Abstract
Despite the proven effective approach to targeting the phosphatidylinositol-3-kinase (PI3K) pathway in B-cell malignancies, the approved PI3K inhibitors idelalisib and duvelisib have been less commonly selected for patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), given the availability of other more tolerable agents. However, patients with CLL/SLL can experience a disease course that is multiply relapsed, refractory, or intolerant to treatment, and PI3K inhibitors can achieve meaningful responses. This article reviews the common early- and late-onset (considered immune-mediated) toxicities with PI3K inhibitors, including infections, hepatotoxicity, diarrhea and/or colitis, and pneumonitis. Data on pretreatment considerations, toxicity management, and drug rechallenge are presented. In addition, next-generation PI3K inhibitors and novel treatment approaches with PI3K inhibitors, including combinations, time-limited treatments, and intermittent dosing, are highlighted.
Learning Objectives
Identify and manage common early and late onset toxicities that can occur in treatment of patients with CLL/SLL on PI3K inhibitors
Discuss next generation PI3K inhibitors and other novel treatment approaches with PI3K Inhibitors in CLL/SLL
Clinical case
A 72-year-old man with chronic kidney disease (creatinine, 2.2 mg/dL) was referred for relapsed, high-risk chronic lymphocytic leukemia (CLL). He had been diagnosed with CLL 4 years prior (normal fluorescence in situ hybridization findings; IGHV mutational analysis not performed) and had received frontline treatment with bendamustine plus rituximab (BR). Eighteen months after BR, he had symptomatic relapse of his disease (52% deletion of 17p by fluorescence in situ hybridization; IGHV unmutated) and was started on ibrutinib with improved lymphadenopathy. After 5 months, he presented with a severe headache and was found to have a subdural hematoma; his platelet count at the time was 120 × 109 /L, and he denied receiving anticoagulation or other antiplatelet agents. He recovered fully and was followed off therapy for 6 months until progression with symptomatic bulky lymphadenopathy occurred. He declined participation in clinical trials, and, with lack of support to complete venetoclax dose escalation, he elected to start idelalisib plus rituximab. He has returned for a scheduled visit 6 weeks after idelalisib initiation. Upon examination, he is well appearing with reduced lymphadenopathy. His complete blood count demonstrates improvement in his hemoglobin and platelets, and he is not neutropenic. His creatinine is stable (2.0 mg/dL), and his alkaline phosphatase and bilirubin are within normal limits, but his aspartate aminotransferase (AST) is increased to 410 IU/L, and his alanine aminotransferase (ALT) is 520 IU/L.
Introduction
The phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway is a well-recognized biologic target in malignancy governing key oncogenic processes such as survival, proliferation, and migration.1,2 Class I PI3Ks are activated by surface receptor tyrosine kinases, including the B-cell receptor (BCR) and chemokine receptors (CXCR4, CXCR5), implicated in the pathobiology of CLL/small lymphocytic lymphoma (SLL). There are 4 isoforms of class I PI3Ks according to the catalytic domain: α (p110α/PI3Kα), β (p110β/PI3Kβ), δ (p110δ/PI3Kδ), and γ (p110γ/PI3Kγ). The isoforms are differentially expressed, with γ and δ dominant in hematopoietic cells; PI3Kδ mediates BCR-driven proliferation and chemotaxis, and PI3Kγ is important in diverse immune processes, including T-cell function. Therefore, in addition to direct antitumor effects from inhibition of PI3K isoforms in CLL cells, inhibition appears to exert antitumor activity indirectly through interruptions within the CLL microenvironment.3-5
Idelalisib (formerly GS1101, CAL101) is a first-in-class oral PI3Kδ-specific inhibitor that demonstrated promising clinical efficacy in very high-risk relapsed/refractory (R/R) CLL/SLL. In 2014, idelalisib was approved with rituximab in patients with R/R CLL for whom rituximab monotherapy would be appropriate and in patients with SLL receiving ≥2 prior therapies. Duvelisib (formerly IPI-145), a dual oral PI3Kγ/δ inhibitor, was approved in 2018 for patients with R/R CLL/SLL receiving ≥2 prior therapies. Despite early efficacy results, PI3K inhibitor (PI3Ki) use has been limited largely by toxicities that can lead to permanent discontinuation and the availability of other drugs with tolerability that is more favorable.6 Like many of the BCR pathway tyrosine kinase inhibitors, the ability to apply continuous administration of the PI3Ki, especially early in the treatment course, is necessary for long-term disease control.
In the frontline and R/R settings, randomized studies have demonstrated superiority of Bruton tyrosine kinase (BTK) inhibitors (BTKis; ibrutinib, acalabrutinib) and venetoclax (a BCL-2 inhibitor) over chemoimmunotherapy, with rapid incorporation into practice. However, CLL/SLL remains an incurable disease for most patients, leaving an unmet need. Given the effectiveness of targeting the PI3K pathway, efforts remain underway to consider best use of current agents and next-generation PI3Kis and/or alternative dosing regimens. Early recognition and treatment of PI3Ki toxicities is imperative for safe and effective treatment with these agents.
Clinical efficacy and safety: idelalisib and duvelisib
The recommended 150-mg twice-daily dosing of idelalisib is derived from observations of a dose plateau in the pharmacodynamic target (pAkt) and treatment response, combined with recognition of grade 3+ treatment-emergent adverse events, including diarrhea/colitis, transaminitis, and pneumonitis.7 Table 1 summarizes the efficacy results of approved PI3Kis idelalisib and duvelisib in phase 2 and 3 trials for CLL/SLL. The pivotal idelalisib phase 3, double-blind, placebo-controlled trial in R/R CLL randomized 220 patients in whom rituximab monotherapy would have been appropriate to receive rituximab plus idelalisib (idela + R) or rituximab (R) plus placebo. The primary endpoint of progression-free survival (PFS) was met (not reached [NR]) at 12 months for the idelalisib regimen vs 5.5 months in the placebo arm (P < .001), and the study was stopped early due to significant efficacy.8 At a median of 18 months of follow-up, the benefit still held for patients treated with idela + R (followed by idelalisib monotherapy) with a median PFS of 20.3 months (95% confidence interval, 17.3-26.3); the median overall survival in the idela + R arm was 40.6 months (95% confidence interval, 28.5-57.3 months) vs 34.6 months (95% confidence interval, 16.0-NR) in the placebo group.9
Efficacy in select phases 2 and 3 clinical trials of idelalisib and duvelisib in CLL/SLL
| Trial . | Phase 3, idelalisib + rituximab vs rituximab in R/R CLL8,9 . | Phase 3, idelalisib vs placebo + BR in R/R CLL14 . | Phase 3, idelalisib + ofatumumab vs ofatumumab in previously treated CLL13 . | Phase 3, acalabrutinib vs investigator’s choice (BR or idelalisib + rituximab) in R/R CLL10 . | Phase 2, treatment-naïve older patients with CLL, idelalisib + rituximab11 . | Phase 2, treatment-naïve CLL with idelalisib + ofatumumab12 . | Phase 2, DYNAMO trial, double-refractory FL, SLL, MZL22 . | Phase 3, DUO trial, duvelisib vs ofatumumab in R/R CLL/SLL21 . |
|---|---|---|---|---|---|---|---|---|
| Population | R/R CLL | R/R CLL | R/R CLL | R/R CLL | Treatment-naïve older patients with CLL/SLL | Treatment-naïve patients with CLL | iNHL (FL, SLL, or MZL) double-refractory to rituximab + chemoimmunotherapy or radioimmunotherapy | R/R CLL/SLL |
| Treatment | Idelalisib 150 mg by mouth twice daily plus rituximab IV 375 mg/m2 in week 0, day 1, and 500 mg/m2 day 1 of weeks 2, 4, 6, 8, 12, 16, and 20 vs rituximab + placebo | Bendamustine 70 mg/m2 IV on days 1 and 2 C1-6 plus rituximab: 375 mg/m2 on C1D1, and 500 mg/m2 D1C2–6 plus idelalisib 150 mg twice daily vs BR plus placebo | Ofatumumab 300 mg in week 1, day 1 followed by 2000 mg weekly for 7 wk, then every 4 wk for 16 wk vs idelalisib 150 mg by mouth twice daily plus ofatumumab on same week schedule as control group but at 1000 mg from week 2 | Acalabrutinib 100 mg by mouth twice daily vs investigator’s choice of idelalisib 150 mg by mouth twice daily plus rituximab 375 mg/m2 on C1D1 and 500 mg/m2 D1C2–6 or bendamustine 70 mg/m2 IV on days 1 and 2 C1-6 plus rituximab | Idelalisib 150 mg by mouth twice daily plus rituximab 375 mg/m2 IV weekly for 8 wk | Idelalisib 150 mg by mouth twice daily plus ofatumumab 300 mg C3D1 followed by 1000 mg weekly for 7 wk, then 100 mg every 4 wk for 16 wk (6 mo total) | Duvelisib 25 mg by mouth twice daily | Duvelisib 25 mg twice daily or ofatumumab IV for up to 12 doses |
| Number of patients | 220 | 416 | 261 | 398 | 64 | 27 | 129 | 159 |
| Primary endpoint | PFS | PFS | PFS | PFS | ORR | ORR | ORR | PFS |
| mPFS | Not reached at 12 mo for idelalisib + R vs 5.5 mo in placebo arm; P < .001; 20.3 mo (17.3−26.3 mo) at 18-mo follow-up | 20.8 mo (16.6-26.4) for idelalisib arm vs 11.1 mo (8.9-11.1) in placebo arm (P < .0001) at 14-mo follow-up | 16.3 mo (13.6-17.8) in idelalisib plus ofatumumab arm vs 8.0 mo (5.7-8.2) with ofatumumab (P < .0001) | Acalabrutinib monotherapy (PFS NR) vs investigator’s choice (16.5 mo; hazard ratio, 0.31; P < .0001) at 16.1-mo follow-up | mPFS was not reached; PFS at 12, 18, and 24 mo was 92.9%, at 36 mo was 82% (64%-92%) | 23 mo (18-36) | 9.5 mo (8.1-11.8) | 13.3 mo in duvelisib arm vs 9.9 mo in ofatumumab arm (P < .0001) |
| ORR (CRs) in PI3Ki arm | 85.5% (1 patient with CR) | 70% (1%) | 75.3% (1 patient; <1%) | NR separately for idela + R vs BR in investigator’s choice arm | 96.9% (14.1%) | 88.9% (1 patient with CR) | 47.3% (1.6%) entire population SLL, 67.9% FL, 42.2%; MZL: 38.9% | 73.8% (1 patient with CR) |
| OS | mOS was 40.6 mo (28.5-57.3) vs 34.6 mo (16.0-NR) | Not adequately powered to show OS benefit | mOS not reached and not different from control | mOS not reached | mOS not reached; at 36 mo, was 90% (82%-99%) | mOS not reached; at 36 mo, was 88% (68%-96%) | mOS was 28.9 mo (21.4-NE); 1-y OS estimate of 77% | mOS not reached in either arm, with 12-mo OS of 86% (0.65-1.50) for both treatment arms |
| Trial . | Phase 3, idelalisib + rituximab vs rituximab in R/R CLL8,9 . | Phase 3, idelalisib vs placebo + BR in R/R CLL14 . | Phase 3, idelalisib + ofatumumab vs ofatumumab in previously treated CLL13 . | Phase 3, acalabrutinib vs investigator’s choice (BR or idelalisib + rituximab) in R/R CLL10 . | Phase 2, treatment-naïve older patients with CLL, idelalisib + rituximab11 . | Phase 2, treatment-naïve CLL with idelalisib + ofatumumab12 . | Phase 2, DYNAMO trial, double-refractory FL, SLL, MZL22 . | Phase 3, DUO trial, duvelisib vs ofatumumab in R/R CLL/SLL21 . |
|---|---|---|---|---|---|---|---|---|
| Population | R/R CLL | R/R CLL | R/R CLL | R/R CLL | Treatment-naïve older patients with CLL/SLL | Treatment-naïve patients with CLL | iNHL (FL, SLL, or MZL) double-refractory to rituximab + chemoimmunotherapy or radioimmunotherapy | R/R CLL/SLL |
| Treatment | Idelalisib 150 mg by mouth twice daily plus rituximab IV 375 mg/m2 in week 0, day 1, and 500 mg/m2 day 1 of weeks 2, 4, 6, 8, 12, 16, and 20 vs rituximab + placebo | Bendamustine 70 mg/m2 IV on days 1 and 2 C1-6 plus rituximab: 375 mg/m2 on C1D1, and 500 mg/m2 D1C2–6 plus idelalisib 150 mg twice daily vs BR plus placebo | Ofatumumab 300 mg in week 1, day 1 followed by 2000 mg weekly for 7 wk, then every 4 wk for 16 wk vs idelalisib 150 mg by mouth twice daily plus ofatumumab on same week schedule as control group but at 1000 mg from week 2 | Acalabrutinib 100 mg by mouth twice daily vs investigator’s choice of idelalisib 150 mg by mouth twice daily plus rituximab 375 mg/m2 on C1D1 and 500 mg/m2 D1C2–6 or bendamustine 70 mg/m2 IV on days 1 and 2 C1-6 plus rituximab | Idelalisib 150 mg by mouth twice daily plus rituximab 375 mg/m2 IV weekly for 8 wk | Idelalisib 150 mg by mouth twice daily plus ofatumumab 300 mg C3D1 followed by 1000 mg weekly for 7 wk, then 100 mg every 4 wk for 16 wk (6 mo total) | Duvelisib 25 mg by mouth twice daily | Duvelisib 25 mg twice daily or ofatumumab IV for up to 12 doses |
| Number of patients | 220 | 416 | 261 | 398 | 64 | 27 | 129 | 159 |
| Primary endpoint | PFS | PFS | PFS | PFS | ORR | ORR | ORR | PFS |
| mPFS | Not reached at 12 mo for idelalisib + R vs 5.5 mo in placebo arm; P < .001; 20.3 mo (17.3−26.3 mo) at 18-mo follow-up | 20.8 mo (16.6-26.4) for idelalisib arm vs 11.1 mo (8.9-11.1) in placebo arm (P < .0001) at 14-mo follow-up | 16.3 mo (13.6-17.8) in idelalisib plus ofatumumab arm vs 8.0 mo (5.7-8.2) with ofatumumab (P < .0001) | Acalabrutinib monotherapy (PFS NR) vs investigator’s choice (16.5 mo; hazard ratio, 0.31; P < .0001) at 16.1-mo follow-up | mPFS was not reached; PFS at 12, 18, and 24 mo was 92.9%, at 36 mo was 82% (64%-92%) | 23 mo (18-36) | 9.5 mo (8.1-11.8) | 13.3 mo in duvelisib arm vs 9.9 mo in ofatumumab arm (P < .0001) |
| ORR (CRs) in PI3Ki arm | 85.5% (1 patient with CR) | 70% (1%) | 75.3% (1 patient; <1%) | NR separately for idela + R vs BR in investigator’s choice arm | 96.9% (14.1%) | 88.9% (1 patient with CR) | 47.3% (1.6%) entire population SLL, 67.9% FL, 42.2%; MZL: 38.9% | 73.8% (1 patient with CR) |
| OS | mOS was 40.6 mo (28.5-57.3) vs 34.6 mo (16.0-NR) | Not adequately powered to show OS benefit | mOS not reached and not different from control | mOS not reached | mOS not reached; at 36 mo, was 90% (82%-99%) | mOS not reached; at 36 mo, was 88% (68%-96%) | mOS was 28.9 mo (21.4-NE); 1-y OS estimate of 77% | mOS not reached in either arm, with 12-mo OS of 86% (0.65-1.50) for both treatment arms |
C3D1, cycle 3, day 1; C1-6, cycles 1-6; C1D1, cycle 1, day 1; CR, complete response; D1C2-6, day 1 cycles 2-6; FL, follicular lymphoma; iNHL, indolent non-hodgkin lymphoma; mPFS, median progression free survival; mOS, median overall survival; MZL, marginal zone lymphoma; NR, not reached.
In the first reporting, adverse events (AEs) were similar between the idelalisib- and placebo-treated patients, with a 40% incidence of serious AEs (vs 35% in the placebo group) and grade ≥3 AEs occurring in 56% (vs 48%), with only 9 patients (8%) receiving idelalisib discontinuing due to toxicity. However, initial follow-up was short (3.8 months, with only 35% of patients receiving idelalisib >6 months), and at 18-month follow-up, the longer exposure increased the incidence of all-grade and grade ≥3 diarrhea/colitis and pneumonitis, highlighting the risk for early- and late-onset toxicities with PI3Kis.
Initial studies in R/R CLL (Table 1) compared the PI3Kis with chemotherapy or anti-CD20 antibody, considered standard of care at the time; currently patients with R/R CLL have multiple targeted novel therapeutic options, including BTKis (ibrutinib, acalabrutinib). The recently reported ASCEND trial randomized patients with R/R CLL to acalabrutinib or investigator’s choice (idela + R or BR; 77% received idela + R) and demonstrated significantly longer PFS at a median follow-up of 16.1 months with acalabrutinib monotherapy (PFS NR) vs investigator’s choice (16.5 months; hazard ratio, 0.31; P < .0001).10 Because the overall response rate (ORR) was similar between the acalabrutinib and investigator’s choice arms, toxicity and early drug discontinuation may have contributed to the improved PFS with acalabrutinib; AEs led to discontinuation less frequently with acalabrutinib (11%) than with idela + R (47%), and at the time of the data cutoff, 80% continued on acalabrutinib vs only 32% remaining on idelalisib.
In the first frontline idelalisib study, previously untreated patients ≥65 years old (n = 64) received idela + R with an encouraging ORR (97%; 19% complete response), including in patients with high-risk TP53 aberrations (n = 9; ORR, 100%; 33% complete response).11 In treatment-naïve patients receiving idela + R, however, AEs were much higher than in patients with R/R disease.9 In frontline treatment, AST and/or ALT elevations were observed in 67% of all-grade AEs (23% grade ≥3) vs AST elevations in patients with R/R disease in 36% of all-grade AEs (6% grade ≥3) or ALT elevations in patients with R/R disease in 46% of all-grade AEs (9% grade ≥3). Diarrhea and/or colitis in frontline treatment were observed in 64% of all-grade AEs (42% grade ≥ 3) vs diarrhea in patients with R/R disease of 46% of all-grade (16% grade ≥3) or colitis in patients with R/R disease of 11% of all-grade (8% grade ≥3). Similar higher serious AEs were seen in the frontline treatment in the phase 2 idelalisib + ofatumumab12 trial, which led to early closure due to hepatotoxicity (79% with AST/ALT elevations and 54% grade ≥3 AEs).
Toxicities of idelalisib across trials for CLL and other non-Hodgkin lymphomas are detailed in Table 2. In the phases 2 and 3 trials of idelalisib in CLL, grade ≥3 hepatotoxicity occurred in 2% to 54% of patients, grade 2 or higher diarrhea occurred in 5% to 42% of patients, and pneumonitis occurred in 1% to 17% of patients; these were more frequent for idelalisib in the frontline treatment or in combination with drugs besides anti-CD20 immunotherapy.8,11,13-17 In a phase 2 trial of idelalisib and the Syk inhibitor entospletinib for R/R CLL and non-Hodgkin lymphoma, grade ≥3 or higher pneumonitis occurred in 17% of patients (5 patients [8%] required mechanical ventilation and 2 patients died). The combination of idelalisib with lenalidomide appeared especially toxic in 2 Alliance trials for relapsed mantle cell lymphoma and follicular lymphoma, both of which closed early.16
AEs in idelalisib and duvelisib clinical trials for CLL/SLL and indolent lymphomas
| . | Phase 1, R/R CLL, single-agent idelalisib7 . | Phase 1, R/R MCL44 . | Phase 1, R/R MCL and follicular lymphoma, idelalisib, lenalidomide, rituximab16 . | Phase 1, R/R indolent lymphoma, single-agent idelalisib45 . | Phase 2, R/R classical Hodgkin lymphoma, single-agent idelalisib46 . | Phase 2, treatment-naïve older patients with CLL, idelalisib + rituximab11 . |
|---|---|---|---|---|---|---|
| Number of patients | 54 | 40 | 11 | 64 | 25 | 64 |
| AEs | ||||||
| Colitis/diarrhea | 29.6% Grade ≥3, 5.6% | 40% Grade ≥3, 17.5% | 38% | 36% Grade ≥3, 9.4% | 4% | 64% Grade 3, ≥42% |
| Hepatotoxicity | 33-18% Grade ≥3, ∼2% | 60% Grade ≥3, 20% | 63% Grade ≥3, 18% | 53% Grade ≥3, 23% | Grade ≥3, 16% | 67% Grade ≥3, 23% |
| Infections | 44% Grade ≥3, 20% | 32.5% Grade ≥3, 10% | 25%, All grade ≥3 | 36% Grade ≥3, 17.2% | 16% | 44% Grade ≥3, 25% |
| Cutaneous reactions | 22% | 22.5% Grade ≥3, 2.5% | 63% Grade ≥3, 54% | 25% Grade ≥3, 3.1% | 8% | 58% Grade ≥3, 13% |
| Pneumonitis | 4% | 3%, All grade ≥3 | ||||
| Dose reductions | 36% | 45% | ||||
| Drug discontinuation due to AEs | 13% | 18% | 73% | 21% | 8% | 29.7% |
| Phase 2, double-refractory indolent lymphoma, single-agent idelalisib47 | Phase 2, idelalisib + entospletinib in R/R CLL and NHL17 | Phase 2, treatment-naïve CLL with idelalisib + ofatumumab12 | Phase 3, idelalisib + rituximab vs rituximab in R/R CLL8 | Phase 3, idelalisib vs placebo + BR in R/R CLL14 | Phase 3: idelalisib + ofatumumab in previously treated CLL13 | |
| Number of patients | 125 | 66 | 24 | 220 | 416 | 261 |
| AEs | ||||||
| Colitis/diarrhea | 43% Grade ≥3, 16% | 29% Grade ≥3, 2% | 46% Grade ≥3, 17% | 19% Grade ≥3, 4%* | 38% Grade ≥3, 9% | 54% Grade ≥3, 23% |
| Hepatotoxicity | 47% Grade ≥3, 13% | 23% | 79% Grade ≥3, 54% | 35% Grade ≥3, 5%* | 61% Grade ≥3, 21% | 20% Grade ≥3, 13% |
| Infections | 25% Grade ≥3, 7% | ∼18% | Grade ≥3, 13%* | 32% Grade ≥3, 12.5%-16% | 78% Grade ≥3, 22% | |
| Cutaneous reactions | 13% Grade ≥3, 2% | 30% Grade ≥3, ∼17% | 10% Grade ≥3, 3%* | 16% Grade ≥3, 3% | 18% Grade ≥3, 1% | |
| Pneumonitis | 17%; study terminated early | 13% | 4%* | 1.4% | 6% Grade ≥3, 5% | |
| Dose reductions | 34% | 13% | 58% | |||
| Drug discontinuation due to AEs | 20% | Study terminated early due to AEs | 5% | 27% | 39% | |
| Phase 1, duvelisib monotherapy, R/R CLL48 | Phase 1, duvelisib monotherapy, treatment-naïve CLL48 | Phase 1, duvelisib monotherapy, R/R CLL, iNHL, TCL49 | Phase 1, duvelisib monotherapy, R/R TCL50 | Phase 1, duvelisib monotherapy, R/R iNHL51 | Phase 1, duvelisib + rituximab vs BR, CLL or iNHL52 | |
| Number of patients | 55 | 18 | 210 | 35 | 31 | 46 |
| AEs | ||||||
| Colitis/diarrhea | 47.3% Grade ≥3, 9.1% | 77.8% Grade ≥3, 22.2% | 41.9% Grade ≥3, 11.4% | 31% | 54.8% Grade ≥3, 25.8% | 37% Grade ≥3, 13% |
| Hepatotoxicity | 30.9% Grade ≥3, 10.9% | 33.3% Grade ≥3, 16.7% | 38.6% Grade ≥3, 19.5% | 57% Grade ≥3, 40% | 58.1% Grade ≥3, 38.7% | 21.7% Grade ≥3, 6.5% |
| Infections | >62% Grade ≥3, >23.6% | 22% | 29.5% Grade ≥3, 105 | 23% Grade ≥3, 17% | 19.4% | 34.7% Grade ≥3, 6.5% |
| Cutaneous reactions | 18.2% | 38.9% Grade ≥3, 5.6% | 16.2% Grade ≥3, 5.2% | 23% Grade ≥3, 17% | 42% Grade ≥3, 6.5% | 41.3% Grade ≥3, 19.6% |
| Pneumonitis | 7% | 11% | 4% | 6.5% | ||
| Dose reductions | ||||||
| Drug discontinuation due to AEs | 36% | 33% | ∼30% | 37% | 19% | 23.9% |
| Phase 2, DYNAMO trial, double-refractory FL, SLL, MZL22 | Phase 3 DUO trial, duvelisib vs ofatumumab, R/R CLL/SLL21 | |||||
| Number of patients | 129 | 319 | ||||
| AEs | ||||||
| Colitis/diarrhea | 56.6% Grade ≥3, 20.1 | 64% Grade ≥3, 27% | ||||
| Hepatotoxicity | 14% Grade ≥3, 5.4% | Grade ≥3, 3% | ||||
| Infections | 7.8% Grade ≥3, 5.4% | 48% Grade ≥3 | ||||
| Cutaneous reactions | 18.6% Grade ≥3, 4.7% | 10% Grade ≥3, 2% | ||||
| Pneumonitis | 4.7% | 3% | ||||
| Dose reductions | 66% | |||||
| Drug discontinuation due to AEs | 24% | 35% |
| . | Phase 1, R/R CLL, single-agent idelalisib7 . | Phase 1, R/R MCL44 . | Phase 1, R/R MCL and follicular lymphoma, idelalisib, lenalidomide, rituximab16 . | Phase 1, R/R indolent lymphoma, single-agent idelalisib45 . | Phase 2, R/R classical Hodgkin lymphoma, single-agent idelalisib46 . | Phase 2, treatment-naïve older patients with CLL, idelalisib + rituximab11 . |
|---|---|---|---|---|---|---|
| Number of patients | 54 | 40 | 11 | 64 | 25 | 64 |
| AEs | ||||||
| Colitis/diarrhea | 29.6% Grade ≥3, 5.6% | 40% Grade ≥3, 17.5% | 38% | 36% Grade ≥3, 9.4% | 4% | 64% Grade 3, ≥42% |
| Hepatotoxicity | 33-18% Grade ≥3, ∼2% | 60% Grade ≥3, 20% | 63% Grade ≥3, 18% | 53% Grade ≥3, 23% | Grade ≥3, 16% | 67% Grade ≥3, 23% |
| Infections | 44% Grade ≥3, 20% | 32.5% Grade ≥3, 10% | 25%, All grade ≥3 | 36% Grade ≥3, 17.2% | 16% | 44% Grade ≥3, 25% |
| Cutaneous reactions | 22% | 22.5% Grade ≥3, 2.5% | 63% Grade ≥3, 54% | 25% Grade ≥3, 3.1% | 8% | 58% Grade ≥3, 13% |
| Pneumonitis | 4% | 3%, All grade ≥3 | ||||
| Dose reductions | 36% | 45% | ||||
| Drug discontinuation due to AEs | 13% | 18% | 73% | 21% | 8% | 29.7% |
| Phase 2, double-refractory indolent lymphoma, single-agent idelalisib47 | Phase 2, idelalisib + entospletinib in R/R CLL and NHL17 | Phase 2, treatment-naïve CLL with idelalisib + ofatumumab12 | Phase 3, idelalisib + rituximab vs rituximab in R/R CLL8 | Phase 3, idelalisib vs placebo + BR in R/R CLL14 | Phase 3: idelalisib + ofatumumab in previously treated CLL13 | |
| Number of patients | 125 | 66 | 24 | 220 | 416 | 261 |
| AEs | ||||||
| Colitis/diarrhea | 43% Grade ≥3, 16% | 29% Grade ≥3, 2% | 46% Grade ≥3, 17% | 19% Grade ≥3, 4%* | 38% Grade ≥3, 9% | 54% Grade ≥3, 23% |
| Hepatotoxicity | 47% Grade ≥3, 13% | 23% | 79% Grade ≥3, 54% | 35% Grade ≥3, 5%* | 61% Grade ≥3, 21% | 20% Grade ≥3, 13% |
| Infections | 25% Grade ≥3, 7% | ∼18% | Grade ≥3, 13%* | 32% Grade ≥3, 12.5%-16% | 78% Grade ≥3, 22% | |
| Cutaneous reactions | 13% Grade ≥3, 2% | 30% Grade ≥3, ∼17% | 10% Grade ≥3, 3%* | 16% Grade ≥3, 3% | 18% Grade ≥3, 1% | |
| Pneumonitis | 17%; study terminated early | 13% | 4%* | 1.4% | 6% Grade ≥3, 5% | |
| Dose reductions | 34% | 13% | 58% | |||
| Drug discontinuation due to AEs | 20% | Study terminated early due to AEs | 5% | 27% | 39% | |
| Phase 1, duvelisib monotherapy, R/R CLL48 | Phase 1, duvelisib monotherapy, treatment-naïve CLL48 | Phase 1, duvelisib monotherapy, R/R CLL, iNHL, TCL49 | Phase 1, duvelisib monotherapy, R/R TCL50 | Phase 1, duvelisib monotherapy, R/R iNHL51 | Phase 1, duvelisib + rituximab vs BR, CLL or iNHL52 | |
| Number of patients | 55 | 18 | 210 | 35 | 31 | 46 |
| AEs | ||||||
| Colitis/diarrhea | 47.3% Grade ≥3, 9.1% | 77.8% Grade ≥3, 22.2% | 41.9% Grade ≥3, 11.4% | 31% | 54.8% Grade ≥3, 25.8% | 37% Grade ≥3, 13% |
| Hepatotoxicity | 30.9% Grade ≥3, 10.9% | 33.3% Grade ≥3, 16.7% | 38.6% Grade ≥3, 19.5% | 57% Grade ≥3, 40% | 58.1% Grade ≥3, 38.7% | 21.7% Grade ≥3, 6.5% |
| Infections | >62% Grade ≥3, >23.6% | 22% | 29.5% Grade ≥3, 105 | 23% Grade ≥3, 17% | 19.4% | 34.7% Grade ≥3, 6.5% |
| Cutaneous reactions | 18.2% | 38.9% Grade ≥3, 5.6% | 16.2% Grade ≥3, 5.2% | 23% Grade ≥3, 17% | 42% Grade ≥3, 6.5% | 41.3% Grade ≥3, 19.6% |
| Pneumonitis | 7% | 11% | 4% | 6.5% | ||
| Dose reductions | ||||||
| Drug discontinuation due to AEs | 36% | 33% | ∼30% | 37% | 19% | 23.9% |
| Phase 2, DYNAMO trial, double-refractory FL, SLL, MZL22 | Phase 3 DUO trial, duvelisib vs ofatumumab, R/R CLL/SLL21 | |||||
| Number of patients | 129 | 319 | ||||
| AEs | ||||||
| Colitis/diarrhea | 56.6% Grade ≥3, 20.1 | 64% Grade ≥3, 27% | ||||
| Hepatotoxicity | 14% Grade ≥3, 5.4% | Grade ≥3, 3% | ||||
| Infections | 7.8% Grade ≥3, 5.4% | 48% Grade ≥3 | ||||
| Cutaneous reactions | 18.6% Grade ≥3, 4.7% | 10% Grade ≥3, 2% | ||||
| Pneumonitis | 4.7% | 3% | ||||
| Dose reductions | 66% | |||||
| Drug discontinuation due to AEs | 24% | 35% |
MCL, mantle cell lymphoma; TCL, T-cell lymphoma.
In longer follow-up (median, 18 mo),9 prolonged exposure saw increases in any grade, grade 2, and grade ≥3 diarrhea (46.4%, 17.3%, and 16.4%, respectively); any grade and grade ≥3 colitis (10.9% and 8.2%, respectively); and any grade and grade ≥ 3 pneumonitis, respectively (10.0% and 6.4%). The incidence of elevated hepatic aminotransferases did not increase with time.
Toxicities from idelalisib may be higher for patients treated outside of clinical trials. A cohort study compared outcomes of Medicare beneficiaries aged ≥65 years treated with idelalisib for R/R follicular lymphoma and R/R CLL with those of patients in clinical trials.18 The Medicare patients with CLL outside of trials were older, had more comorbidities, and had a significantly shorter time on treatment (173 days vs 472 days; P < .001) and a significantly higher fatal infection rate (18.4 vs 9.8 per 100 person-years; P = .04). In this study, the use of Pneumocystis jirovecii pneumonia (PJP) prophylaxis, despite recommendations for use, was low (∼33%), and dose reductions were significantly lower than in many trials. Several large multicenter retrospective studies also reported higher “real-world” discontinuation rates of idelalisib due to toxicities.19,20
Duvelisib demonstrated efficacy in high-risk R/R CLL in the phase 3 DUO trial randomizing patients (n = 319) 1:1 to duvelisib 25 mg twice daily vs ofatumumab. At a median follow-up of 22.4 months, a significant improvement in the primary endpoint of independent review committee–assessed PFS (13.3 months vs 9.9 months; P < .0001) was observed, including in patients with TP53 aberrations (n = 31; P = .0002); ORR was also significantly higher in the duvelisib arm (74% vs 45%; P < .0001).21
The toxicity profile of duvelisib in clinical trials has been comparable to idelalisib in the R/R CLL/SLL population. In DUO, grade 3+ AEs occurred in 87% of duvelisib-treated patients (vs 48% in ofatumumab-treated patients), with the most common grade 3+ AEs being neutropenia (30%), diarrhea (15%), and pneumonia (14%). Grade ≥3 AEs of special interest included colitis (12%), AST and/or ALT increase (3% each), and pneumonitis (3%). It is notable that in this trial, discontinuation due to AEs was higher than discontinuation due to disease progression (35% vs 22%).21,22
Across phases 1 to 3 trials of idelalisib and duvelisib (Table 2), ∼5% to 73% of patients discontinued drug due to AEs, and toxicity was the most common reason for drug discontinuation in many studies.23 Correlatives support an immune-mediated mechanism for many of the severe toxicities observed with idelalisib and duvelisib. In the phase 2 frontline idela + R trial,11 there was an infiltration of T lymphocytes in colonic biopsies performed for persistent diarrhea, and in the frontline idelalisib + ofatumumab trial, lymphoid aggregates and increased activated CD3+ T cells were present in liver biopsies of patients with persistent transaminitis.12 In addition, elevated proinflammatory cytokines/chemokines were higher in frontline idelalisib patients with hepatotoxicity12 and in idelalisib plus entospletinib patients with pneumonitis.17 Idelalisib can preferentially inhibit regulatory T cells (Tregs) important for self-tolerance, leading to unchecked T-effector cells.24,25 Both CD4+ and CD8+ T-cell numbers remain less than half of pretreatment levels for several years after chemoimmunotherapy. The increased number and function of the T-cell repertoire in treatment-naïve patients with CLL may explain the increased immune-related toxicity with PI3Kis in this setting.12,26
Management of common toxicities for idelalisib and duvelisib
Idelalisib carries a black box warning for hepatotoxicity, severe diarrhea/colitis, pneumonitis, infection, and intestinal perforation.27 Similarly, duvelisib has a black box warning for infections, diarrhea/colitis, cutaneous reactions, and pneumonitis.28 Here, we review recommendations for the management of the most common and severe toxicities observed in patients treated with the approved PI3Kis in CLL: idelalisib and duvelisib. These recommendations incorporate product labels27,28 and prior expert guidelines, safety reports, and special considerations within the National Comprehensive Cancer Network guidelines.29-35 Where data are lacking or evolving, our approach to practical management in these scenarios is also included. Treatment with PI3Kis in clinical trials should always follow protocol-specific grading and management.
Neutropenia and infections
Neutropenia is common during the first months of PI3Ki use (∼50% any grade; grade 3 or 4 in >20%). Though several idelalisib trials employed growth factor support for grade 4 neutropenia, interruption or discontinuation due to neutropenia was rare.29 Monitoring with complete blood count is recommended every 2 weeks for the first 3 months of PI3Ki treatment and weekly if grade ≥3 neutropenia occurs.
Severe and fatal infections, including PJP and cytomegalovirus (CMV), have been observed, mainly in patients without prophylaxis. Therefore, patients should receive sulfamethoxazole/trimethoprim (preferred agent) or equivalent PJP prophylaxis until 2 to 6 months after cessation of the PI3Ki; an alternative approach is to use a quantitative measure of CD4+ count >200/μL to account for differences in recovery after treatment. Because sulfamethoxazole/trimethoprim can be associated with myelosuppression, patients with refractory cytopenias while receiving PI3Kis should consider alternative PJP prophylaxis (options and National Comprehensive Cancer Network guidelines). CMV monitoring at baseline and approximately monthly while receiving PI3Kis should be employed, with consultation with an infectious disease specialist for treatment if CMV RNA is >100 000 copies or rising over several measurements. Acyclovir or an equivalent is often recommended for viral prophylaxis, given cases of severe cutaneous or systemic varicella zoster virus reaction. Baseline HIV, hepatitis B virus, and hepatitis C virus status is important, given the risk for severe infections and hepatotoxicity while receiving PI3Kis; if the patient has a positive test result, gastrointestinal and infectious disease specialist consultation should occur before starting the PI3Ki.
Diarrhea/colitis
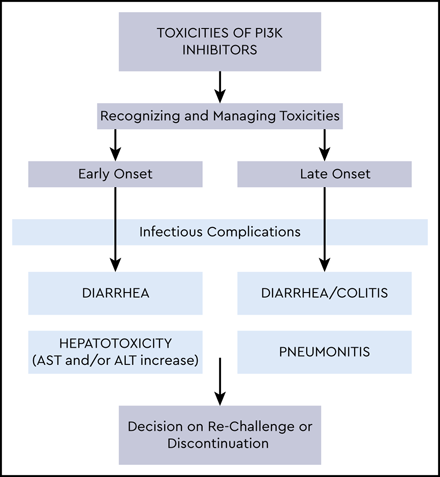
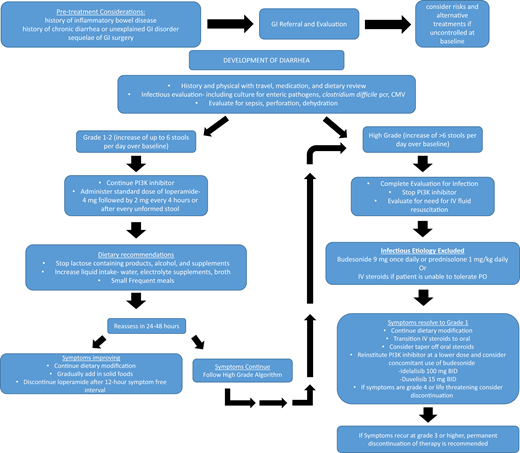
Diarrhea is a common AE with use of PI3Kis, occurring in 4% to 77% across all grades (5% to 42% grade ≥3) and more commonly in treatment-naïve patients (Figure 1, Table 2). Colitis was defined separately from diarrhea in some trials when evidence of inflammation was seen on mucosal biopsies; however, biopsy was not required, and often the terms were reported together or overlapping. Two distinct entities of diarrhea/colitis occurring during PI3Ki use are recognized. The first, usually within the first 8 weeks, is often responsive to supportive care and antimotility agents. In contrast, late-onset (median time, ∼7 months) diarrhea/colitis is typically more severe and believed to be immune mediated.29 In all incidences, development of diarrhea while using a PI3Ki should be evaluated urgently with close follow-up until improvement. Though some consider recommendations for diarrhea/colitis management on the basis of traditional toxicity grading severity (ie, grade 1 or 2 or grade ≥3 by Common Terminology Criteria for Adverse Events criteria),36 grade 2 late-onset diarrhea not responding to antimotility agents after 36 to 48 hours is significant and should be managed as a higher-grade event with withholding of the PI3Ki while completing workup and supportive care. For grade 4 or life-threatening diarrhea, the PI3Ki should be discontinued permanently. Though rechallenge (including overlapping with steroids) can be successful in select cases of grade 3 or grade 2 AEs of late onset/refractory to antimotility agents, we include a full discussion of alternative treatment options, including clinical trials, before rechallenge in these cases, given the risk of diarrhea/colitis recurrence.
Hepatotoxicity
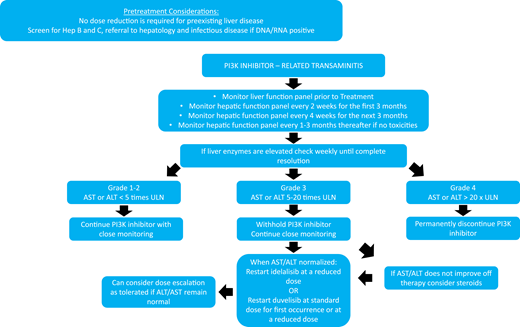
Hepatotoxicity, characterized by a hepatocellular type of injury (elevated AST or ALT rather than elevated bilirubin or alkaline phosphatase), is among the most commonly reported PI3Ki AEs; it was noted in 14% to 70% of patients with grade ≥3 AEs in 3% to 40% of patients across all trials (Figure 2, Table 2) and was more common in treatment-naïve patients. Unlike PI3Ki-associated diarrhea and pneumonitis, which can increase in incidence with longer drug exposure, hepatotoxicity is most often seen during the first 12 weeks with grade ≥3 AE occurrence, plateauing by 20 weeks in the phase 3 R/R disease idela + R trial.9
Given this risk and timing, hepatic function testing is recommended every 2 weeks during the first 3 months, then monthly for 3 months, and then every 1 to 3 months thereafter, depending on any toxicities that develop. A monitoring and treatment algorithm for hepatotoxicity based on AST/ALT elevation over the upper limit of normal is outlined in Figure 2. Severe and fatal cases of hepatotoxicity have occurred, but most AST/ALT increases resolve with withholding of the PI3K and supportive care.
Pneumonitis
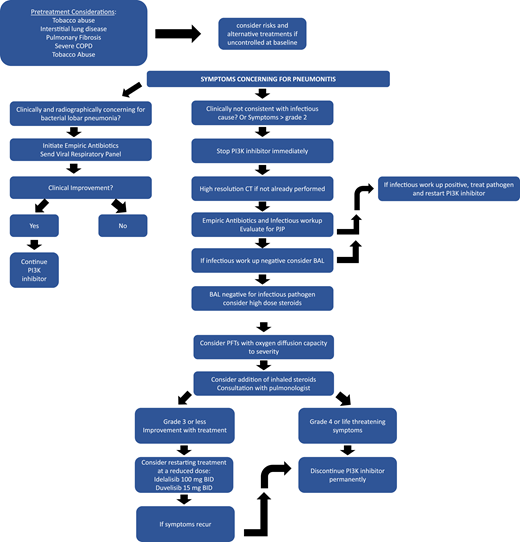
Noninfectious, likely immune-mediated pneumonitis characterized by acute/subacute cough, dyspnea, and/or fever (similar to reports of hypersensitivity pneumonitis with mammalian target of rapamycin inhibitors)29 occurred in 1.4% to 17% PI3Ki-treated patients, with some cases being fatal (Figure 3). A chest x-ray can show bilateral infiltrates, and a computed tomographic scan can demonstrate diffuse ground-glass opacities; alveolar consolidations; and, in some cases, pleural effusions. Given that these findings are nonspecific and that infectious complications are common with a PI3Ki, patients should receive empiric antibiotics and appropriate infectious evaluation (considering bacterial, viral, and opportunistic infections, including PJP), with pneumonitis as a diagnosis of exclusion. High-dose corticosteroids while withholding the PI3Ki may be helpful in severe cases.29,37
Return to the clinical case
The patient developed grade 3 hepatotoxicity (Figure 2; AST/ALT, 5 to 20 times the upper limit of normal), and the idelalisib was withheld. His AST/ALT trended toward normal over the course of 2 weeks without intervention, and idelalisib was successfully reinitiated at a reduced 100-mg twice-daily dose. Eight months later, however, he presented after 2 days of profuse diarrhea (8 to 10 stools per day), requiring hospitalization. Despite his improvement to grade 1 while withholding idelalisib plus providing supportive care and oral budesonide, his diarrhea recurred on rechallenge, and idelalisib was permanently discontinued (Figure 1).
Patients stopping PI3Ki for toxicity can be sequenced successfully to other targeted therapies, including ibrutinib or venetoclax.20,38 In this case, the patient had already experienced severe ibrutinib toxicity (subdural hemorrhage) that would raise concern about returning to BTKi treatment; venetoclax remained an approved therapeutic option. Thirty-six patients treated in a trial with venetoclax after idelalisib had a 67% ORR and a 12-month PFS of 79%.39 However, patients do not always require next-line therapy immediately after treatment is discontinued for intolerance and should be followed until International Workshop on Chronic Lymphocytic Leukemia treatment indications are present.40
The patient was monitored for several months after cessation of idelalisib until he required treatment and was started on venetoclax therapy. With his tumor lysis syndrome risk and reduced renal function, he met criteria for hospitalization during the venetoclax dose escalation.
Moving forward: future directions for PI3Kis in CLL/SLL
Next-generation PI3Kis (Table 3) may have improved tolerability through different off-target effects and/or employing alternative dosing. Umbralisib (TGR-1202), a selective PI3Kδ inhibitor, demonstrated favorable safety and ORR in patients with CLL/SLL in a phase 1 trial for R/R hematologic malignancies (n = 20; 50% objective response by International Workshop on Chronic Lymphocytic Leukemia criteria) and led to a randomized phase 3 frontline CLL/SLL study of umbralisib plus the anti-CD20 antibody ublituximab vs obinutuzumab plus chlorambucil. A press release announced meeting the primary endpoint (PFS), but data were pending at the time of publication of the present article. Preclinical work hypothesizes that CK1ε, inhibited also by umbralisib, may prevent depletion of Tregs and limit immune toxicities. In the phase 2 trial of umbralisib, which included 49 patients with previous intolerance to idelalisib or a BTKi, 58% of patients received umbralisib longer than the original tyrosine kinase inhibitor (median follow-up, 15.7 months) with an estimated median PFS of 23.5 months; only 6 patients discontinued due to umbralisib AEs in a population defined entirely by prior drug intolerance.41
Currently approved and select investigational PI3Kis in CLL and other hematologic malignancies
| Drug . | PI3K isoform selectivity . | Status . | Approved dosing . |
|---|---|---|---|
| Idelalisib | PI3Kδ | FDA approved for patients with R/R CLL for whom rituximab monotherapy is appropriate and in patients with SLL or FL after ≥2 prior therapies | 150 mg by mouth twice daily |
| Duvelisib | PI3Kγ/δ | FDA approved for R/R CLL, SLL, and FL after ≥2 prior therapies | 25 mg by mouth twice daily |
| Copanlisib | PI3Kα/δ | FDA approved for relapsed follicular lymphoma after ≥2 prior therapies | 60 mg IV on days 1, 8, and 15 of a 28-d treatment cycle |
| Umbralisib | PI3Kδ | Investigational | Not applicable |
| MEI-401 | PI3Kδ | Investigational | Not applicable |
| Parsaclisib | PI3Kδ | Investigational | Not applicable |
| Drug . | PI3K isoform selectivity . | Status . | Approved dosing . |
|---|---|---|---|
| Idelalisib | PI3Kδ | FDA approved for patients with R/R CLL for whom rituximab monotherapy is appropriate and in patients with SLL or FL after ≥2 prior therapies | 150 mg by mouth twice daily |
| Duvelisib | PI3Kγ/δ | FDA approved for R/R CLL, SLL, and FL after ≥2 prior therapies | 25 mg by mouth twice daily |
| Copanlisib | PI3Kα/δ | FDA approved for relapsed follicular lymphoma after ≥2 prior therapies | 60 mg IV on days 1, 8, and 15 of a 28-d treatment cycle |
| Umbralisib | PI3Kδ | Investigational | Not applicable |
| MEI-401 | PI3Kδ | Investigational | Not applicable |
| Parsaclisib | PI3Kδ | Investigational | Not applicable |
FDA, U.S. Food and Drug Administration.
ME-401 is a selective PI3Kδ inhibitor with longer PI3Kδ occupancy.42 Considering the pharmacokinetics/pharmacodynamics and observed toxicities in the phase 1 dose escalation studies, intermittent dosing after continuous induction has demonstrated promising reduction in observed immune-mediated toxicities, potentially through allowing Treg recovery (grade ≥3 in 34% with continuous dosing vs 12% with continuous to intermittent dosing strategy) while maintaining efficacy.43
CLL/SLL remains an incurable disease for most patients, despite recent targeted therapy approvals, leaving an unmet need for patients with resistance, intolerance, and/or comorbidities that complicate available treatment options. Given the effectiveness of targeting the PI3K pathway, efforts remain critical to consider best use of current and next-generation PI3Kis in development and alternative PI3Ki regimens, including novel–novel combinations, fixed duration, and intermittent dosing to improve toxicities (Table 4). Even as these strategies are studied, early recognition of and intervention for PI3Ki toxicities remains crucial to mitigate risks and maintain meaningful disease control without compromising quality of life.
Select clinical trials of PI3K in CLL/SLL or other hematologic malignancies with focus on next-generation agents, novel–novel combinations, and/or alternative dosing
| PI3Ki . | Phase (ClinicalTrials.gov identifier) . | Population . | Dosing/combination . | Notes . |
|---|---|---|---|---|
| Duvelisib | Phase 1 (NCT03534323) | R/R CLL | Duvelisib plus venetoclax | Fixed duration; dosing can be stopped if reaching MRD negativity at 1 y |
| Duvelisib | Phase 2 (NCT03961672) | R/R CLL | Duvelisib is administered at standard dosing during a 3-mo induction followed by twice-weekly maintenance | Intermittent dosing after induction continuous cycles |
| Umbralisib | Phase 3 UNITY (NCT02612311) | Frontline CLL | Umbralisib + ublituximab vs obinutuzumab + chlorambucil | Press release for meeting primary endpoint |
| Umbralisib | Phase 1 (NCT02268851) | R/R CLL and MCL | Umbralisib and ibrutinib | First clinical data on safety of doublet BTKi and PI3Kδi53 |
| Umbralisib | Phase 1 ((NCT02006485) | Frontline and R/R CLL/SLL and B-cell NHL | Umbralisib, ublituximab, and ibrutinib | Triplet therapy combination safety data for anti-CD20 plus BTKi and PI3Kδi54 |
| Umbralisib | Phase 2 (NCT04016805) | Patients with CLL currently on ibrutinib or venetoclax | Umbralisib and ublituximab | Addition of PI3Ki combination to increase MRD rates with addition of umbralisib and ublituximab to ibrutinib or venetoclax |
| Umbralisib | Phase 2 (NCT03801525) | Frontline CLL | Ublituximab, umbralisib, and venetoclax | Frontline triplet with limited treatment duration |
| ME-401 | Phase 1 | ME-401 as monotherapy or in combination with R | Alternate dosing strategy (continuous → intermittent dosing) effective with reduced toxicities43 | |
| ME-401 | Phase 1 (NCT02914938) | R/R CLL/SLL or B-cell NHL | ME-401 alone or in combination with rituximab or zanubrutinib in R/R CLL/SLL or other |
| PI3Ki . | Phase (ClinicalTrials.gov identifier) . | Population . | Dosing/combination . | Notes . |
|---|---|---|---|---|
| Duvelisib | Phase 1 (NCT03534323) | R/R CLL | Duvelisib plus venetoclax | Fixed duration; dosing can be stopped if reaching MRD negativity at 1 y |
| Duvelisib | Phase 2 (NCT03961672) | R/R CLL | Duvelisib is administered at standard dosing during a 3-mo induction followed by twice-weekly maintenance | Intermittent dosing after induction continuous cycles |
| Umbralisib | Phase 3 UNITY (NCT02612311) | Frontline CLL | Umbralisib + ublituximab vs obinutuzumab + chlorambucil | Press release for meeting primary endpoint |
| Umbralisib | Phase 1 (NCT02268851) | R/R CLL and MCL | Umbralisib and ibrutinib | First clinical data on safety of doublet BTKi and PI3Kδi53 |
| Umbralisib | Phase 1 ((NCT02006485) | Frontline and R/R CLL/SLL and B-cell NHL | Umbralisib, ublituximab, and ibrutinib | Triplet therapy combination safety data for anti-CD20 plus BTKi and PI3Kδi54 |
| Umbralisib | Phase 2 (NCT04016805) | Patients with CLL currently on ibrutinib or venetoclax | Umbralisib and ublituximab | Addition of PI3Ki combination to increase MRD rates with addition of umbralisib and ublituximab to ibrutinib or venetoclax |
| Umbralisib | Phase 2 (NCT03801525) | Frontline CLL | Ublituximab, umbralisib, and venetoclax | Frontline triplet with limited treatment duration |
| ME-401 | Phase 1 | ME-401 as monotherapy or in combination with R | Alternate dosing strategy (continuous → intermittent dosing) effective with reduced toxicities43 | |
| ME-401 | Phase 1 (NCT02914938) | R/R CLL/SLL or B-cell NHL | ME-401 alone or in combination with rituximab or zanubrutinib in R/R CLL/SLL or other |
MRD, minimal residual disease defined as by less than one CLL cell in the peripheral blood or bone marrow per 10,000 leukocytes (<10-4)
Acknowledgment
A.H. is supported by an institutional NIH T32 training grant.
References
Competing Interests
Conflict-of-interest disclosure: D.M.B. discloses the following: AbbVie: consultant, scientific advisory board, site principal investigator (PI) of a clinical trial (grant paid to institution); ArQule: scientific advisory board, site PI of a clinical trial (grant paid to institution); Ascentage Pharma: site PI of a clinical trial (grant paid to institution); AstraZeneca: consultant, site PI of a clinical trial (grant paid to institution); BeiGene: site PI of a clinical trial (grant paid to institution); DTRM Biopharma: site PI of a clinical trial (grant paid to institution); Genentech: consultant, scientific advisory board, site PI of a clinical trial (grant paid to institution); Juno/Celgene/Bristol-Myers Squibb: site PI of a clinical trial (grant paid to institution); MEI Pharma: site PI of a clinical trial (grant paid to institution); Pharmacyclics: consultant, site PI of a clinical trial (grant paid to institution); Pfizer: consultant, scientific advisory board; Teva: consultant; TG Therapeutics: scientific advisory board, site PI of a clinical trial (grant paid to institution); Tolero Pharmaceuticals: site PI of a clinical trial (grant paid to institution); and Verastem Oncology: consultant, scientific advisory board, site PI of a clinical trial (grant paid to institution). Other guidelines/registry memberships (when sponsored or consultant also included under sponsor above): National Comprehensive Cancer Network panel member, informCLL Registry steering committee (AbbVie), Observational Trial of Real-World Treatment Utilization and Effectiveness of PI3K-inhibitors in CLL/SLL and FL (REAL) registry steering committee (Verastem Oncology), and a biosimilars outcomes research panel (Pfizer). A.H. declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.
CorrespondenceDanielle M. Brander, Duke University Health System, DUMC 3961, 2400 Pratt St, Suite 5000, Durham, NC 27705; e-mail: danielle.brander@duke.edu.