Abstract
Women with bleeding disorders suffer from multiple bleeding symptoms, including easy bruising, epistaxis, bleeding from minor wounds and the oral cavity, and bleeding after dental work or surgery. However, women with bleeding disorders especially suffer from gynecologic and obstetrical bleeding. These symptoms often are not recognized as abnormal, and many women are left undiagnosed and without access to appropriate medical care. Additional challenges to diagnosing women with bleeding disorders include lack of access to appropriate laboratory testing and issues around disease classification and nomenclature. Efforts have been undertaken to address these challenges, including the development and validation of bleeding assessment tools and strategies to clarify diagnostic thresholds and algorithms for von Willebrand disease (VWD) and platelet function disorders. Efforts to improve communication with the nomenclature used for hemophilia carriers are also underway.
Learning Objectives
Understand the burden of disease for women with bleeding disorders
Explore the barriers facing women in achieving an accurate bleeding disorder diagnosis
Clinical case
An 18-year-old woman seeks medical attention from her rural family physician for heavy periods. They typically last 8 to 10 days, with the heaviest bleeding on days 2 and 3. She has struggled with iron deficiency since menarche and had excessive bleeding following extraction of wisdom teeth at age 14 that required a return to the dentist. She bruises easily, and her gums bleed when she brushes her teeth. She has no family history of bleeding disorders.
Background
Up to 30% of all women report heavy menstrual bleeding (HMB) at some point during their reproductive years and up to half seek medical attention for this symptom.1,2 Multiple studies have shown that fully 15% to 30% of those with HMB have an underlying inherited bleeding disorder.2-4 Among women known to have a bleeding disorder, HMB is the most common symptom.3 These women are also at risk for postpartum hemorrhage and postoperative bleeding as well as hemorrhagic ovarian cysts.5 HMB is defined as loss of more than 80 mL menstrual blood per cycle; however, this is difficult to determine clinically. Studies have shown that having to change sanitary protection more often than every hour, soaking through pajamas and sheets at night, passing clots >1 inch in diameter, and low ferritin all correlate with menstrual blood loss of more than 80 mL.6 HMB is an important cause of work and school absenteeism and historically has led to two thirds of the hysterectomies in women of reproductive age.3,5 It has also been shown to have a profoundly negative impact on quality of life.7
The population prevalence of a symptomatic inherited bleeding disorder is approximately 1 in 10008,9 ; however, far fewer have ever been diagnosed. In Canada, a predicted 37 500 are affected but only ∼8000 (∼20%) have been diagnosed (http://fhs.mcmaster.ca/chr/; data up to the end of 2018). This problem particularly affects women, and in addition to patients not being diagnosed, those who are diagnosed report delays of up to 15 years after the onset of symptoms until they receive appropriate medical attention.10 There are multiple barriers to diagnosis, including the lack of recognition of the difference between normal and abnormal bleeding (especially gynecologic and obstetric), challenges in terms of laboratory testing, and issues around disease classification and nomenclature.
Bleeding assessment tools
In the past decade, significant work has been done to develop and validate bleeding assessment tools (BATs).11-14 BATs are questionnaires about bleeding that result in a quantitative bleeding score. In addition to standardizing a bleeding history, BATs have been shown to accurately distinguish normal from abnormal bleeding.15 Several studies have validated an abnormal bleeding score as a screening test for inherited bleeding disorders, particularly von Willebrand disease (VWD).11-14 In addition, BATs have been shown to be an effective measure of bleeding severity in patients known to have a bleeding disorder.12,14-17 BATs contain a series of questions about the presence or absence of bleeding symptoms and the level of medical attention and treatment required for each (ie, epistaxis that required medical consultation with a medical professional, treatment with desmopressin, or blood transfusion). Each symptom is then scored, with higher levels of medical intervention receiving a higher score. An overall bleeding score is then calculated by adding together the scores for each symptom (see Table 1 for an example of a BAT scoring key).
ISTH-BAT scoring key for bleeding episodes
| Type of bleeding . | BAT score . | ||||
|---|---|---|---|---|---|
| 0 . | 1 . | 2 . | 3 . | 4 . | |
| Epistaxis | None/trivial | >5 per year or more than 10 min | Consultation only | Packing, cauterization, or antifibrinolytics | Blood transfusion or replacement therapy (use of hemostatic blood components or rFVIIa) or desmopressin |
| Cutaneous | None/trivial | ≥5 bruises (>1 cm) in exposed areas | Consultation only | Extensive | Spontaneous hematoma requiring blood transfusion |
| Bleeding from minor wounds | None/trivial | >5 per year or more than 10 min | Consultation only | Surgical hemostasis | Blood transfusion, replacement therapy, or desmopressin |
| Oral cavity | None/trivial | Present | Consultation only | Surgical hemostasis or antifibrinolytics | Blood transfusion, replacement therapy, or desmopressin |
| Gastrointestinal bleeding | None/trivial | Present (not associated with ulcer, portal hypertension, hemorrhoids, angiodysplasia) | Consultation only | Surgical hemostasis, antifibrinolytics | Blood transfusion, replacement therapy, or desmopressin |
| Hematuria | None/trivial | Present (macroscopic) | Consultation only | Surgical hemostasis, iron therapy | Blood transfusion, replacement therapy, or desmopressin |
| Tooth extraction | None/trivial or none performed | Reported in ≤25% of all procedures, no intervention | Reported in >25% of all procedures, no intervention | Resuturing or packing | Blood transfusion, replacement therapy, or desmopressin |
| Surgery | None/trivial or none performed | Reported in ≤25% of all procedures, no intervention | Reported in >25% of all procedures, no intervention | Surgical hemostasis or antifibrinolytics | Blood transfusion, replacement therapy or desmopressin |
| Menorrhagia | None/trivial | Consultation only or changing pads more frequently than once every 2 hours or clot and flooding or pictorial bleeding assessment chart score >100 | Time off work or school more than twice per year or requiring antifibrinolytics or hormonal or iron therapy | Requiring combined treatment with antifibrinolytics and hormonal therapy or present since menarche and for more than 12 months | Acute menorrhagia requiring admission and emergency treatment or requiring blood transfusion, replacement therapy, desmopressin, or requiring dilatation and curettage or endometrial ablation or hysterectomy |
| Postpartum hemorrhage | None/trivial or no deliveries | Consultation only or use of syntocin or lochia for >6 weeks | Iron therapy or antifibrinolytics | Requiring blood transfusion, replacement therapy, desmopressin or requiring examination under anesthesia and/or the use of a uterine balloon or package to tamponade the uterus | Any procedure requiring critical care or surgical intervention (eg, hysterectomy, internal iliac artery ligation, uterine artery embolization, uterine brace sutures |
| Muscle hematomas | Never | Posttrauma, no therapy | Spontaneous, no therapy | Spontaneous or traumatic, requiring desmopressin or replacement therapy | Spontaneous or traumatic, requiring surgical intervention or blood transfusion |
| Hemarthrosis | Never | Posttrauma, no therapy | Spontaneous, no therapy | Spontaneous or traumatic, requiring desmopressin or replacement therapy | Spontaneous or traumatic, requiring surgical intervention or blood transfusion |
| Central nervous system bleeding | Never | — | — | Subdural, any intervention | Intracerebral, any intervention |
| Other bleeding | None/trivial | Present | Consultation only | Surgical hemostasis, antifibrinolytics | Blood transfusion or replacement therapy or desmopressin |
| Type of bleeding . | BAT score . | ||||
|---|---|---|---|---|---|
| 0 . | 1 . | 2 . | 3 . | 4 . | |
| Epistaxis | None/trivial | >5 per year or more than 10 min | Consultation only | Packing, cauterization, or antifibrinolytics | Blood transfusion or replacement therapy (use of hemostatic blood components or rFVIIa) or desmopressin |
| Cutaneous | None/trivial | ≥5 bruises (>1 cm) in exposed areas | Consultation only | Extensive | Spontaneous hematoma requiring blood transfusion |
| Bleeding from minor wounds | None/trivial | >5 per year or more than 10 min | Consultation only | Surgical hemostasis | Blood transfusion, replacement therapy, or desmopressin |
| Oral cavity | None/trivial | Present | Consultation only | Surgical hemostasis or antifibrinolytics | Blood transfusion, replacement therapy, or desmopressin |
| Gastrointestinal bleeding | None/trivial | Present (not associated with ulcer, portal hypertension, hemorrhoids, angiodysplasia) | Consultation only | Surgical hemostasis, antifibrinolytics | Blood transfusion, replacement therapy, or desmopressin |
| Hematuria | None/trivial | Present (macroscopic) | Consultation only | Surgical hemostasis, iron therapy | Blood transfusion, replacement therapy, or desmopressin |
| Tooth extraction | None/trivial or none performed | Reported in ≤25% of all procedures, no intervention | Reported in >25% of all procedures, no intervention | Resuturing or packing | Blood transfusion, replacement therapy, or desmopressin |
| Surgery | None/trivial or none performed | Reported in ≤25% of all procedures, no intervention | Reported in >25% of all procedures, no intervention | Surgical hemostasis or antifibrinolytics | Blood transfusion, replacement therapy or desmopressin |
| Menorrhagia | None/trivial | Consultation only or changing pads more frequently than once every 2 hours or clot and flooding or pictorial bleeding assessment chart score >100 | Time off work or school more than twice per year or requiring antifibrinolytics or hormonal or iron therapy | Requiring combined treatment with antifibrinolytics and hormonal therapy or present since menarche and for more than 12 months | Acute menorrhagia requiring admission and emergency treatment or requiring blood transfusion, replacement therapy, desmopressin, or requiring dilatation and curettage or endometrial ablation or hysterectomy |
| Postpartum hemorrhage | None/trivial or no deliveries | Consultation only or use of syntocin or lochia for >6 weeks | Iron therapy or antifibrinolytics | Requiring blood transfusion, replacement therapy, desmopressin or requiring examination under anesthesia and/or the use of a uterine balloon or package to tamponade the uterus | Any procedure requiring critical care or surgical intervention (eg, hysterectomy, internal iliac artery ligation, uterine artery embolization, uterine brace sutures |
| Muscle hematomas | Never | Posttrauma, no therapy | Spontaneous, no therapy | Spontaneous or traumatic, requiring desmopressin or replacement therapy | Spontaneous or traumatic, requiring surgical intervention or blood transfusion |
| Hemarthrosis | Never | Posttrauma, no therapy | Spontaneous, no therapy | Spontaneous or traumatic, requiring desmopressin or replacement therapy | Spontaneous or traumatic, requiring surgical intervention or blood transfusion |
| Central nervous system bleeding | Never | — | — | Subdural, any intervention | Intracerebral, any intervention |
| Other bleeding | None/trivial | Present | Consultation only | Surgical hemostasis, antifibrinolytics | Blood transfusion or replacement therapy or desmopressin |
Table adapted from Rodeghiero et al.13
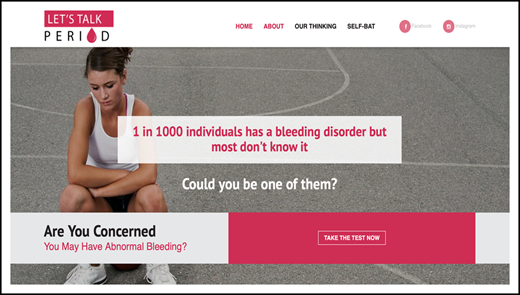
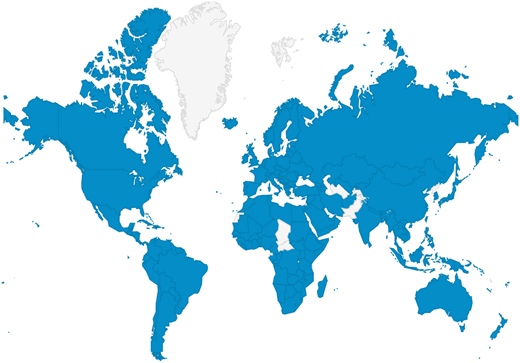
The majority of BATs are administered by experts; however, a self-administered version (the Self-BAT) was published in 2015.14 The Self-BAT has been validated for use as a screening tool for VWD and has been studied in hemophilia carriers.14,18 It was designed to be made widely and freely available to the general public and can be found at https://letstalkperiod.ca. The Let’s Talk Period website (Figure 1) was launched in May 2016, and in September 2016, 2 complementary social media accounts were also launched on Facebook (https://www.facebook.com/letstalkperiod) and Instagram (https://www.instagram.com/lets_talk_period/). Four years after launch, the website has had 168 855 page views from 200 countries (see Figure 2 for global reach). A total of 19 365 individuals have completed the Self-BAT, and 8512 (44%) had a positive or abnormal bleeding score. Anyone with an abnormal score is advised to speak with a physician about the result. The Facebook page has 4428 followers and a reach of 866 520 individuals; the Instagram account has 385 followers. Preliminary studies show that patients referred to a hematologist because of a positive Self-BAT bleeding score had more significant bleeding symptoms and were more likely to require intervention (ie, iron replacement; referral to an ear, nose, and throat specialists; or a gynecologist) than those referred by their primary care provider for bleeding or bruising symptoms, abnormal laboratory results, or a positive family history of a bleeding disorder.19 The project has been expanded to include a grade 9 outreach program to raise awareness about normal vs abnormal menstruation, iron deficiency, and bleeding disorders.20 Future plans include launching toolkits for teachers and nurses as well as providing resources for primary care practitioners.
Importantly, Let’s Talk Period is not the only initiative of its kind. There are worldwide efforts to raise awareness about HMB and bleeding disorders in women. Other examples include the Irish Know Your Flow website (https://www.knowyourflow.ie), Better You Know (https://betteryouknow.org) from the National Hemophilia Foundation in the United States, and the work of the Foundation for Women & Girls with Blood Disorders (https://www.fwgbd.org).
Laboratory testing
An important barrier to the diagnosis of an underlying bleeding disorder is the lack of access to accurate laboratory testing, even if bleeding symptoms are recognized as abnormal. Unfortunately, most bleeding disorders cannot be diagnosed by the commonly available screening tests of coagulation, the complete blood count, prothrombin time/international normalized ratio (PT/INR), and activated partial thromboplastin time (aPTT), which can be normal even in affected individuals. Therefore, special coagulation assays that include coagulation factor levels and/or platelet aggregation and release must be performed. These are available only in special coagulation laboratories and require significant expertise and experience to perform. In addition, pre-analytical variables are known to have a major impact on the results.21 Thus, patients often need to be referred to a center with a special coagulation laboratory which, in many cases, necessitates travel and missing work or school. This issue was highlighted very clearly in a recent publication by Jaffray et al,22 which showed that <40% of post-menarchal females referred with abnormal von Willebrand factor (VWF) offsite test results were confirmed to have VWD with onsite testing. Even when assays are performed properly, there remain challenges in terms of interpretation and lack of international agreement on diagnostic thresholds, classification, and nomenclature.
Issues with diagnosis, classification, and nomenclature for bleeding disorders that affect women
von Willebrand disease
VWD is the result of deficiency or dysfunction of VWF, a hemostatic protein essential for normal hemostasis. It is characterized by excessive mucocutaneous bleeding such as HMB, epistaxis, easy bruising, prolonged bleeding from minor wounds, oral cavity and gastrointestinal bleeding, and bleeding after dental work, childbirth, or surgery, with musculoskeletal bleeding seen in more severe cases.23 It is the most common inherited bleeding disorder in humans, with prevalence estimates ranging from ∼1 in 100 to 1 in 10 000.23-26 At the level of primary care, ∼1 in 1000 individuals is affected and requires medical attention for bleeding.9,27 Although VWD is autosomally inherited, only women suffer from the gynecologic and obstetrical manifestations. The current International Society on Thrombosis and Haemostasis (ISTH) classification recognizes 3 types: type 1 is a partial quantitative deficiency of VWF, type 2 is caused by qualitative abnormalities of VWF, and type 3 is a virtual absence of the VWF protein with associated very low levels of factor VIII (FVIII). Type 2 VWD is further divided into 4 subtypes: type 2A is characterized by a loss of high molecular weight VWF, type 2B results from a gain of function in VWF that increases its affinity to platelets, type 2M is caused by reduced VWF interactions with platelets or collagen, and type 2N results from reduced binding of VWF to FVIII.28 Although it is not included in the ISTH classification, type 1C VWD which is caused by increased VWF clearance, is also recognized.29,30
Even within the bleeding disorder community, there remains debate about diagnostic cutoffs for diagnosis and classification of VWD, especially for type 1 VWD. In addition to the classification reviewed above, the term “low VWF” has been used for patients with milder reductions in VWF levels (ie, VWF antigen [VWF:Ag] and/or VWF ristocetin cofactor [VWF:RCo] between 0.30 and 0.50 IU/mL). Importantly, the work of Lavin et al18,31 has shown that the bleeding phenotype is similar in patients with VWF levels <0.30 IU/mL and those with milder reductions, including gynecologic bleeding. The American Society of Hematology/International Society on Thrombosis and Haemostasis/National Hemophilia Foundation/World Federation of Hemophilia (ASH/ISTH/NHF/WFH) Guideline on VWD diagnosis has taken an evidence-based approach to this problem with publication of the final guideline expected in December 2020. Priorities for the guideline were informed by >600 responses from international stakeholders, including health care providers, patients, and caregivers.32
Hemophilia carriers
The hemophilias are X-linked bleeding disorders, with a frequency of 1 in 4000 live male births for hemophilia A and 1 in 20 000 live male births for hemophilia B.33 In contrast, the true prevalence of hemophilia carriers is not known because many come to the attention of physicians only as the result of a male relative being diagnosed, although it has been estimated that for every male with hemophilia, there are 3 to 5 hemophilia carriers.34 Approximately 30% of hemophilia carriers manifest low FVIII/FIX levels.35,36 A number of variables have been proposed to explain these low levels, including skewed X-chromosome inactivation (lionization), ABO blood type, VWF level, and F8/F9 mutation severity, although published studies show conflicting results about the relative contribution of each.35-38
In a multinational study of 168 hemophilia carriers, 65 (38%) had abnormal or positive bleeding scores (BS) with the mean BS in carriers of 5.7 compared with a BS of 1.43 in normal controls (P < .0001). The correlation between coagulation factor levels and abnormal bleeding was weak (r2 = −0.36; P < .001), and even carriers with normal levels of factors were shown to have excessive bleeding.37 Many other studies have also evaluated the bleeding symptoms experienced by hemophilia carriers by using a variety of other bleeding assessment tools, and it is clear that these patients experience multiple bleeding symptoms, including menorrhagia, postpartum hemorrhage, excessive postsurgical bleeding, epistaxis, easy bruising, oral cavity bleeding, and musculoskeletal bleeding.35,36,39-41 Despite this, the underlying pathophysiology of bleeding is not completely understood, although recent work by Candy et al42 suggests that a decreased and less sustained response to hemostatic stress is a contributor. Unfortunately, many of these women continue to receive suboptimal care and have an impaired quality of life.43,44
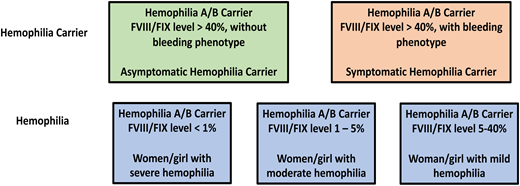
To improve communication around the issue of bleeding in hemophilia carriers, a joint initiative of the Factor VIII and Factor IX as well as Women’s Issues in Thrombosis and Hemostasis ISTH Scientific and Standardization Committees was undertaken. This group recommends that the term “hemophilia carrier” be reserved for discussions regarding genetic counseling and terms such as “symptomatic/asymptomatic hemophilia carrier” and “women and girls with hemophilia” be used in clinical management. Thus, a hemophilia carrier with factor levels >5% to 40% should be referred to as a woman or girl with mild hemophilia, 1% to 5% as moderate hemophilia, and <1% as severe hemophilia. Carriers with factor levels >40% should be referred to as symptomatic or asymptomatic, depending on their bleeding phenotype (see Figure 3 for a schematic of this nomenclature).
Proposed nomenclature for hemophilia carriers and women and girls with hemophilia.
Proposed nomenclature for hemophilia carriers and women and girls with hemophilia.
Platelet function disorders
Disorders of platelet function present with a similar pattern of clinical bleeding in women, including HMB. In a study of adolescents presenting to a tertiary care center with HMB, platelet function disorders were second only to VWD in terms of the those who were found to have an underlying bleeding disorder.44 Although the severe disorders, such as should be Glanzmann Thrombasthenia and Bernard-Soulier Syndrome are straightforward to identify from a laboratory perspective, the diagnosis of the more common, milder forms presents many challenges similar to those of other inherited bleeding disorders. Assays of platelet aggregation and release are not widely standardized, are technically challenging to perform, and have poor reproducibility. International efforts by ISTH and the International Society for Laboratory Hematology (ISLH) provide guidance for addressing these issues.45,46
Return to the clinical case
This young woman had heard about the Let’s Talk Period website at her school, so she went online and took the Self-BAT. Her quantitative BS was calculated to be 7 (≥6 is positive or abnormal). Her family physician performed initial blood work that showed a hemoglobin of 112 g/L (normal, 120-160 g/L) with a mean corpuscular volume of 75 fL (normal, 81-98 fL). Her ferritin was low at 8 μg/L (normal, 15-205 μg/L), so she was started on oral iron supplementation. PT and aPTT were normal, so she was referred to an urban center for hematologic consultation. There, the hematologist determined that her bleeding symptoms were indeed abnormal (ISTH-BAT BS of 7 [≥6 is abnormal or positive]) and that she was not able to tolerate the oral iron because of abdominal pain and constipation. Blood work showed a hemoglobin of 105 g/L with a ferritin of 5 μg/L, so intravenous iron was arranged. PT and aPTT were again normal, and special coagulation blood work was sent to the local laboratory. VWF:Ag was 0.25 IU/mL (normal, 0.50-1.50 IU/mL), VWF:GPIbM was 0.21 IU/mL (normal, 0.50-1.50 IU/mL), and FVIII:C was 0.56 IU/mL (normal, 0.50-1.50 IU/mL), with normal VWF multimers. Platelet aggregation and release testing were normal. Therefore, she was diagnosed with type 1 VWD and a desmopressin trial was arranged. She was started on tranexamic acid 1 g orally twice per day during menses pending an appointment with a gynecologist to discuss other strategies for managing her menstrual cycles.
Conclusion
The accurate diagnosis of women with bleeding disorders is critical to ensuring appropriate medical care. An accurate assessment of bleeding symptoms is critical, as is access to high-quality laboratory assays. There are several effective treatments for women with bleeding disorders that can vastly improve quality of life.
Correspondence
Paula D. James, Queen’s University, Etherington Hall, Room 2015, 94 Stuart St, Kingston, ON Canada K7L 3N6; e-mail: jamesp@queensu.ca.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.