Abstract
Inhibition of Bruton’s tyrosine kinase (BTK) has revolutionized the treatment landscape for patients with chronic lymphocytic leukemia (CLL). By targeting this critical kinase in proximal B-cell receptor signaling, BTK inhibitors (BTKis) impair cell proliferation, migration, and activation of NF-κB. Clinically, because indefinite inhibition is a mainstay of therapy, there is an extended period of exposure in which adverse effects can develop. Given the impressive efficacy and activity of BTKis in the treatment of patients with CLL, appropriate management of treatment-emergent adverse events (AEs) is of paramount importance. Here we review the BTKi landscape and present the available toxicity and safety data for each agent. The long-term toxicity profile of ibrutinib, a first-in-class inhibitor, is well characterized and includes a clinically significant incidence of cardiac arrhythmias, bleeding, infection, diarrhea, arthralgias, and hypertension. Acalabrutinib, the initial second-generation BTKi to earn approval from the US Food and Drug Administration, demonstrates improved kinase selectivity for BTK, with commonly observed adverse reactions including infection, headache, and diarrhea. Mediated by both on-target inhibition of BTK and variable off-target inhibition of other kinases including interleukin-2–inducible T-cell kinase (ITK), tyrosine-protein kinase (TEC), and endothelial growth factor receptor (EGFR), the toxicity profile of BTKis is closely linked to their pattern of kinase binding. Other emerging BTKis include second-generation agents with variable degrees of kinase selectivity and third-generation agents that exhibit reversible noncovalent binding to BTK. We also highlight critical considerations for the prevention and monitoring of AEs and offer practical management strategies for treatment-emergent toxicities.
Learning Objectives
Increase familiarity with treatment-emergent toxicities commonly observed with BTKis, calling attention to their incidence, mechanism (where known), and appropriate clinical management
Review the landscape of currently approved and in-development BTKis in CLL, highlighting differences in the kinase-binding and toxicity profiles of agents in this class
Introduction
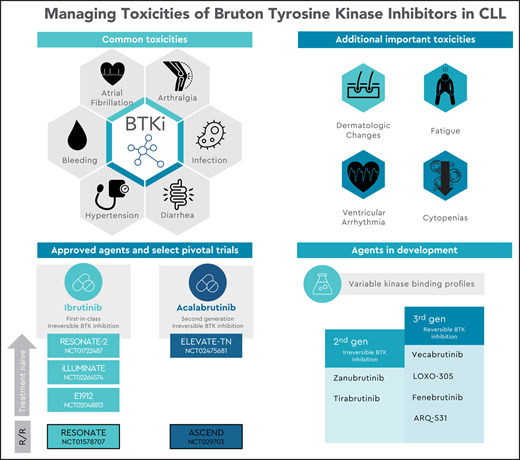
Inhibition of Bruton’s tyrosine kinase (BTK) has revolutionized the treatment landscape for patients with chronic lymphocytic leukemia (CLL). By targeting BTK, a critical kinase in proximal B-cell receptor (BCR) signaling, this class of small molecule inhibitors impairs BCR signaling and activation of NF-KB and inhibits cell proliferation and migration.1,2 Clinically, because indefinite inhibition is a mainstay of therapy, there is an extended period of exposure in which adverse effects can develop, often leading to discontinuation after several years. Mediated by both on-target inhibition of BTK and variable off-target inhibition of other kinases including interleukin-2–inducible T-cell kinase (ITK), tyrosine-protein kinase (TEC), and endothelial growth factor receptor (EGFR), the toxicity profile of BTK inhibitors (BTKis) is closely linked to their pattern of kinase binding.
With 8 years of follow-up data since its initial pivotal study,3 the long-term toxicity profile of ibrutinib (a first-in-class, irreversible inhibitor of BTK) is well characterized. It includes cardiac arrhythmias, bleeding, infection, diarrhea, arthralgias, and hypertension.4-8 Acalabrutinib, the initial second-generation BTKi to earn approval from the US Food and Drug Administration (FDA), demonstrates improved kinase selectivity for BTK, with commonly observed adverse reactions including infection, headache, and diarrhea.9-12 Other emerging BTKis include second-generation agents with variable degrees of kinase selectivity13-16 and third-generation agents that exhibit reversible noncovalent binding to BTK.17-20 At present, the relevant differences in the toxicity profiles of individual BTKis are challenging to discern based on limited preliminary data that consist primarily of nonrandomized studies with limited direct comparisons. Notably, experience with ibrutinib has demonstrated that patient-specific risk factors (eg, age, comorbidities) can also influence the likelihood of adverse effects and that significant variation exists between rates of adverse effects documented in initial pivotal clinical trial studies6 and subsequent real-world analyses of these agents, where AEs were responsible for 63% and 50% of discontinuations in the frontline and relapsed or refractory (R/R) settings, respectively.21 To maximize the safety and long-term tolerability of BTKis, it is crucial to carefully consider each patient’s clinical history before therapy initiation and to pay careful attention to patient-reported signs and symptoms observed during therapy.
Herein we review the BTKi landscape, present the available toxicity and safety data for each agent, and highlight critical considerations for the management of treatment-related toxicities in patients with CLL on BTKis.
Clinical case
A 68-year-old man without significant comorbidities received a diagnosis of CLL 10 years ago. He was previously treated with fludarabine, cyclophosphamide, and rituximab, achieving a complete response to therapy. After a multiyear period of observation, he has developed progressive fatigue and dyspnea on exertion over the past 6 months. His physical examination is notable for diffuse adenopathy (3 to 4 cm) and a spleen palpable 4 cm below the costal margin (white blood cell count 160,000/μL; 95% lymphocytes; hemoglobin 10 g/dL, platelets 95,000/μL; fluorescence in situ hybridization del(17p), del(11q); TP53 sequencing mutated; immunoglobulin heavy chain variable gene VH1-69, unmutated). The need for therapy was discussed with the patient.
Overview of the BTKi landscape
Irreversible inhibitors
Irreversible BTKis act via covalent binding to a cysteine residue at position 481, found in the adenosine triphosphate–binding pocket of BTK. At the time of this writing, two BTKis are FDA approved for use in CLL, ibrutinib and acalabrutinib, with multiple other agents under active investigation (Table 1). Clinical experience with long-term follow-up is greatest with ibrutinib, which has been extensively studied as a standard of care option for patients with CLL, demonstrating greater efficacy than comparator therapies in registration trials in both the frontline (RESONATE-2, FDA approval in 2016) and R/R settings (RESONATE, FDA approval in 2014) and in patients with deletion 17p. As a first-generation inhibitor, ibrutinib irreversibly inhibits ≥10 other kinases, with a half-maximal inhibitory concentration of <11 nmol/L.22 Acalabrutinib is more selective for BTK than ibrutinib and notably demonstrates less inhibition of related kinases with a conserved cysteine residue; of these, relative inhibition of ITK and EGFR is less than that of TEC.23,24 Initial studies demonstrated 97% BTK occupancy (the percentage of total BTK bound by drug),9 with recent work highlighting more potent inhibition of the BCR and NF-KB pathways at 100 mg twice-daily dosing compared with daily administration.24 The drug has been subsequently studied in phase 3 trials in both the treatment-naive (as monotherapy and in combination with obinutuzumab, ELEVATE-TN)10 and R/R setting (single-agent, ASCEND),12 earning FDA approval in 2019.
BTK inhibitors currently approved and under development
| Compound . | Indication . | Stage of development . | Clinical study . | Mechanism . | TEC family kinase inhibition half-maximal inhibitory concentration (nm) . | ||
|---|---|---|---|---|---|---|---|
| BTK . | ITK . | TEC . | |||||
| Ibrutinib22 | Newly diagnosed and R/R | Approved | RESONATE25 | Irreversible C481 binding | 0.5 | 10.7 | 78 |
| RESONATE4 | |||||||
| Acalabrutinib9 | Newly diagnosed and R/R | Approved | ELEVATE-TN10 | Irreversible C481 binding | 5.1 | >1,000 | 93 |
| ASCEND12 | |||||||
| Zanubrutinib13 (BGB-3111) | In development | 3 (approved in mantle cell lymphoma) | SEQUOIA14 ALPINE15 | Irreversible C481 binding | 0.22 | 30 | 1.9 |
| Vecabrutinib17 (SNS-062) | Early development | 1b (CLL/B-NHL) | NCT03037645 | Noncovalent reversible | 3 | 4 | 14 |
| Halted in CLL | |||||||
| LOXO-30519 | Early development | 1/2 (CLL/B-NHL) | NCT03740529 | Noncovalent reversible | 3.15 | >5,000 | 1,234 |
| Fenebrutinib18 (GDC-0853) | Early development | 1 (CLL/B-NHL) | NCT01991184 | Noncovalent reversible | 0.91 | >1,000 | >1,000 |
| ARQ-53120 | Early development | 1 (CLL/B-NHL) | NCT03162536 | Noncovalent reversible | 4.23 | >10,000 | 5.8 |
| Compound . | Indication . | Stage of development . | Clinical study . | Mechanism . | TEC family kinase inhibition half-maximal inhibitory concentration (nm) . | ||
|---|---|---|---|---|---|---|---|
| BTK . | ITK . | TEC . | |||||
| Ibrutinib22 | Newly diagnosed and R/R | Approved | RESONATE25 | Irreversible C481 binding | 0.5 | 10.7 | 78 |
| RESONATE4 | |||||||
| Acalabrutinib9 | Newly diagnosed and R/R | Approved | ELEVATE-TN10 | Irreversible C481 binding | 5.1 | >1,000 | 93 |
| ASCEND12 | |||||||
| Zanubrutinib13 (BGB-3111) | In development | 3 (approved in mantle cell lymphoma) | SEQUOIA14 ALPINE15 | Irreversible C481 binding | 0.22 | 30 | 1.9 |
| Vecabrutinib17 (SNS-062) | Early development | 1b (CLL/B-NHL) | NCT03037645 | Noncovalent reversible | 3 | 4 | 14 |
| Halted in CLL | |||||||
| LOXO-30519 | Early development | 1/2 (CLL/B-NHL) | NCT03740529 | Noncovalent reversible | 3.15 | >5,000 | 1,234 |
| Fenebrutinib18 (GDC-0853) | Early development | 1 (CLL/B-NHL) | NCT01991184 | Noncovalent reversible | 0.91 | >1,000 | >1,000 |
| ARQ-53120 | Early development | 1 (CLL/B-NHL) | NCT03162536 | Noncovalent reversible | 4.23 | >10,000 | 5.8 |
NHL, non-Hodgkin lymphoma.
Multiple irreversible BTKis are at an earlier development stage and are under active investigation. Zanubrutinib (formerly BGB-3111) is an irreversible inhibitor with greater selectivity for BTK than for ITK. Extended follow-up from a phase 1/2 study was presented at ASH 2019,25 and the drug is being compared with ibrutinib in the phase 3 setting (ALPINE).15 It also gained FDA approval in 2019 in the treatment of patients with mantle cell lymphoma who have received ≥1 prior therapy. Tirabrutinib (formerly ONO/GS-4059) has a high degree of selectivity for BTK compared with the other TEC family kinases. It has been studied in the phase 1 setting as monotherapy for R/R patients26 and in combination with spleen tyrosine kinase (SYK) and phosphoinositol 3-kinase (PI3K) inhibitors.16
Reversible inhibitors
Mutations in the C481 binding site are known to confer clinical resistance to irreversible BTK inhibition, contributing to ∼65% of ibrutinib failures due to CLL progression (∼40% with mutations in BTK alone and an additional ∼25% with co-occurring mutation in PLCG2).27-29 Reversible third-generation BTKis act independently of covalent binding to C481 and can achieve target inhibition of both wild-type and C481S-mutated BTK. Therefore, they theoretically offer the potential to overcome resistance mediated by this mutation. Several agents in this class are in development. Vecabrutinib (formerly SNS-062) is a selective, reversible inhibitor that was initially evaluated in a phase 1b trial in patients with CLL who received ≥2 previous regimens and progressed on therapy with an irreversible BTKi.30 Based on a recent press release, its further development in CLL appears to be halted.31 LOXO-305 is a highly selective noncovalent BTKi with minimal off-target kinase inhibition and binding to BTK C481S, with a half-maximal inhibitory concentration of 1.42 nM.19 Preliminary data from 13 patients enrolled in a first-in-human phase 1 trial were presented at ASH 2019.32 Fenebrutinib (formerly GDC-0853) was assessed for safety, tolerability, and pharmacokinetics in a cohort of 24 patients with B-cell malignancy and was generally well tolerated by 14 patients with R/R CLL.33 In contrast to other noncovalent inhibitors, ARQ 531 maintains downregulation of the BCR pathway in the case of C481S BTK or autoactivating PLCγ2 mutations by concurrently inhibiting additional kinases, including LYN and the Src family kinase MEK1.20 Final results of a phase 1 trial demonstrated that the drug was well tolerated at 65 mg daily dosing in a cohort that included 26 patients with CLL or small lymphocytic lymphoma (SLL).34
The patient was initiated on ibrutinib 420 mg/day (monotherapy). His initial response was notable for a partial response with lymphocytosis (white blood cell count 180,000/μL; 79% lymphocytes; hemoglobin 12 g/dL; platelets 105,000/μL). His symptoms improved, with resolution of splenomegaly and lymphadenopathy. However, after 6 months on ibrutinib therapy, he now reports migratory arthralgias and fatigue that are impairing his activities of daily living.
AEs necessitating specialized management
Rates of discontinuation
Here we consider the incidence, mechanisms of action, and management of AEs that arise throughout therapy with BTKis, focusing on the 2 currently approved agents (Table 2). On the whole, AEs lead to clinically significant rates of discontinuation or dosage reduction seen in landmark clinical studies of ibrutinib (eg, 12% in RESONATE at initial publication,4 16% at final follow-up35 ), and discontinuation rates appear to be lower with acalabrutinib (eg, 9% to 11% at shorter 28.3-month follow-up).10 Integrated analysis of multiple ibrutinib studies reveals that AEs contribute to a 14% rate of dosage reduction and rates of discontinuation of therapy of 10% in year 1, 5% in year 2, and 6% in year 3.6 Outside the context of clinical trials, real-world analysis demonstrates an even more significant impact of AEs on treatment cessation (eg, contributing to half of an overall 42% discontinuation rate during early treatment),21 highlighting the need for careful management of treatment-emergent toxicities.
Management of selected adverse events
| Adverse event . | Management recommendations . |
|---|---|
| Atrial fibrillation | • Obtain a baseline clinical risk assessment of cardiovascular risk factors before initiating therapy. |
| • New AF: Interdisciplinary risk–benefit assessment. CHA2DS2-VASc 0-1, most clinicians favor continuing BTKi therapy; ≥2, consider temporary drug hold until AF control or discontinuation. | |
| • Consider beta-blockade, often preferred as the first choice over CYP3A4 inhibitors (eg, verapamil and diltiazem) or P-glycoprotein substrates (amiodarone), which interact with BTKis. | |
| • Anticoagulation strategies include either low-dose apixaban (2.5 mg twice daily given CYP3A4 interaction) or enoxaparin (at regular doses in patients with a platelet count >50,000/μL). Where possible, avoid combination with vitamin K antagonists. | |
| Ventricular arrhythmia | • Obtain a detailed cardiac history and baseline electrocardiogram for all patients; reserve echocardiogram for patients with significant cardiac history or risk factors. |
| • Instruct patients to remain vigilant for potential early warning signs of ventricular arrhythmia and immediately investigate incident lightheadedness, palpitations, or syncope. | |
| Bleeding risk | • Commonly encountered bruising seen with BTKis does not confer an increased risk of major hemorrhage and does not necessitate cessation of therapy. |
| • When possible, send patients for necessary procedures before starting therapy. | |
| • Hold BTKis for either 3 days (minor procedure) or 7 days (major procedure) both before and after invasive procedures because of increased periprocedural bleeding risk. | |
| • For minor bleeding, holding BTKi results in the resolution of bleeding tendency in 2-3 days. For severe bleeds, transfuse platelets as appropriate to overcome clinical bleeding, regardless of platelet count. | |
| • Encourage patients with bleeding to abstain from over-the-counter supplements that may exacerbate bleeding risk, such as vitamin E or fish oil. | |
| • Consider treatment options other than BTKi when dual antiplatelet therapy is indicated. | |
| Infection | • Obtain a complete workup with an appropriate index of suspicion for opportunistic infections such as Aspergillus fumigatus and PJP. |
| • In the case of severe infection, hold BTKi until a definitive diagnosis is determined and restart after the start of clinical improvement, except in the case of fungal infections. | |
| • Provide clinically indicated vaccinations (eg, against influenza and pneumococcus) of patients before treatment initiation. | |
| • Consider PJP prophylaxis for patients deemed at high risk of infection (eg, R/R or heavily pretreated patients) or patients with a prior history of infection. | |
| Hypertension | • Optimize pharmacotherapy for control of baseline hypertension before treatment initiation. |
| • Routinely monitor and begin appropriate medical therapy for incident hypertension in conjunction with the patient’s primary care provider. | |
| Diarrhea | • Most BTKi-related diarrhea can be managed with supportive care, antimotility agents, and evening dosing of ibrutinib to mitigate symptoms. |
| • Consider temporary drug holds in the case of grade ≥3 diarrhea. | |
| Fatigue, arthralgia, and myalgia | • Avoid dosage reductions for fatigue early in the course of therapy. Search for other potential causes of fatigue when observed later in the treatment course; consider drug holiday or dosage reduction only when severe and truly drug related. |
| • Rule out other causes of arthralgia. When grade 1-2, we favor observation and supportive care. Consider dosage reduction when symptoms affect ADLs, with dose holds for grade ≥3 AEs (affecting self-care ADLs) and rechallenge at lower doses if resolution of symptoms. | |
| • Arthralgias can be adjunctively treated with pharmacotherapy, although evidence is anecdotal. Approaches include magnesium supplementation and quinine-containing tonic water. Severe arthralgias demonstrate a variable response to short-course steroids and anti-inflammatory agents. | |
| Cytopenias | • Treatment-emergent flare of autoimmune cytopenias can be managed with short-course corticosteroids or CD20 monoclonal antibody treatment, and most patients can continue BTKi therapy. |
| Dermatologic manifestations | • BTKi-related skin manifestations are often responsive to corticosteroids or dose holds. |
| • Textural changes in hair or nails can be treated with biotin supplementation and the application of nail oil. | |
| Headache | • Acalabrutinib-associated headache resolves with extended treatment and is often responsive to caffeine. |
| Adverse event . | Management recommendations . |
|---|---|
| Atrial fibrillation | • Obtain a baseline clinical risk assessment of cardiovascular risk factors before initiating therapy. |
| • New AF: Interdisciplinary risk–benefit assessment. CHA2DS2-VASc 0-1, most clinicians favor continuing BTKi therapy; ≥2, consider temporary drug hold until AF control or discontinuation. | |
| • Consider beta-blockade, often preferred as the first choice over CYP3A4 inhibitors (eg, verapamil and diltiazem) or P-glycoprotein substrates (amiodarone), which interact with BTKis. | |
| • Anticoagulation strategies include either low-dose apixaban (2.5 mg twice daily given CYP3A4 interaction) or enoxaparin (at regular doses in patients with a platelet count >50,000/μL). Where possible, avoid combination with vitamin K antagonists. | |
| Ventricular arrhythmia | • Obtain a detailed cardiac history and baseline electrocardiogram for all patients; reserve echocardiogram for patients with significant cardiac history or risk factors. |
| • Instruct patients to remain vigilant for potential early warning signs of ventricular arrhythmia and immediately investigate incident lightheadedness, palpitations, or syncope. | |
| Bleeding risk | • Commonly encountered bruising seen with BTKis does not confer an increased risk of major hemorrhage and does not necessitate cessation of therapy. |
| • When possible, send patients for necessary procedures before starting therapy. | |
| • Hold BTKis for either 3 days (minor procedure) or 7 days (major procedure) both before and after invasive procedures because of increased periprocedural bleeding risk. | |
| • For minor bleeding, holding BTKi results in the resolution of bleeding tendency in 2-3 days. For severe bleeds, transfuse platelets as appropriate to overcome clinical bleeding, regardless of platelet count. | |
| • Encourage patients with bleeding to abstain from over-the-counter supplements that may exacerbate bleeding risk, such as vitamin E or fish oil. | |
| • Consider treatment options other than BTKi when dual antiplatelet therapy is indicated. | |
| Infection | • Obtain a complete workup with an appropriate index of suspicion for opportunistic infections such as Aspergillus fumigatus and PJP. |
| • In the case of severe infection, hold BTKi until a definitive diagnosis is determined and restart after the start of clinical improvement, except in the case of fungal infections. | |
| • Provide clinically indicated vaccinations (eg, against influenza and pneumococcus) of patients before treatment initiation. | |
| • Consider PJP prophylaxis for patients deemed at high risk of infection (eg, R/R or heavily pretreated patients) or patients with a prior history of infection. | |
| Hypertension | • Optimize pharmacotherapy for control of baseline hypertension before treatment initiation. |
| • Routinely monitor and begin appropriate medical therapy for incident hypertension in conjunction with the patient’s primary care provider. | |
| Diarrhea | • Most BTKi-related diarrhea can be managed with supportive care, antimotility agents, and evening dosing of ibrutinib to mitigate symptoms. |
| • Consider temporary drug holds in the case of grade ≥3 diarrhea. | |
| Fatigue, arthralgia, and myalgia | • Avoid dosage reductions for fatigue early in the course of therapy. Search for other potential causes of fatigue when observed later in the treatment course; consider drug holiday or dosage reduction only when severe and truly drug related. |
| • Rule out other causes of arthralgia. When grade 1-2, we favor observation and supportive care. Consider dosage reduction when symptoms affect ADLs, with dose holds for grade ≥3 AEs (affecting self-care ADLs) and rechallenge at lower doses if resolution of symptoms. | |
| • Arthralgias can be adjunctively treated with pharmacotherapy, although evidence is anecdotal. Approaches include magnesium supplementation and quinine-containing tonic water. Severe arthralgias demonstrate a variable response to short-course steroids and anti-inflammatory agents. | |
| Cytopenias | • Treatment-emergent flare of autoimmune cytopenias can be managed with short-course corticosteroids or CD20 monoclonal antibody treatment, and most patients can continue BTKi therapy. |
| Dermatologic manifestations | • BTKi-related skin manifestations are often responsive to corticosteroids or dose holds. |
| • Textural changes in hair or nails can be treated with biotin supplementation and the application of nail oil. | |
| Headache | • Acalabrutinib-associated headache resolves with extended treatment and is often responsive to caffeine. |
Cardiac arrhythmia: atrial fibrillation
CLL is a disease of older adults, with a median age of 72 years at diagnosis. Beyond the rates of atrial fibrillation (AF) present in the general population of similar age (1% to 2%),36 there is a higher rate of AF in treatment-naive patients with CLL (6.1% prevalence at diagnosis, with a subsequent incidence of 1% per year).37 An early signal suggesting an association between AF and BTK inhibition was observed in 7% to 10% of patients in initial randomized trials of ibrutinib4,5 (Table 3). This association persisted in extended follow-up and pooled analyses of multiple studies. For example, in a pooled safety analysis of 4 randomized trials of ibrutinib in CLL/SLL and mantle cell lymphoma,38 new-onset AF was reported in 6% of patients on ibrutinib and 2% of patients treated with comparator agents; grade ≥3 AF occurred in 3% and <1% of these patients, respectively. The prevalence of AF associated with ibrutinib was greatest in the first 3 months on therapy (median time of onset 2.8 months), with late events (onset at month 18 or later) occurring in a minority (1%) of patients. More recent randomized comparisons of ibrutinib with chemoimmunotherapy (CIT) highlight the relevance of patient age to the risk of AF. In younger patients, the E1912 study39,40 (median age 58) reported an incidence of grade ≥3 AF in 2.9% of patients on ibrutinib (vs 0% on CIT). In contrast, the Alliance 041202 study41 (median age 71) reported a 9% incidence of AF on ibrutinib monotherapy (vs 3% for CIT). Compared with ibrutinib, acalabrutinib has a lower rate of AF in several series. For example, with a median follow-up of 41 months, AF was observed in 7% of R/R patients (3% grade ≥3) receiving acalabrutinib monotherapy.11 Although the mechanism of BTKi-related AF remains unclear, inhibition of PI3K signaling—a critical regulator of cardiac protection under stress that is regulated by BTK and TEC—has been implicated.42 Although the kinase binding profile of agents in earlier development may a priori be thought to affect their risk of AF, an exact estimate of incidence is difficult to determine because of smaller study populations.
Frequency of adverse events in landmark studies of currently approved BTKis
| Adverse events . | Ibrutinib . | Acalabrutinib . | ||
|---|---|---|---|---|
| RESONATE25 . | RESONATE83,84 . | ELEVATE-TN10 . | ASCEND12 . | |
| TN n = 135 . | RR n = 195 . | TN n = 179 . | RR n = 154 . | |
| f/u = 18.4 mo . | f/u = 19 mo . | f/u = 28.3 mo . | f/u = 22 mo . | |
| Atrial fibrillation | ||||
| All grades | 14 (10) | 13 (7) | 7 (3.6) | 9 (6) |
| Grade ≥ 3 | 6 (4) | 7 (4) | NR | 2 (1) |
| Bleeding | ||||
| All grades | 9 (7) | NR | 70 (39) | 44 (29) |
| Grade ≥ 3 | 8 (6) | 4 (2) | 4 (2) | 4 (3) |
| Hypertension | ||||
| All grades | 18 (14) | NR | 9 (5) | 7 (5) |
| Grade ≥ 3 | 5 (4) | 8 (4) | 4 (2) | 4 (3) |
| Arthralgia | ||||
| All grades | 27 (20) | 36 (19) | 28 (16) | 23 (15)* |
| Grade ≥3 | 3 (2) | NR | 1 (0.6) | 2 (1)* |
| Infection | ||||
| All grades | NR | NR | 116 (65) | 97 (63) |
| Grade ≥3 | 21 (23) | 59 (30) | 25 (14) | 30 (20) |
| Diarrhea | ||||
| All grades | 57 (42) | 105 (54) | 62 (35) | 30 (20) |
| Grade ≥3 | 5 (4) | 9 (5) | 1 (0.6) | 3 (2) |
| Adverse events . | Ibrutinib . | Acalabrutinib . | ||
|---|---|---|---|---|
| RESONATE25 . | RESONATE83,84 . | ELEVATE-TN10 . | ASCEND12 . | |
| TN n = 135 . | RR n = 195 . | TN n = 179 . | RR n = 154 . | |
| f/u = 18.4 mo . | f/u = 19 mo . | f/u = 28.3 mo . | f/u = 22 mo . | |
| Atrial fibrillation | ||||
| All grades | 14 (10) | 13 (7) | 7 (3.6) | 9 (6) |
| Grade ≥ 3 | 6 (4) | 7 (4) | NR | 2 (1) |
| Bleeding | ||||
| All grades | 9 (7) | NR | 70 (39) | 44 (29) |
| Grade ≥ 3 | 8 (6) | 4 (2) | 4 (2) | 4 (3) |
| Hypertension | ||||
| All grades | 18 (14) | NR | 9 (5) | 7 (5) |
| Grade ≥ 3 | 5 (4) | 8 (4) | 4 (2) | 4 (3) |
| Arthralgia | ||||
| All grades | 27 (20) | 36 (19) | 28 (16) | 23 (15)* |
| Grade ≥3 | 3 (2) | NR | 1 (0.6) | 2 (1)* |
| Infection | ||||
| All grades | NR | NR | 116 (65) | 97 (63) |
| Grade ≥3 | 21 (23) | 59 (30) | 25 (14) | 30 (20) |
| Diarrhea | ||||
| All grades | 57 (42) | 105 (54) | 62 (35) | 30 (20) |
| Grade ≥3 | 5 (4) | 9 (5) | 1 (0.6) | 3 (2) |
f/u, follow-up; NR, not reported in original publication; RR, relapsed or refractory; TN, treatment-naive.
Reported numbers reflect 16.1-month follow-up.85
The management of BTKi-related AF begins with a baseline clinical risk assessment of cardiovascular risk factors before initiating therapy. For patients with a long history of poorly controlled AF, we favor consideration of other treatment modalities (eg, BCL2 inhibition). Once AF arises, an interdisciplinary effort should be made to manage this complication along with the patient’s underlying CLL while balancing the individualized risk of bleeding with that of stroke. Clinical risk assessments, such as the CHA2DS2-VASc and HAS-BLED scoring systems, can guide this approach. For patients with limited risk factors (CHA2DS2-VASc 0-1), most clinicians favor continuing BTKi therapy.43,44 For patients with ≥2 risk factors, multiple strategies have been reported on, with limited data to clearly favor one approach over another. Management is therefore clinician dependent, with some authors advocating discontinuing BTKi and initiating anticoagulation.44 In contrast, others prefer to hold drugs only temporarily and reinitiate therapy once control of AF is achieved.43 Pharmacologically, it is crucial to keep in mind potential interactions between BTKis and AF-directed therapy or anticoagulation. In terms of the former, beta-blockade is often preferred as the first choice over CYP3A4 inhibitors (eg, verapamil and diltiazem) or P-glycoprotein substrates (amiodarone). Considering the latter, we favor expert management strategies for anticoagulation with either low-dose apixaban43 (2.5 mg twice daily given CYP3A4 interaction) or enoxaparin44 (at regular doses in patients with a platelet count >50,000/μL). We note that the pathophysiology of bleeding on ibrutinib is complex and that this risk, both during BTK inhibition alone and when coadministered with anticoagulation, is greatest early in the BTKi treatment course.45 Because early phase 1 clinical trials of ibrutinib included fatal subdural hematoma on warfarin and subsequent BTKi trials excluded these patients,4 we generally avoid the combination of BTKi and vitamin K antagonists. When considering anticoagulation, we place extra emphasis on the instruction, recommended to all our patients on BTKis, to abstain from over-the-counter supplements that may exacerbate bleeding risk, such as vitamin E or fish oil.45
Ventricular arrhythmia
With longer-term follow-up of patients receiving ibrutinib, rare unexplained cases of incident ventricular arrhythmias and sudden cardiac death have emerged.46 Independent analysis has confirmed this association in data from US-based46,47 and international registries.48 For example, in a global study of cardiovascular adverse drug reactions, ibrutinib was associated with a higher reporting of ventricular arrhythmias (odds ratio 4.7; 95% confidence interval, 3.7-5.9).48 An increased rate of sudden death was observed in pooled data from ∼1,000 patients participating in randomized trials of ibrutinib, with 788 events found per 100,000 person-years, more than the 200 to 400 events seen in 65-year-olds in the general population.47 QTc prolongation does not appear to be a likely contributor, because ibrutinib has been associated with a reduced QT duration.48 For now, clinicians should maintain a high index of suspicion when encountering cardiac symptoms (eg, syncope, palpitations, light-headedness) in patients on all BTKis.
Bleeding risk
Ibrutinib is associated predominantly with minor bleeding (grade ≤2, low-grade ecchymoses and petechiae) in up to two-thirds of patients. Major bleeding (grade ≥3, necessitating transfusion or hospitalization) occurs less frequently in 2% to 9% of patients.4,5,7 An integrated analysis of 15 ibrutinib studies (n = 1,768 patients) demonstrated a higher proportion of major bleeding on ibrutinib compared with comparator therapy (4.4% vs 2.8%). Nevertheless, this difference did not persist after adjustment for more prolonged exposure with ibrutinib (incidence of 3.2 vs 3.1 per 1,000 person-months).38 Interestingly, a higher risk of major bleeding was observed with the use of anticoagulants or antiplatelet agents in both the ibrutinib-treated and comparator populations. In contrast with ibrutinib, in the ELEVATE-TN trial, only 2% major bleeding was seen on acalabrutinib monotherapy, with minor bleeding observed in 37% of patients.10 In the relapsed setting, major bleeding was observed in 5% of patients in long-term follow-up of phase 2 data and 1% of patients in ASCEND.11
Although the mechanism of pathogenesis is complex and incompletely understood, both on- and off-target kinase inhibition are implicated. In platelets, BTK and other related Tec family kinases play an important role in platelet aggregation mediated via the collagen receptor glycoprotein VI. Patients with CLL not on BTKi therapy exhibit less robust platelet aggregation than healthy controls45,49,50 and greater impairment in collagen-induced aggregation responses on ibrutinib.45,49 More recent work has also implicated ibrutinib-mediated shedding of GPIb-IX and integrin αIIbβ3.51
Our approach to managing bleeding risk on BTKis is as follows. Bruising, commonly seen with BTKis, does not confer an increased risk of major hemorrhage and does not warrant cessation of therapy. BTKis should be held for either 3 days (minor procedure) or 7 days (major procedure) both before and after invasive procedures because of the increased periprocedural bleeding risk. When possible, we recommend completing necessary surgical procedures before starting therapy. For patients who have already been on long-term ibrutinib, there is evidence that interruptions to therapy are less impactful later in the treatment course. For minor bleeding, holding ibrutinib results in the resolution of bleeding tendency in 2 to 3 days. For severe bleeds, we advocate providing platelet transfusions as appropriate to overcome clinical bleeding, regardless of platelet count. When considering anticoagulation, we follow the strategy outlined in the section on AF, above. We also consider alternative CLL therapy, if available, for patients needing dual antiplatelet therapy. Although second- and third-generation agents may ultimately confer less risk for all bleeding-related AEs, we currently manage bleeding risk agnostic to the particular BTKi agent used.
Infection
Patients receiving BTK inhibitors are immunocompromised and are at risk of infectious complications despite receiving effective therapy. Infection (of any grade) occurs in >50% of patients on BTKis (Table 3), particularly during the early period after starting treatment, and R/R patients are at greater risk. In real-world analysis, 11% of R/R patients needed treatment discontinuation because of infectious complications, with a median time to therapy cessation of 6 months.21 Of all infectious complications, pneumonia was the most common, observed in an integrated analysis of landmark ibrutinib studies in 12% of patients (grade ≥3 infection).6
These data suggest that in many instances, infectious complications seen on BTKis may be largely attributable to the biology of the disease itself. Yet aside from CLL-related immune dysfunction, changes on BTKi therapy, including inhibition of ITK52 and impairment of macrophages,53,54 have also been implicated in the pathogenesis of susceptibility to infection. Ibrutinib has also been associated with multiple functional defects in neutrophil function (decreased reactive oxygen species production).55 In a recent cross-trial comparison, similar immunologic changes during BTK inhibition were observed with both ibrutinib and acalabrutinib; both were associated with a sustained increase in serum immunoglobulin A, with patients exhibiting greater levels of this immunoglobulin at lower risk of infectious complications.56
Recent series have also highlighted the prevalence of opportunistic infections, including Aspergillus fumigatus and Pneumocystis jirovecii (PJP), in patients with CLL on BTKis.57-60 A single-institution report of 566 patients (44.9% of whom received prophylaxis) documented no cases of PJP.57 Another center reported no PJP infections in 130 patients receiving prophylaxis, with a 2.4% prevalence of PJP in 85 patients receiving BTKi monotherapy without prophylaxis.58 In view of limited data, at present, current international workshop on CLL guidelines do not specifically address the role of PJP prophylaxis,61 and practices vary across institutions. In our practice, PJP prophylaxis should be considered in patients deemed at high risk of infection (eg, R/R or heavily pretreated patients) or patients with a known prior history of infection. Troublingly, Aspergillus fumigatus infection has been reported at a higher-than-expected rate in patients on BTKis.57,59,62 Data suggest that the risk of aspergillosis is highest early in the treatment course57 and is higher among patients receiving corticosteroids.60
When an infection occurs, management begins with a complete workup, with an appropriate index of suspicion for opportunistic infections. During acute infection, some experts advocate holding kinase inhibitors until a definitive diagnosis is determined,43 whereas others hold specifically in the case of grade 4 infection.44 Regardless of approach, pharmacotherapy can be reinitiated after the start of clinical improvement when deemed appropriate by the caring provider. Particular attention should be paid to drug interactions (eg, with CYP3A4 inhibitors voriconazole or posaconazole).
We favor the administration of indicated vaccines (eg, against influenza and pneumococcus) before treatment initiation. In addition, based on preliminary data, we also consider recombinant, adjuvanted varicella-zoster virus vaccine.63 Additionally, we reserve intravenous immunoglobulin therapy for patients with recurrent infections and known hypogammaglobulinemia.
Finally, at the time of this writing, preliminary data are emerging regarding the use of BTKis in the setting of coronavirus disease 2019 (COVID-19). There is concern that patients with CLL may be at particularly high risk of infection or poor outcome given their average age and comorbidities and the profound immune changes seen with this disease. To this end, early in the pandemic professional societies (based on expert consensus) recommended limiting their exposure to the health care system and postponing the initiation of treatment where feasible. An international case series of patients with symptomatic COVID-19 (n = 198) revealed high mortality rates in both watch-and-wait and treated patients with CLL during hospital admission.64 Importantly, in this study, therapy with BTKi at the time of hospitalization did not affect the overall case fatality rate. Because this is a rapidly developing story with more data forthcoming, we point the reader toward the latest international guidelines65,66 for the most up-to-date consensus practices regarding CLL, BTK inhibition, and COVID-19.
Other adverse events
Hypertension
The most extended follow-up data currently available for ibrutinib (PCYC-1102) report grade ≥3 hypertension (≥160/100 mm Hg) in 28% of patients.3 Multiple series have documented that the prevalence of hypertension (any grade) increases over time (eg, 11% year 1, 15% year 2; 20% year 3 in a pooled analysis). Strikingly, in a series of 562 patients with lymphoid malignancies treated on ibrutinib, the incidence of new hypertension was 71.6%, 13 times higher than in a comparable Framingham cohort. In this analysis, 80% of patients exhibited a 10–mm Hg increase from baseline, and more than 10% of patients demonstrated an increase of ≥50 mm Hg.67 By comparison, at a median follow-up of 53 months in ACE-CL-001, the rate of grade ≥3 hypertension on acalabrutinib was 11%, with minimal late increases in incidence.68 Accordingly it remains unclear whether hypertension is a broad class effect of BTKi inhibition. Proposed mechanisms include PI3k/Akt inhibition, leading to downregulation of PI3K-p110alpha and downregulation of nitrous oxide production.42,67 Management involves judicious optimization of baseline hypertension before treatment initiation, regular monitoring of blood pressure at clinic visits, and appropriate medical therapy for incident hypertension in collaboration with the patient’s primary care provider.
Diarrhea
Diarrhea is the most commonly observed AE on BTKi therapy, occurring early in treatment (before month 6) with a predominantly self-limited course.4,8,10 Although the prevailing view is that ibrutinib-related diarrhea is probably EGFR mediated, rates of diarrhea seen on acalabrutinib are similar to those with ibrutinib11,24 (despite differences in EGFR binding). It is our practice to manage most BTKi-related diarrhea with supportive care, antimotility agents, and nighttime dosing of drug (in the case of ibrutinib) to mitigate symptoms. We also consider temporary drug holds in the case of grade ≥3 diarrhea.
Fatigue, arthralgia, and myalgia
Fatigue is a commonly reported symptom seen early in the course of BTK inhibition and is usually self-limited. It has been reported in 36% of patients on ibrutinib (in a pooled analysis, 0% to 3% grade 3)6 and in 28% to 34% of patients on acalabrutinib (0% to 2% grade 3).10,11,24 Given the propensity for preexisting disease-related fatigue and the close temporal link between symptom onset and treatment start, we do not typically advocate dose interruptions on account of this toxicity, particularly if it occurs early after initiation of therapy. If it persists or occurs significantly later during the course of therapy, then an evaluation to search for other potential causes of fatigue should be undertaken, and a drug holiday or dosage reduction should be considered to assess whether it is drug related.
Arthralgia and myalgia are seen in 11% to 36% of patients on ibrutinib, with higher rates compared with comparators in a pooled analysis.4,5,38,69 Patients describe a dynamic pattern of migratory arthralgias that can be debilitating; arthralgias were associated with 42% of ibrutinib discontinuations in real-world data.21 A recent case series focusing on this toxicity noted that the majority of arthralgias developed at 7 months after treatment initiation, and risk factors included female sex and treatment-naive status.69 After ruling out other possible etiologies of arthralgia, these authors recommended observation for grade 1 to 2 arthralgia, with dosage reduction considered when symptoms affect activities of daily living (ADLs). Dose holds were recommended for grade ≥3 AEs (affecting self-care ADLs), with rechallenge at lower dosages if symptoms resolve. Evidence for specific pharmacotherapy has not been studied formally but is anecdotal, and approaches include magnesium supplementation and quinine-containing tonic water. Severe arthralgias demonstrate a variable response to short-course steroids (we recommend an abbreviated course at low dosages given the increased risk for fungal infection) and anti-inflammatory agents (which must be used cautiously given the potentially higher risk of bleeding on BTKis).
Cytopenias
CLL is associated with autoimmune cytopenias (AICs) which occur in ∼4% to 10% of patients; autoimmune hemolytic anemia is most common (∼7%), followed by immune thrombocytopenia (<1% to 2%), with pure red cell aplasia occurring less frequently.70 Most AICs are thought to be caused by a variety of complex mechanisms of immune dysregulation that are a consequence of CLL itself. BTKis are known to decrease the need for immunosuppressive therapy in the setting of preexisting autoimmune cytopenias,71 and true flare or de novo treatment-emergent AICs are uncommon in both the clinical trial and real-world settings. A case series of patients on clinical trials of ibrutinib at 2 major institutions reported 13 total patients who had AICs at treatment start, with the majority (9 patients) continuing on the drug despite experiencing an initial flare.72 Similarly, in real-world data from the Mayo Clinic, of 161 patients, only 11 (6%) developed treatment-emergent AICs, occurring a median of 59 days after therapy. Of these, 7 patients were able to continue therapy after temporary dose hold or reduction.73 In this literature, authors report benefit from early use of additional immunosuppressive therapies, which can often be discontinued upon count recovery. It is our practice to treat treatment-emergent AIC flare with the addition of short-course corticosteroids or anti-CD20 monoclonal antibody treatment and then resume treatment with BTKis. Other cytopenias including neutropenia can be seen74 but rarely necessitate dose interruption or discontinuation, and in our experience, when encountered it usually responds to growth factor support.
Dermatologic manifestations
In addition to the nonpalpable asymptomatic petechial rash thought to result from ibrutinib-induced platelet dysfunction, at least two other forms of skin manifestations of BTKi therapy have been characterized: a palpable rash that is often pruritic, associated with EGFR inhibition and infiltration of inflammatory cells; and erythema nodosum.75,76 Both manifestations are often responsive to corticosteroids or dose holds. Textural changes in hair and nails are also observed. An early series from the National Institutes of Health recognized a higher incidence of brittle fingernails or toenails with the formation of vertical nail ridges in two-thirds of patients treated with ibrutinib.77 These manifestations appear gradually, with a median reported onset of 9 months, and do not represent a dose-limiting toxicity. They can be treated with biotin supplementation and the application of nail oil.
Headache
Headache is seen uniquely with the second-generation inhibitor acalabrutinib. In pivotal studies, nearly 70% of patients experienced grade 1 or 2 headaches, most often during the first cycles (particularly weeks 1 to 3).10,11 Headache has been reported to resolve with extended treatment, and the administration of acetaminophen with or without caffeine is often effective for symptomatic relief.
The patient demonstrated clinical improvement with ibrutinib therapy but developed impairment in his ADLs later in the course of treatment. His dosage was reduced initially, and magnesium and quinine were tried, but he had persistent arthralgias that did not improve. His ibrutinib was discontinued, and changing therapy to an alternative BTKi or initiating therapy with a venetoclax-based regimen was discussed. His arthralgias resolved off therapy, and he subsequently began treatment with acalabrutinib without recurrence of arthralgias.
Additional considerations
Impact of dose modification and temporary interruption of therapy on clinical outcomes
The influence of dose modification and temporary interruption on clinical outcomes remains an active area of interest in CLL. Biologically, BTK target occupancy, a pharmacodynamic measure of covalent binding, is associated with the degree of CLL tumor cell inhibition.24 Although BTK target occupancy is frequently measured in the setting of clinical trials, no optimal absolute threshold has emerged, and measurement outside this setting is not routine. Several analyses have looked at the impact of dose interruptions and reductions on clinical outcomes. In an analysis of the RESONATE data, higher dose intensity during the first 8 weeks of therapy was associated with longer median progression-free survival (NR vs 6.9 months), and dose interruptions of ≥8 consecutive days (reported with 9 months of follow-up) were associated with a shorter median progression-free survival (10.8 months vs NR).78 Host-related factors may be a confounder here, because in other analyses patients with early alterations tended to have poor performance status and had a higher likelihood of permanent early discontinuation.79,80 The timing of these events also probably plays an important role. For example, a clinically indicated reduction in starting dose (eg, patients receiving concurrent strong or moderate CYP3A inhibitors should have their ibrutinib starting dose reduced to 140 mg or 280 mg, respectively) does not appear to be associated with differences in event-free survival or overall survival.81
In general, given that many early secondary effects are likely to decrease in severity with time, we recommend a strategy where low-grade toxicities are treated with expectant management and supportive care when possible. It is our practice to try to avoid dose reductions or cessation of therapy within the first 6 months of starting treatment. However, evidence suggests that clinically indicated dose interruptions (eg, to mitigate significant adverse events grade ≥3) are unlikely to facilitate the emergence of drug-resistant clones and do not appear to compromise long-term clinical outcomes.80 For this reason, in this setting we do not hesitate to hold drugs where appropriate.
Sequential therapy and toxicity of emerging second- and third-generation BTKis
As data continue to accumulate on the use of novel BTKIs, we will gain greater insight into the extent to which classic BTK-associated toxicities are influenced by kinase binding properties. Idiosyncratic drug-specific toxicities (eg, acalabrutinib headache) may continue to appear with newer inhibitors. These agents may ultimately prove to be an attractive option for patients intolerant to ibrutinib because of off-target toxicities. Although current data are limited, in a nonrandomized study of patients (n = 33) receiving acalabrutinib after discontinuing ibrutinib due to “intolerance,” of 61 AEs, 72% of ibrutinib-related AEs did not occur during acalabrutinib treatment, suggesting continued disease control with improved tolerability.82 Randomized data evaluating the impact of restarting ibrutinib (at dose reduction) compared with switching to acalabrutinib are not currently available. Studies of newer agents in previously BTKi-intolerant patients (eg, zanubrutinib NCT04116437) are ongoing, as are head-to-head phase 3 trials (eg, ELEVATE-RR, NCT02477696, and ALPINE NCT03734016), which will provide us with direct comparison data.
Conclusion
A thorough clinical history encompassing patient-specific comorbidities is of the utmost importance when therapy is considered for patients with CLL. Patients should be appropriately informed about the risk of adverse effects when initiating therapy. Patients with arrhythmias or poorly controlled hypertension will probably do better with other treatment modalities (eg, BCL2 inhibition). As data continue to emerge on the use of BTKi in combination with other therapeutic agents, opportunities to treat patients with CLL with a fixed duration of treatment rather than indefinite therapy may reduce the potential for longer-term toxicities. Data from ongoing phase 3 trials evaluating BTKis in combination with other agents will help discern the potential for overlapping toxicities and better define the role of combinations in the management of CLL. Although there may be some reason to prefer later-generation tyrosine kinase inhibitors (particularly for patients at higher risk of cardiovascular AEs), inference from cross-trial comparisons is inherently limited. Ongoing randomized clinical trial data will provide a more direct comparison to assess for advantages in the toxicity profile of one agent over another. Given the impressive efficacy and activity of BTKis in the treatment of upfront and R/R CLL, it is vital that caregivers become as familiar as possible with the management of BTKi-emergent toxicities, because this class will probably remain a mainstay of treatment either as monotherapy or in combination with other agents for quite some time.
Acknowledgments
The authors thank Adrian Wiestner for critical reading of the manuscript and colleagues for helpful discussions.
Conflict-of-interest disclosure
A. L. has no conflicts to disclose. N. L. is on advisory board committees at: Abbvie, AstraZeneca, Bei-Gene, Juno, Loxo, Oncternal, Mingsight, and TG Therapeutics. Additionally, N. L. provides research support to the following institutions: Abbvie, AstraZeneca, Bei-Gene, Celgene, Genentech, Janssen, Pharmacyclics, and Verastem.
Off-label drug disclosure
None disclosed.
Correspondence
Nicole Lamanna, Columbia University Medical Center, 161 Fort Washington Ave, 9th Floor, Room 9-965, New York, NY 10032; email: nl2129@cumc.columbia.edu.