid use of dasatinib in patients with pulmonary or pericardial disease and use a lower starting dose (50 mg daily) in older patients on the basis of a recent report.35 However, whether effusion or PAH risks are lower requires longer follow-up. The topic of lower first-line doses and dose de-escalation after MMR or deeper responses is an important area with emerging data.36,37 Relevant to patient 2 with T2DM and an ASCVD risk score of 15.8%, recent reports suggest that cardiovascular risk is also higher with dasatinib.27 Whether intermediate-risk patients should receive first-line second-generation TKIs is an area of debate. On the basis of age and comorbidities, I favor imatinib in patients such as patient 2. Last, her response was in keeping with the ELTS low-risk prediction. Although it has not been tested prospectively, I also calculate ELTS score at diagnosis to guide first-line TKI decision making.
How I choose between first- and second-generation TKIs as first-line therapy
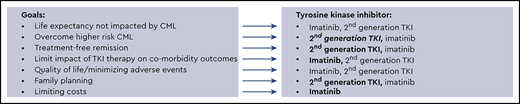
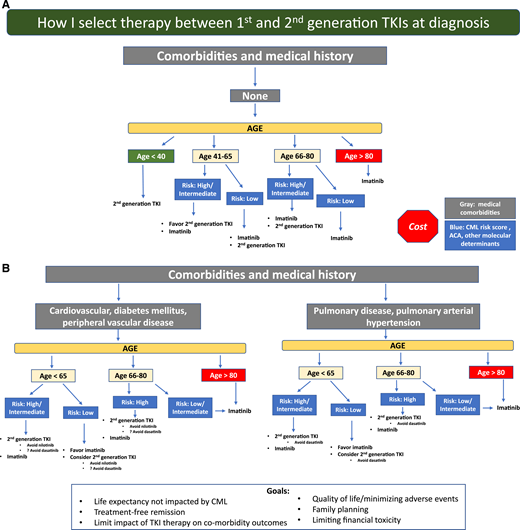
Life expectancy not impacted by CML is the overarching goal, and my general approach is shown in Figure 2. This approach is in no way definitive, and individual patient goals and preferences matter. I use second-generation TKIs whenever feasible for patients at higher risk for treatment failure (eg, high clinical risk scores, high-risk ACA, and p190), although an OS benefit has not clearly been established. Response monitoring, particularly for EMR achievement, is critical. New molecular tools for risk stratification at diagnosis, including somatic mutation panels and a recently reported 17-gene expression signature associated with absence of EMR and poorer event-free survival and OS, are becoming available38 and may help clarify who benefits from second-generation TKIs as first-line treatment. For younger patients, particularly women who have not embarked on family planning, second-generation TKI use with the goal of TFR as rapidly as possible is reasonable, and I typically select second-generation TKIs, which may also be tolerated better. Because cardiovascular complications are age related, for older patients (aged >50) without comorbidities, the discussion is more nuanced and includes ASCVD risk calculation. However, it is difficult to predict who will experience events. As patients age, my enthusiasm for first-line second-generation TKIs declines, and a switch, if needed, due to resistance or intolerance is my typical approach, particularly for low-risk patients. In addition, I am cautious when using second-generation TKIs in patients with specific comorbidities (Figure 2). Aiming for TFR is an expressed goal of almost all my patients, although those in successful TFR are still a minority, and safety with long-term drug use matters. Imatinib-treated patients achieve deeper molecular responses, as indicated by IRIS and German CML Study IV updates, although the timelines are longer. Although careful discussion of first-line therapy selection is appropriate, for some, choices are either not available or not affordable. TKI costs are a significant burden on patients and health care systems. Although significantly less expensive in some countries, the cost of generic imatinib has been high in the United States. In a recent Kaiser Family Foundation report of older patients enrolled in Medicare part D who are not eligible for low-income subsidies, median out-of-pocket costs in 2019 for generic imatinib were above the catastrophic threshold (by $3883).39 The cost of second-generation TKIs is even higher. Although generic drug prices are declining and the possibility of generic dasatinib is on the horizon, a recent cost-effectiveness analysis simulating 10 years of CML treatment identified that an imatinib-first approach was the most cost-effective one, even when TFR was considered.40
A general overview of my approach is shown. A first step is to assess patient medical comorbidities because this influences the risks and benefits of imatinib vs specific second-generation tyrosine kinase inhibitors (TKIs). (A) Patients without comorbidities. (B) Patients with particular comorbidities. Because comorbidities, in particular cardiovascular comorbidities, increase with age, selecting a second-generation TKI in older individuals with or without comorbidities requires a careful discussion and monitoring. Patient goals and preferences are also important and influence decision making.
A general overview of my approach is shown. A first step is to assess patient medical comorbidities because this influences the risks and benefits of imatinib vs specific second-generation tyrosine kinase inhibitors (TKIs). (A) Patients without comorbidities. (B) Patients with particular comorbidities. Because comorbidities, in particular cardiovascular comorbidities, increase with age, selecting a second-generation TKI in older individuals with or without comorbidities requires a careful discussion and monitoring. Patient goals and preferences are also important and influence decision making.
Correspondence
Vivian G. Oehler, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D4-100, P.O. Box 19024, Seattle, WA 98109-1024; e-mail: voehler@u.washington.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.