Abstract
The paradigm for managing patients with chronic myeloid leukemia is evolving. In the recent past, restoring a normal life expectancy while patients are receiving never-ending targeted therapy with BCR–ABL1 tyrosine kinase inhibitors through prevention of progression to blast phase and mitigation of iatrogenic risks was considered the best achievable outcome. Now, long-term treatment-free remission with continued response off tyrosine kinase inhibitor therapy is recognized as the most optimal benefit of treatment. Indeed, numerous independent clinical trials provided solid proof that tyrosine kinase inhibitor discontinuation was feasible in patients with deep and sustained molecular responses. This article discusses when tyrosine kinase inhibitors may be safely stopped in clinical practice on the basis of the best and latest available evidence.
Learning Objectives
Know factors influencing deep molecular response achievement
Understand appropriate selection criteria of tyrosine kinase inhibitor discontinuation
Understand safety aspects after end of treatment
Introduction
During treatment with tyrosine kinase inhibitors (TKIs) targeting BCR-ABL1, the driving oncoprotein of chronic myeloid leukemia (CML), obtaining an at least 3-log reduction in BCR-ABL1 transcripts, which defines a major molecular response (MMR) (MMR/BCR-ABL1 internationally standardized [IS] ratio, ≤0.1%), is an important step toward a favorable outcome. Indeed, stable MMR represents a robust surrogate marker for long-term progression-free survival.1 However, patients in MMR but not achieving deep molecular responses (DMRs), such as a 4-log (MR4), 4.5-log (MR4.5), or even 5-log-(MR5) reduction in leukemia load, must receive TKIs continuously to maintain CML under control because treatment-free remission (TFR) is unlikely (Table 1).2,3 On the contrary, a large body of clinical research has established that the long-term success rate of TKI discontinuation in patients with sustained DMR was ≥50%, with success defined as remaining in DMR or MMR.4-8 Furthermore, it was demonstrated that, provided proper residual disease monitoring and rules for resuming therapy were followed, CML sensitivity to TKIs was largely preserved. DMR was restored soon after treatment reintroduction in almost all patients with molecular relapse. TFR is now a new goal of CML therapy, although with the current TKI arsenal and standard treatment-switching strategies, only 10% to 30% of patients with CML may achieve TFR.9 Nonetheless, when TFR is set as a high-priority objective, DMR is a prominent clinically meaningful endpoint of treatment.
Definition of molecular responses by peripheral blood real time quantitative polymerase chain reaction
| Deep molecular response levels . | Definition . |
|---|---|
| MR4 | BCR-ABL1 IS ratio ≤0.01% or undetectable transcripts with ≥10 000 copies of ABL1 or ≥24 000 copies of GUS |
| MR4.5 | BCR-ABL1 IS ratio ≤0.0032% or undetectable transcripts with ≥32 000 copies of ABL1 or ≥77 000 copies of GUS |
| MR5 | BCR-ABL1 IS ratio ≤0.001% or undetectable transcripts with ≥100 000 copies of ABL1 or ≥240 000 copies of GUS |
| Deep molecular response levels . | Definition . |
|---|---|
| MR4 | BCR-ABL1 IS ratio ≤0.01% or undetectable transcripts with ≥10 000 copies of ABL1 or ≥24 000 copies of GUS |
| MR4.5 | BCR-ABL1 IS ratio ≤0.0032% or undetectable transcripts with ≥32 000 copies of ABL1 or ≥77 000 copies of GUS |
| MR5 | BCR-ABL1 IS ratio ≤0.001% or undetectable transcripts with ≥100 000 copies of ABL1 or ≥240 000 copies of GUS |
IS, internationally standardized; MR4, 4-log molecular response; MR4.5, 4.5-log molecular response; MR5, 5-log molecular response.
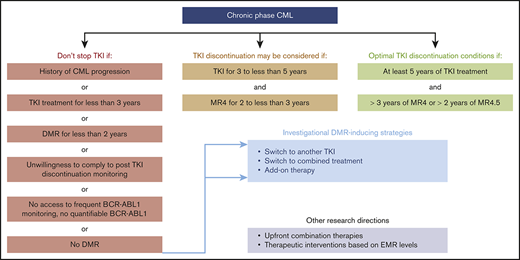
The European LeukemiaNet (ELN), the National Comprehensive Cancer Network (NCCN), and other cooperative working groups have built clinical practice recommendations to guide physicians regarding selection of patients for TKI discontinuation.10 The ELN set minimal criteria for safely stopping TKIs as follows: (1) CML in first chronic phase (CP); (2) TKI provided as first- or second-line treatment, provided that the treatment change was driven by intolerance; (3) ≥5 years of treatment with the first-generation TKI imatinib or 4 years with second-generation TKI dasatinib, nilotinib, or bosutinib; and (4) ≥2 years of sustained MR4 or better (Table 2). In the NCCN version 3.2020 guidelines for TKI discontinuation, slightly less stringent selection criteria than those of the ELN were chosen.11 At least 3 years of treatment are requested, including ≥2 years of sustained MR4 or better, and patients with DMR to salvage TKI for resistant CP-CML are not excluded from TKI discontinuation attempts (Table 3). In the latter situation, expected TFR rates are less favorable than when DMRs are readily obtained but patient safety seems preserved.12,13 As an illustration, the 4-year cumulative incidences of MMR loss after dasatinib or nilotinib discontinuation in the STOP 2G-TKI study were 76.9% in patients with prior suboptimal response or resistance to imatinib and only 35.5% in those lacking such a history, but all relapses were successfully controlled after recommencing the original second-generation TKI.13
European LeukemiaNet 2020 recommendations: requirements for tyrosine kinase inhibitor discontinuation
| Mandatory . | Minimal . | Optimal . |
|---|---|---|
| CP-CML | First-line TKI or second-line TKI if motivated by intolerance to first-line drug | Duration of therapy >5 y |
| Real time quantitative polymerase chain reaction on the IS scale | Typical e13a2 or e14a2 BCR-ABL1 transcripts | DMR duration >3 y if MR4 |
| Patient motivation and adherence | Duration of therapy >5 y if imatinib >4 y if second-generation TKI | DMR duration >2 y if MR4.5 |
| DMR duration (MR4 or M4.5) >2 y |
| Mandatory . | Minimal . | Optimal . |
|---|---|---|
| CP-CML | First-line TKI or second-line TKI if motivated by intolerance to first-line drug | Duration of therapy >5 y |
| Real time quantitative polymerase chain reaction on the IS scale | Typical e13a2 or e14a2 BCR-ABL1 transcripts | DMR duration >3 y if MR4 |
| Patient motivation and adherence | Duration of therapy >5 y if imatinib >4 y if second-generation TKI | DMR duration >2 y if MR4.5 |
| DMR duration (MR4 or M4.5) >2 y |
CML, chronic myeloid leukemia; CP, chronic phase; DMR, deep molecular response; IS, internationally standardized; MR4, 4-log molecular response; MR4.5, 4.5-log molecular response; TKI, tyrosine kinase inhibitor.
National Comprehensive Cancer Network 2020 guidelines: criteria for discontinuation of tyrosine kinase inhibitor therapy
| TKI discontinuation may be considered if all criteria below are met . |
|---|
| Age ≥18 y |
| CP-CML, no history of accelerated or blast phase CML |
| Quantifiable BCR-ABL1 transcripts |
| TKI therapy for ≥3 y |
| Stable MR4 for ≥2 y |
| Access to real time quantitative polymerase chain reaction with sensitivity of at least MR4.5 |
| TKI discontinuation may be considered if all criteria below are met . |
|---|
| Age ≥18 y |
| CP-CML, no history of accelerated or blast phase CML |
| Quantifiable BCR-ABL1 transcripts |
| TKI therapy for ≥3 y |
| Stable MR4 for ≥2 y |
| Access to real time quantitative polymerase chain reaction with sensitivity of at least MR4.5 |
CML, chronic myeloid leukemia; CP, chronic phase; MR4.5, 4.5-log molecular response; TKI, tyrosine kinase inhibitor.
Clinical case
A 34-year-old woman complaining of fatigue was referred for absolute leukocytosis of 44 000/μL. Blood and marrow smear, cytogenetics, and molecular biology tests revealed CP-CML. The Philadelphia chromosome was not accompanied by additional cytogenetic abnormalities; BCR-ABL1 transcripts were of the p210 e13a2 type; and the patient’s Sokal risk group was low. The therapeutic goal and the principle of using BCR-ABL1 TKIs were explained, and one of the pressing questions raised by the patient was when therapy would end. At the time of CML diagnosis, forecasting on an individual basis if and when TKIs may be stopped is not possible. Nevertheless, maximizing chances of achieving DMR through individualized TKI selection and dynamic molecular response–based switching strategies may open the door for removal of therapy.
DMR as a key milestone in the path to TKI discontinuation: first-line treatment choices
In the frontline setting, the likelihood of gaining DMR depends on ≥3 parameters: TKI generation, CP-CML risk score, and early molecular responses (EMRs), as detailed below.
TKI generation and DMR
Second-generation TKIs produce significantly higher rates of DMR than standard-dose imatinib in newly diagnosed CP-CML. In the phase 3 DASISION trial, the cumulative incidences of MR4.5 with first-line imatinib were 3% by 1 year, 8% by 2 years, 13% by 3 years, 23% by 4 years, and 33% by 5 years.14 The cumulative incidences of MR4.5 obtained in the first-line dasatinib 100 mg daily arm were 5% by 1 year, 19% by 2 years, 24% by 3 years, 34% by 4 years, and 42% by 5 years. In the phase 3 ENESTnd study, the cumulative incidences of MR4.5 on imatinib were 1% by 1 year, 9% by 2 years, 15% by 3 years, 23% by 4 years, 31% by 5 years, and 45.2% by 10 years.15,16 The cumulative incidences of MR4.5 obtained in the nilotinib 300-mg twice-daily arm were 11% by 1 year, 25% by 2 years, 32% by 3 years, 40% by 4 years, 54% by 5 years, and 63.8% by 10 years. The phase 3 BFORE trial comparing bosutinib 400 mg daily with standard-dose imatinib in the frontline setting is not mature enough to draw informative conclusions.17
CP-CML risk scores and DMR
In DASISION and ENESTnd, the best DMR rates were obtained with second-generation TKIs, regardless of baseline CP-CML risk.14,15 As an example, in ENESTnd, the 5-year cumulative incidences of MR4.5 were 53.4% with nilotinib 300 mg twice daily versus 36.5% with imatinib 400 mg daily in patients with a low Sokal score, 60.4% with nilotinib 300 mg twice daily versus 32.7% with imatinib in patients with an intermediate Sokal score, and 44.6% with nilotinib 300 mg twice daily versus 23.1% with imatinib in patients with a high Sokal score.15
EMR to TKIs and DMR
The 3-month evaluation of response to TKIs is an important step during CML management. Achievement of an optimal EMR, corresponding to BCR-ABL1 transcript levels ≤10% IS, indicates a favorable overall and progression-free survival as well as a very low risk of transformation.14 15 Furthermore, several studies found that EMR was an early predictor of DMR, regardless of first-line TKI type and CP-CML risk score.14,15,18,19 In ENESTnd, cumulative incidences of MR4.5 by 5 years in patients with <1%, between 1% and 10%, and >10% BCR-ABL1 IS at 3 months were 70%, 51.7%, and 8.3%, respectively, with nilotinib 300 mg twice daily and 67.4%, 33.8%, and 15.9%, respectively, with imatinib.18 In a large single-center study, Sasaki et al20 found that best fit average real time quantitative polymerase chain reaction values for sustained MR4.5 for ≥2 years at any time during first-line TKI treatment were 0.051% IS at 3 months, 0.019% IS at 6 months, 0.007% IS at 9 months, and 0.003% IS at 12 months. Minimum acceptable RT quantitative polymerase chain reaction values for sustained MR4.5 for ≥2 years at any time during first-line TKI treatment were 1.561% IS at 3 months, 0.592% IS at 6 months, 0.295% IS at 9 months, and 0.085% IS at 12 months (95th percentile). Altogether, these findings suggest that a drastic BCR-ABL1 reduction strategy soon after TKI onset may increase the probability of and reduce the time to TKI discontinuation.
DMR as a key milestone in the path to TKI discontinuation: switching strategies
For patients incapable of reaching DMR or deemed to have a low likelihood of DMR, a change of TKI or a combination therapy as a way to achieve DMR is being explored. Such strategies are not approved by health authorities and remain within the scope of research. The randomized ENESTcmr trial showed that in patients lacking DMR after ≥3 years of first-line imatinib, a switch to nilotinib was more efficient at inducing DMR than was remaining on imatinib.21,22 By 2 years, MR4.5 was obtained in 42.9% of patients who received nilotinib and in 20.8% of patients who stayed on imatinib.21 The randomized DASCERN trial assessed the benefit of a switch from imatinib to dasatinib in patients lacking EMR on first-line imatinib, and DMR achievement was explored as a secondary endpoint. By 3 years, MR4 but not MR4.5 was more frequently attained with dasatinib (42%) than with imatinib (26%).23 Importantly, nilotinib exposes patients to ischemic cardiovascular events, and dasatinib is well known to frequently cause pleural effusion; thus, a balanced evaluation of potential benefits and harms of switching approaches to achieve DMR is necessary.14,15 For patients lacking DMR on a second-generation TKI, using the more potent third-generation TKI ponatinib has some theoretical interest; however, this strategy has not been considered, owing to the cardiovascular toxicity profile of ponatinib.24 Other approaches are underway in the context of clinical trials, such as combining adenosine triphosphate–competitive TKIs with the allosteric TKI asciminib or with other therapies targeting residual CML cells or boosting the antileukemic immune response.25
Clinical case: follow-up
The patient had no prohibitive comorbid condition that could adversely affect TKI safety. It was thus decided to start a first-line second-generation TKI. We must recognize that second-generation TKIs do not offer an overall survival advantage over imatinib and that these drugs mostly benefit to intermediate- or high-risk patients with CP-CML in terms of reduction of progression events. Nevertheless, second-generation TKIs benefit low-risk patients because they significantly enhance chances of DMR and speed up DMR time as compared with imatinib. The patient obtained an MMR at 3 months, an MR4 at 6 months, then an MR4.5 at 12 months. Once achieved, the estimated durability of DMR is ∼70%.26 The patient maintained MR4.5 after 2 more years of continuous treatment, thus fulfilling minimal criteria for TKI discontinuation.
When is the right time to discontinue TKIs in patients with DMR?
Although some patients may obtain DMR rapidly, stopping TKIs before the third year of therapy is not advisable, because most CML progression events occur during the first 2 to 3 years of treatment. In the NCCN guidelines, a minimum of 3 years of TKI exposure, including 2 years in MR4 or better, is sufficient to envisage treatment discontinuation.11 For the ELN, the optimal duration of treatment is ≥5 years, including ≥3 years in MR4 or ≥2 years in MR4.5 (Table 2).10 Differences between ELN recommendations and NCCN guidelines highlight the fact that optimal durations of TKI therapy and DMR and best DMR levels before TKI discontinuation remain under debate. Possible predictors of TFR have been investigated because these might guide decision making regarding if and when to stop treatment on an individual basis. DMR level and total duration of TKI and, more important, that of DMR appear to play an important role.27 The international EUROSKI trial revealed that the estimated risk of molecular relapse after imatinib removal continuously decreased as DMR duration increased.28 To what extent this holds true for second-generation TKIs remains to be determined. Overall, accurately foreseeing TFR chances in individual patients remains difficult, and the choice between minimal stopping criteria or postponing TKI discontinuation until optimal conditions are obtained requires weighing the benefits against potential collateral damage of extended TKI therapy. Patient preferences may be taken into account as well.29 In the future, biomarkers such as immunological parameters may help predict the success of TKI cessation.30
Risks associated with TKI discontinuation
Patients in DMR while receiving therapy have a negligible risk of secondary resistance, disease progression, or CML-related death; thus, the safety of TKI discontinuation is of utmost importance. Both the ELN and the NCCN have agreed to consider that a loss of MMR after TKI removal appropriately defines a molecular relapse and warrants clinical intervention in a timely fashion, namely within 4 weeks.10,11 About 85% of molecular relapses occur within a short time window of 3 to 12 months and are characterized by a 0.5- to 1-log increase per month in the leukemia load, suggesting that hematological relapses will likely follow molecular relapses in the absence of rapid TKI resumption. Molecular relapses occurring beyond the first 12 months usually display slower kinetics.31 Thus, surveillance in the post-treatment setting relies on adaptation of BCR-ABL1 transcript assessment to the time to onset of molecular relapse (Table 4). Those with molecular relapse are sensitive to the same TKI as the one used before discontinuation because MMR and DMR are regained within a median time of 3 to 6 months with a few exceptions, underscoring the importance of BCR-ABL1 transcript monitoring after treatment resumption.31 The feasibility of second TKI discontinuation attempts in patients recovering sustained DMR is currently under investigation but is not advisable yet in clinical practice.32 Occasional cases of CML transformation have been reported either during the treatment-free phase or just after therapy restart and resemble sudden blast phase.33 Although these are exceptional cases, they do occur in optimal responders to TKIs. Thus, vigilance is required because any risk level exceeding that existing in optimal responders while receiving treatment may put into question real-life TKI discontinuation opportunities.
National Comprehensive Cancer Network 2020 guidelines: BCR-ABL1 transcript monitoring policy after tyrosine kinase inhibitor removal
| NCCN 2020 . |
|---|
| Monthly during the first year |
| Every 2 mo during the second year |
| Every 3 mo after the second year |
| After TKI reintroduction in case of molecular relapse: monthly until MMR recovery, then every 3 mo |
| NCCN 2020 . |
|---|
| Monthly during the first year |
| Every 2 mo during the second year |
| Every 3 mo after the second year |
| After TKI reintroduction in case of molecular relapse: monthly until MMR recovery, then every 3 mo |
MMR, major molecular response; NCCN, National Comprehensive Cancer Network; TKI, tyrosine kinase inhibitor.
The disappearance of possible drug-related adverse events is an obvious expectation after TKI removal, and, indeed, most regress during the treatment-free phase.34 However, ∼30% of patients may experience newly occurring or worsening of preexisting musculoskeletal pain within several weeks after TKI discontinuation and for up to several months.35,36 Although unrelated to the molecular status, information about this so-called TKI withdrawal syndrome is important to communicate to patients because quality of life may be transiently altered, and painkillers may be needed. Whether this phenomenon may be minimized by tapering TKI doses over several months before discontinuation is an open question. Other aspects of patient safety after TKI removal should also be looked at, such as relevant effects of the suppression of TKI either on selected biological parameters, such as glycemia in patients with diabetes stopping imatinib, or on other-drug metabolism.37
Conclusion
Twenty years after approval of the first TKI against CML, followed by the expansion of the lifesaving BCR-ABL1 TKI arsenal, integration of TFR as a new goal of CML management represents a huge step toward a cure. Of course, there is significant room for improvement in determining durable TFR predictability and achievability. Currently, it seems reasonable to wait until optimal conditions are met before stopping TKIs, namely ≥4 to 5 years of treatment and ≥2 to 3 years of DMR, in line with current recommendations and in the absence of iatrogenic issue. There is a long way to go before all patients may be eligible for TKI cessation. First-line adenosine triphosphate–competitive TKIs in combination with pegylated interferon are being compared with TKI monotherapy as a potential way to increase DMR and TFR.38,39 Investigating the effect of therapeutic interventions on the basis of EMR levels on DMR achievement, such as early switches in favor of a more potent TKI or early add-on strategies, may also be of clinical interest. In addition, the issue of when BCR-ABL1 transcript monitoring in patients who do not relapse may be stopped needs to be resolved because very long-term TFR data are sparse. Challenges over the coming years also include unraveling mechanisms of TFR despite apparent leukemic stem cell persistence and understanding reasons underlying divergent outcomes after TKI discontinuation.
Acknowledgments
The author thanks colleagues from the France Intergroupe des Leucémies Myéloïdes Chroniques, from the European Society of Haematology/International Chronic Myeloid Leukemia Foundation, from European LeukemiaNet, and all other colleagues worldwide for great medical and scientific interactions and exchanges, as well as patients with CML.
Correspondence
Delphine Rea, Département Médico-Universitaire d’Hématologie, Hôpital Saint-Louis, 1 avenue Claude Vellefaux, 75010 Paris, France; e-mail: delphine.rea@aphp.fr.
References
Competing Interests
Conflict-of-interest disclosure: D.R. has received honoraria from and served on advisory boards for Pfizer, Novartis, and Incyte and has been a member of clinical trial steering committees for Bristol Myers Squibb and Novartis.
Author notes
Off-label drug use: None disclosed.
This article was selected by the Blood Advances and Hematology 2020 American Society of Hematology Education Program editors for concurrent submission to Blood Advances and Hematology 2020. It is reprinted from Blood Advances 2020, Volume 4.