Abstract
The treatment of lymphomas has undergone a shift in the last few decades, from traditional cytotoxic chemotherapy toward immune-targeting agents that supplement or, in some cases, even supplant direct tumor killing with activation of antitumor systemic immunity. Since the introduction of the first known immunomodulatory modality, allogeneic hematopoietic cell transplantation, multiple immunotherapeutic approaches have been developed including monoclonal antibodies (mABs), antibody-drug conjugates, bispecific T-cell engagers, checkpoint inhibitors, small molecule inhibitors, chimeric antigen receptor (CAR) T-cell therapies, and vaccines. Many of these agents, either as monotherapies or as a component of a combination strategy, have shown impressive results, combining efficacy with tolerability. Immunotherapy ranging from mABs to checkpoint inhibitors and CAR T-cell therapy are now integrated into lymphoma treatment from the earliest lines of therapy to the relapsed and refractory setting for both Hodgkin (HL) and non-Hodgkin lymphoma (NHL). Although further studies are needed to improve our understanding of the unique side effects of immunomodulation, to determine the optimal sequence and combinations of these agent with targeted therapies and standard chemotherapy, and to identify predictive biomarkers, they clearly represent a growing list of treatment options for both HL and NHL and an important step on our road toward cure of these diseases.
Learning Objectives
Understand different immunotherapeutic approaches being explored in Hodgkin and non-Hodgkin lymphoma, including monoclonal antibodies, antibody-drug conjugates, bispecific T-cell engagers, immune checkpoint inhibitors, and small molecule inhibitors
Review clinical efficacy and safety data for these approaches in frontline and relapsed or refractory settings
Case presentation: part 1
A 53-year-old woman, diagnosed with stage III classical Hodgkin lymphoma (cHL) and treated with 6 cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine, presented with a relapsed disease 7 months after completing therapy. She was treated with 2 cycles of salvage chemotherapy, ifosfamide, carboplatin, and etoposide, followed by an autologous stem cell transplantation (ASCT). One year after ASCT, she had a second relapse that was salvaged with brentuximab vedotin (BV); she subsequently underwent allogeneic hematopoietic cell transplantation (allo-HCT) from a matched related donor. Now relapsed 1 year after allo-HCT, she presents to discuss treatment options for her third relapse.
Introduction
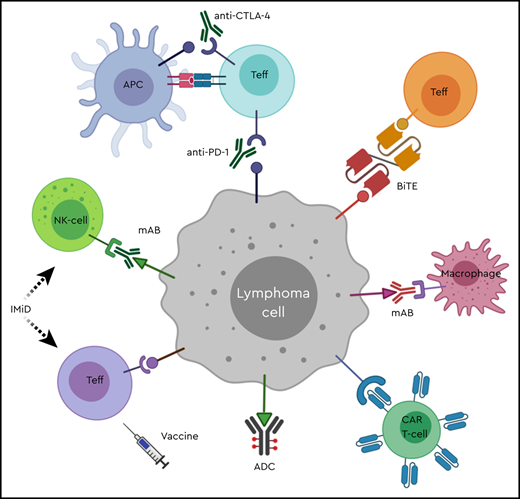
The treatment of lymphomas has undergone a shift in the last few decades, from traditional cytotoxic chemotherapy to chemoimmunotherapy with the addition of rituximab, and more recently toward immune targeting of the tumor cells or the tumor microenvironment (TME). Since the introduction of the first known immunomodulatory modality, allo-HCT, as a treatment option for relapsed or refractory (R/R) lymphoma, multiple immunotherapeutic approaches have been developed including antibodies, checkpoint inhibitors, chimeric antigen receptor (CAR) T-cell therapies, and vaccines (Figure 1). This article focuses on the role of immunologic antitumor therapies in Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) and reviews the clinical results of recently developed agents. Discussion of cell-based approaches including vaccines, CAR T-cell therapies and HCT is covered in separate articles.
Immune optimization in lymphoma. ADC, antibody-drug conjugate; APC, antigen-presenting cell; BiTE, bispecific T-cell engager; CAR T-cell, chimeric antigen receptor T-cell; CTLA-4, cytotoxic T-lymphocyte antigen 4; IMiD, immunomodulatory drug; mAB, monoclonal antibody; NK-cell, natural killer cell; PD-1, programmed cell death-1; Teff, effector T-lymphocyte.
Immune optimization in lymphoma. ADC, antibody-drug conjugate; APC, antigen-presenting cell; BiTE, bispecific T-cell engager; CAR T-cell, chimeric antigen receptor T-cell; CTLA-4, cytotoxic T-lymphocyte antigen 4; IMiD, immunomodulatory drug; mAB, monoclonal antibody; NK-cell, natural killer cell; PD-1, programmed cell death-1; Teff, effector T-lymphocyte.
Monoclonal antibodies
Since the type I anti-CD20 monoclonal antibody (mAB) rituximab changed the therapeutic landscape of B-cell NHL in the 1990s, there have been multiple attempts to improve the efficacy and the duration of tumor killing with CD20-targeting mABs. Ofatumumab, a type I humanized mAB, binds to a different epitope with higher affinity and improved complement-dependent cytotoxicity. Despite these intended enhancements, efficacy in lymphomas has been limited, and ofatumumab’s only approved indication in hematologic malignancies is in chronic lymphocytic leukemia. Selected studies of novel monoclonal antibodies in lymphoma are shown in Table 1. Obinutuzumab is a glycoengineered type II mAB with increased binding affinity to the FcγRIII receptor on immune effector cells1 and decreased FcγRIIb-mediated internalization of CD20 in lipid rafts,2 leading to enhanced antibody-dependent cell-mediated cytotoxicity (ADCC). In the GALLIUM trial, obinutuzumab, combined with chemotherapy followed by 2 subsequent years of maintenance, had a superior 3-year progression-free survival (PFS) compared with rituximab for the frontline treatment of follicular lymphoma (FL), although no overall survival (OS) advantage was observed.3 Improved outcome was also observed in patients with indolent NHL refractory to rituximab in the GADOLIN trial, with a significantly longer PFS for the bendamustine-obinutuzumab combination compared with bendamustine monotherapy.4 Despite promising results in indolent lymphomas, obinutuzumab with cyclophosphamide, doxorubicin, vincristine, prednisone has not demonstrated superior outcome over the rituximab combination (R-CHOP) in the frontline treatment of diffuse large B-cell lymphoma (DLBCL).5
Selected studies of monoclonal antibodies in lymphoma
| mAB . | Target . | Study phase . | Patient population . | Outcome(s) . |
|---|---|---|---|---|
| Obinutuzumab | CD20 | III3 | Previously untreated FL | 3-y PFS: 80.0% (obinutuzumab-based chemotherapy) vs 73.3% (rituximab-based chemotherapy) |
| ORR: 88.5% vs 86.9% | ||||
| Grade 3-5 AEs: 74.6% vs 67.8% | ||||
| SAEs: 46.1% vs 39.9% | ||||
| III4 | Indolent NHL refractory to rituximab | Median PFS: not reached (obinutuzumab plus bendamustine) vs 14.9 mo (bendamustine monotherapy) | ||
| Grade 3-5 AEs: 68% vs 62% | ||||
| SAEs: 38% vs 33% | ||||
| Tafasitamab | CD19 | IIa7 | R/R NHL | Tafasitamab monotherapy |
| ORR: 26% (DLBCL), 29% (FL), 27% (other) | ||||
| AEs: IRR (12%), neutropenia (12%) | ||||
| II8 | R/R DLBCL | Tafasitamab with lenalidomide | ||
| ORR of 54%, CR rate of 32%, PR rate of 22% | ||||
| Treatment-related SAEs: infections (10%), neutropenic fever (5%) | ||||
| Magrolimab | CD47 | Ib/II9 | R/R NHL | Magrolimab with rituximab |
| TRAEs: chills (41%), headache (41%), anemia (41%), IRR (36%) | ||||
| ORR of 50%, CR rate of 36% | ||||
| Mogamulizumab | CCR4 | III10 | R/R CTCL | Median PFS: 7.7 (mogamulizumab) vs 3.1 mo (vorinostat) |
| Grade 3-4 AEs: 41% vs 41% |
| mAB . | Target . | Study phase . | Patient population . | Outcome(s) . |
|---|---|---|---|---|
| Obinutuzumab | CD20 | III3 | Previously untreated FL | 3-y PFS: 80.0% (obinutuzumab-based chemotherapy) vs 73.3% (rituximab-based chemotherapy) |
| ORR: 88.5% vs 86.9% | ||||
| Grade 3-5 AEs: 74.6% vs 67.8% | ||||
| SAEs: 46.1% vs 39.9% | ||||
| III4 | Indolent NHL refractory to rituximab | Median PFS: not reached (obinutuzumab plus bendamustine) vs 14.9 mo (bendamustine monotherapy) | ||
| Grade 3-5 AEs: 68% vs 62% | ||||
| SAEs: 38% vs 33% | ||||
| Tafasitamab | CD19 | IIa7 | R/R NHL | Tafasitamab monotherapy |
| ORR: 26% (DLBCL), 29% (FL), 27% (other) | ||||
| AEs: IRR (12%), neutropenia (12%) | ||||
| II8 | R/R DLBCL | Tafasitamab with lenalidomide | ||
| ORR of 54%, CR rate of 32%, PR rate of 22% | ||||
| Treatment-related SAEs: infections (10%), neutropenic fever (5%) | ||||
| Magrolimab | CD47 | Ib/II9 | R/R NHL | Magrolimab with rituximab |
| TRAEs: chills (41%), headache (41%), anemia (41%), IRR (36%) | ||||
| ORR of 50%, CR rate of 36% | ||||
| Mogamulizumab | CCR4 | III10 | R/R CTCL | Median PFS: 7.7 (mogamulizumab) vs 3.1 mo (vorinostat) |
| Grade 3-4 AEs: 41% vs 41% |
AEs, adverse events; CR, complete response; CTCL, cutaneous T-cell lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; IRR, infusion-related reaction; mAB, monoclonal antibody; NHL, non-Hodgkin lymphoma; ORR, overall response rate; PFS, progression-free survival; PR, partial response; SAEs, serious adverse events; TRAEs, treatment-related adverse events.
In R/R setting, CD19, which is highly expressed on malignant B cells, is an attractive alternative target in B-cell lymphomas as it continues to be expressed in setting of CD20 downregulation, a primary resistance mechanism in rituximab-refractory disease.6 Tafasitamab is a humanized mAB directed against CD19 with an engineered Fc domain to decrease the binding affinity to inhibitory receptor FcγRIIa and increase its binding to stimulatory FcγRIIIa on the effector cells, resulting in more potent ADCC. Single-agent tafasitamab has shown promising antitumor activity and favorable safety profile in a phase IIa study of R/R NHL patients, including those with rituximab-refractory disease, with an overall response rate (ORR) of 26% in DLBCL.7 Tafasitamab has also shown encouraging antitumor activity in combination with lenalidomide in R/R DLBCL in the phase II L-MIND study, with an ORR of 54% and a complete response (CR) rate of 32%.8
CD47 is an antiphagocytic protein with increased expression in NHL cells compared with normal B cells. Overexpression of CD47 by lymphoma cells enables immune evasion of antitumor macrophages and has been shown to be an independent predictor of unfavorable clinical outcomes in multiple NHL subtypes including DLBCL and FL. Antibodies to CD47 block the interaction between CD47 and its ligand SIRPα on macrophages, enhancing recognition and phagocytosis of lymphoma cells. CD47-targeting mABs are under investigation both as single agents and in combination in T- and B-cell NHL. Magrolimab, a humanized mAB against CD47, has demonstrated an ORR of 50% and a CR rate of 36% in combination with rituximab in a heavily pretreated population of DLBCL and FL patients in a phase Ib/II study while causing no clinically significant safety events.9
In T-cell lymphomas, C-C chemokine receptor 4 (CCR4), which is highly expressed on the surface of tumor cells in mature T-cell malignancies including cutaneous T-cell lymphoma (CTCL), is an attractive therapeutic target. Mogamulizumab, a mAB against CCR4, is now approved for R/R mycosis fungoides (MF) and Sézary syndrome (SS) based on the data from a phase 3 randomized trial that demonstrated a superior PFS compared with vorinostat (7.7 vs 3.1 months) and comparable toxicity profile.10
Antibody-drug conjugates
In an effort to deliver cytotoxic agents selectively to tumor cells, antibody-drug conjugates (ADCs) have been developed. An ADC consists of a tumor-specific mAB conjugated to a tumor-killing agent that is released after internalization of the ADC. Several ADCs have shown activity in lymphoid malignancies, selected studies are shown in Table 2. Brentuximab vedotin targets the CD30-expressing HRS cells in HL with the antimicrotubule agent monomethyl auristatin E as its payload. Since its approval by the US Food and Drug Administration in 2011 in R/R cHL, BV has gained additional indications in CD30-expressing lymphomas including in HL (post-HCT consolidation therapy, first-line advanced-stage in combination with chemotherapy) and in peripheral T-cell lymphoma (untreated CD30-expressing peripheral T-cell lymphoma, relapsed systemic anaplastic large cell lymphoma, and relapsed primary cutaneous anaplastic large cell lymphoma and CD30-expressing MF after failure of prior systemic therapy).
Selected studies of antibody-drug conjugates in lymphoma
| ADC . | Target . | Study phase . | Patient population . | Outcome(s) . |
|---|---|---|---|---|
| Brentuximab vedotin | CD30 | II38 | R/R cHL after ASCT failure | BV monotherapy |
| ORR of 75%, CR rate of 34% | ||||
| Median PFS of 5.6 mo | ||||
| Median DoR of 20.5 mo | ||||
| TRAEs: peripheral sensory neuropathy (42%), nausea (35%), fatigue (34%), neutropenia (19%), diarrhea (18%) | ||||
| III39 | R/R cHL after ASCT | Median PFS: 42.9 (BV) vs 24.1 mo (placebo) | ||
| AEs: peripheral sensory neuropathy (56% vs 16%), neutropenia (35% vs 12%) | ||||
| III40 | Previously untreated stage III or IV cHL | 2-y modified PFS: 82.1% (A+AVD) vs 77.2% (ABVD) | ||
| AEs: neutropenia (58% vs 45%), peripheral neuropathy (67% vs 43%), grade 3 or higher pulmonary toxicity (< 1% vs 3%) | ||||
| Moxetumomab pasudotox | CD22 | III11 | R/R HCL after at least 2 therapies | Moxetumomab pasudotox monotherapy |
| Durable CR rate of 30%, CR rate of 41%, ORR of 75% | ||||
| AEs: peripheral edema (39%), nausea (35%), fatigue (34%), headache (33%) | ||||
| Treatment related SAEs: HUS (7.5%), capillary leak syndrome (5%) | ||||
| Polatuzumab vedotin | CD79b | II12 | R/R DLBCL | Nonhematologic AEs: diarrhea (41% with pola+BR vs 21% with BR), infections (39% vs 41%), fatigue (36% vs 28%), pyrexia (33% vs 23%), IRR (31% vs 21%), peripheral neuropathy (39% vs 3%) |
| Hematologic AEs: neutropenia (54% vs 39%), thrombocytopenia (49% vs 23%), anemia (44% vs 15%) | ||||
| Grade 5 AEs: 18% in each arm | ||||
| ORR: 70% vs 33% | ||||
| CR: 58% vs 20% | ||||
| Median DoR: 8.8 vs 3.7 mo |
| ADC . | Target . | Study phase . | Patient population . | Outcome(s) . |
|---|---|---|---|---|
| Brentuximab vedotin | CD30 | II38 | R/R cHL after ASCT failure | BV monotherapy |
| ORR of 75%, CR rate of 34% | ||||
| Median PFS of 5.6 mo | ||||
| Median DoR of 20.5 mo | ||||
| TRAEs: peripheral sensory neuropathy (42%), nausea (35%), fatigue (34%), neutropenia (19%), diarrhea (18%) | ||||
| III39 | R/R cHL after ASCT | Median PFS: 42.9 (BV) vs 24.1 mo (placebo) | ||
| AEs: peripheral sensory neuropathy (56% vs 16%), neutropenia (35% vs 12%) | ||||
| III40 | Previously untreated stage III or IV cHL | 2-y modified PFS: 82.1% (A+AVD) vs 77.2% (ABVD) | ||
| AEs: neutropenia (58% vs 45%), peripheral neuropathy (67% vs 43%), grade 3 or higher pulmonary toxicity (< 1% vs 3%) | ||||
| Moxetumomab pasudotox | CD22 | III11 | R/R HCL after at least 2 therapies | Moxetumomab pasudotox monotherapy |
| Durable CR rate of 30%, CR rate of 41%, ORR of 75% | ||||
| AEs: peripheral edema (39%), nausea (35%), fatigue (34%), headache (33%) | ||||
| Treatment related SAEs: HUS (7.5%), capillary leak syndrome (5%) | ||||
| Polatuzumab vedotin | CD79b | II12 | R/R DLBCL | Nonhematologic AEs: diarrhea (41% with pola+BR vs 21% with BR), infections (39% vs 41%), fatigue (36% vs 28%), pyrexia (33% vs 23%), IRR (31% vs 21%), peripheral neuropathy (39% vs 3%) |
| Hematologic AEs: neutropenia (54% vs 39%), thrombocytopenia (49% vs 23%), anemia (44% vs 15%) | ||||
| Grade 5 AEs: 18% in each arm | ||||
| ORR: 70% vs 33% | ||||
| CR: 58% vs 20% | ||||
| Median DoR: 8.8 vs 3.7 mo |
A+AVD, BV-doxorubicin-vinblastine-dacarbazine; ABVD, doxorubicin-bleomycin-vinblastine-dacarbazine; ADC, antibody-drug conjugate; AEs, adverse events; ASCT, autologous stem cell transplantation; BR, bendamustine-rituximab; BV, brentuximab vedotin; cHL, classical Hodgkin lymphoma; CR, complete response; DLBCL, diffuse large B-cell lymphoma; DoR, duration of response; HCL, hairy cell lymphoma; HUS, hemolytic uremic syndrome; IRR, infusion-related reaction; ORR, overall response rate; PFS, progression-free survival; pola, polatuzumab vedotin; SAEs, serious adverse events; TRAEs, treatment-related adverse events.
In hairy cell leukemia (HCL), moxetumomab pasudotox, a recombinant immunotoxin with anti-CD22 mAB and truncated Pseudomonas exotoxin, is approved for the treatment of HCL relapsed after at least 2 systemic therapies, based on a phase 3 trial that showed a durable CR rate of 30% and 85% of complete responders achieving minimal residual disease negativity by immunohistochemistry.11
Polatuzumab vedotin, recently approved in combination with the alkylating agent bendamustine and rituximab (BR) for R/R DLBCL, uses the same toxic payload as BV and targets CD79b-expressing B cells. The approval was based on phase 2 data demonstrating improved ORR and CR rate with the addition of polatuzumab to BR.12 Polatuzumab is also being studied in a novel combination with the mAB obinutuzumab and the immunomodulatory drug (IMiD) lenalidomide in R/R FL and in R/R DLBCL. For the frontline treatment of DLBCL, polatuzumab is being evaluated in a combination with rituximab, cyclophosphamide, doxorubicin and prednisone compared with R-CHOP in a phase 3 trial13 and may challenge R-CHOP as the long-standing standard of care in newly diagnosed DLBCL patients (Table 5).
Selected ongoing upfront trials of immunotherapy in lymphoma
| Trial ID . | Study regimen . | Phase . | Study objective(s) . |
|---|---|---|---|
| NCT03274492 | Polatuzumab with R-CHP vs R-CHOP in previously untreated DLBCL | III13 | Primary end point: investigator-assessed PFS |
| Secondary end points: IRC-assessed PET/CT CR rate at EOT, EFS, 2-y PFS, and OS | |||
| NCT03907488 | BV vs nivolumab plus AVD in newly diagnosed stage III-IV cHL | III | Primary end point: PFS |
| Secondary end points: OS, EFS, CR rate, and TRAE rate |
| Trial ID . | Study regimen . | Phase . | Study objective(s) . |
|---|---|---|---|
| NCT03274492 | Polatuzumab with R-CHP vs R-CHOP in previously untreated DLBCL | III13 | Primary end point: investigator-assessed PFS |
| Secondary end points: IRC-assessed PET/CT CR rate at EOT, EFS, 2-y PFS, and OS | |||
| NCT03907488 | BV vs nivolumab plus AVD in newly diagnosed stage III-IV cHL | III | Primary end point: PFS |
| Secondary end points: OS, EFS, CR rate, and TRAE rate |
AVD, doxorubicin- vinblastine-dacarbazine; BV, brentuximab vedotin; cHL, classical Hodgkin lymphoma; CR, complete response; DLBCL, diffuse large B-cell lymphoma; EFS, event-free survival; EOT, end of treatment; IRC, independent review committee; OS, overall survival; PFS, progression-free survival; R-CHOP, rituximab-cyclophosphamide-doxorubicin-vincristine-prednisone; R-CHP, rituximab-cyclophosphamide-doxorubicin-prednisone; TRAE, treatment-related adverse event.
Bispecific T-cell engagers
With the improved understanding of the immunomodulatory effects of antibodies on the TME, bispecific antibodies that bind two different antigens have been developed to promote the engagement of peritumoral effector cells with tumor cells. A subtype of bispecific antibodies, bispecific T-cell engager (BiTE), links 2 antibody fragments, one specific for an antigen on tumor cells and the other that binds a surface antigen on T cells, and brings the 2 cells in close proximity, triggering T-cell cytotoxicity without a need for costimulation. Table 3 shows selected studies of bispecific T-cell engagers in lymphoma. Despite its success in R/R B-cell acute lymphoblastic leukemia, the efficacy of blinatumomab, a CD3/CD19 BiTE composed of tandem single-chain variable fragments,14 in NHL, it has been overshadowed by significant neurotoxicity. Mosunetuzumab, a BiTE targeting CD3 and CD20, has shown promising activity and tolerable side effects in a phase 1/1b dose escalation and expansion study in heavily pretreated FL and DLBCL patients, including those who had failed CAR T-cell therapy. Response rates (ORR and CR) were 64.1% and 42.2%, respectively, for indolent NHLs and 34.7% and 18.6%, respectively, for aggressive NHLs.15 Neurotoxicity and cytokine release syndrome (CRS), common sconcern with blinatumomab and CAR T-cell therapy, were reported in 44% and 28.4% of patients, respectively, with most toxicity grades 1 to 2, transient and reversible. The study was modified to include step-up dosing with ascending doses of mosunetuzumab during cycle 1; this appeared to mitigate CRS and neurotoxicity further. The structure of mosunetuzumab, a full-length humanized IgG1 molecule with a near-native antibody structure and an Fc region engineered to enhance its pharmacokinetic properties, increases its serum half-life,16 obviating the need for a continuous infusion that is required with other BiTEs such as blinatumomab. Another CD3/CD20 BiTE, CD20-TBC, is designed to increase avidity for tumor antigen with binding of Fab regions of CD20 and CD3 in a 2:1 configuration and to increase half-life with a modified heterodimeric Fc region. CD20-TCB has shown clinical activity and safety as a single agent in heavily pretreated NHL patients; in a dose-escalation study, the ORR was 47% including CRs in 34% of patients, and CRS occurred in 55% of patients, primarily grades 1 to 2.17 . In addition, CD20-TCB has been safely combined with another CD20-targeted agent, obinutuzumab, in a phase 1b study of R/R B-cell NHL, with only 8% of patients experiencing grade 3 or higher CRS.18 REGN1979, another CD3/C20 BiTE, is also being studied in R/R NHL and has shown activity with a tolerable safety profile as a single agent.19
Selected studies of bispecific T-cell engagers in lymphoma
| BiTE . | Target . | Study phase . | Patient population . | Outcome(s) . |
|---|---|---|---|---|
| Mosunetuzumab | CD20/CD3 | I/Ib15 | R/R B-cell NHL | CRS in 28.4% including 27.1% grade 1-2 |
| NAEs in 44% including 40.8% grade 1-2, headache (14.7%), insomnia (10.1%), and dizziness (9.2%) | ||||
| ORR of 64.1% and CR rate of 42.2% for indolent NHL, ORR of 34.7% and CR rate of 18.6% for aggressive NHLs | ||||
| CD20-TCB | CD20/CD3 | I17 | R/R NHL | CD20-TCB monotherapy |
| CRS in 55.1% including 22% grade 1 and 31% grade 2, pyrexia in 34.7% and neutropenia in 34.7% | ||||
| ORR of 47%, CR rate of 34% in aggressive NHL patient | ||||
| CD20/CD3 | I/Ib18 | R/R B-cell NHL | CD20-TCB with obinutuzumab | |
| AEs: anemia (21%), thrombocytopenia (21%), neutropenia (14%), pyrexia (14%), hypokalemia (14%) | ||||
| CRS in 57% of patients including 50% grade 1-2, rare NAEs | ||||
| ORR of 48% and CR rate of 43% |
| BiTE . | Target . | Study phase . | Patient population . | Outcome(s) . |
|---|---|---|---|---|
| Mosunetuzumab | CD20/CD3 | I/Ib15 | R/R B-cell NHL | CRS in 28.4% including 27.1% grade 1-2 |
| NAEs in 44% including 40.8% grade 1-2, headache (14.7%), insomnia (10.1%), and dizziness (9.2%) | ||||
| ORR of 64.1% and CR rate of 42.2% for indolent NHL, ORR of 34.7% and CR rate of 18.6% for aggressive NHLs | ||||
| CD20-TCB | CD20/CD3 | I17 | R/R NHL | CD20-TCB monotherapy |
| CRS in 55.1% including 22% grade 1 and 31% grade 2, pyrexia in 34.7% and neutropenia in 34.7% | ||||
| ORR of 47%, CR rate of 34% in aggressive NHL patient | ||||
| CD20/CD3 | I/Ib18 | R/R B-cell NHL | CD20-TCB with obinutuzumab | |
| AEs: anemia (21%), thrombocytopenia (21%), neutropenia (14%), pyrexia (14%), hypokalemia (14%) | ||||
| CRS in 57% of patients including 50% grade 1-2, rare NAEs | ||||
| ORR of 48% and CR rate of 43% |
CR, complete response; CRS, cytokine release syndrome; NAEs, neurologic adverse events; NHL, non-Hodgkin lymphoma; ORR, overall response rate; R/R, relapsed or refractory.
Case presentation: part 2
You discuss treatment options with your multiple-relapsed HL patient including BV retreatment, checkpoint inhibitor, standard chemotherapy, or some combination of these agents. She chooses to enroll in a phase 1 trial of the combination of BV, ipilimumab, and nivolumab. She is treated for 24 months per protocol. Now more than 2 years from her last treatment, she remains in CR with no treatment-related toxicity.
Checkpoint inhibitors
Classical HL is an atypical hematologic malignancy, characterized by Hodgkin Reed-Sternberg (HRS) cells that account for less than 1% of cells in the affected lymph node and are dispersed in a background of extensive infiltrate of immune cells. To thrive in an immune-rich TME, HRS cells have developed multiple mechanisms to evade immune surveillance, including a high expression of PD-L1/PD-L2 as a result of copy-number amplification of the PD-L1 and JAK2 gene loci on chromosome 9p24,20 a lack of β2-microglobulin and MHC class I on the HRS cells21 to dampen effector T-cell function, and a lack of MHC class II expression on the surface of HRS cells to suppress helper T-cell function.22 In addition, Epstein-Barr virus, present in 30% to 40% of HRS cells, can promote PD-L1 expression by activating the AP1 transcription factor pathway. Table 4 highlights selected studies of checkpoint inhibitors in lymphoma. The PD-1 inhibitors, pembrolizumab and nivolumab, have shown striking antitumor activity in patients with R/R HL with a manageable safety profile23,24 and are approved for the treatment of patients of cHL after at least 3 prior therapies (pembrolizumab) and who have relapsed or progressed after ASCT and post-ASCT BV (nivolumab). The approvals were based on phase 2 studies that showed an ORR of 69% including 22% CRs, median duration of response (DoR) that was not reached, and 6-month PFS and OS of 72.4% and 99.5%, respectively, for pembrolizumab,23 and an ORR of 69% including 40% CRs and median DoR of 16.6 months for nivolumab.24 Pembrolizumab was superior to BV for patients with R/R cHL who have relapsed after or are ineligible for ASCT in a phase 3 trial, which showed a median PFS of 13.2 months in the pembrolizumab arm compared with 8.3 months in the BV arm; whether this will replace BV as a standard in this space remains to be seen.25 Combination immunotherapies, including BV and nivolumab or the cytotoxic T-lymphocyte antigen 4 (CTLA-4) inhibitor ipilimumab and a triplet regimen of BV plus ipilimumab and nivolumab, are being studied and have shown promising results in early-phase trials of R/R HL.26,27
Selected studies of checkpoint inhibitors in lymphoma
| Checkpoint inhibitor . | Target . | Study phase . | Patient population . | Outcome(s) . |
|---|---|---|---|---|
| Nivolumab | PD-1 | II24 | R/R HL after ASCT failure | Nivolumab monotherapy |
| ORR of 69%, CR rate of 40% CRs | ||||
| Median DoR of 16.6 mo | ||||
| Median PFS of 14.7 mo | ||||
| Grade 3-4 drug-related AEs: lipase increases (5%), neutropenia (3%), ALT increases (3%) | ||||
| I/II27 | R/R HL | Nivolumab with BV | ||
| ORR of 82%, CR rate of 61% | ||||
| AEs: 98%, mostly grades 1-2, IRRs in 44% | ||||
| I/II26 | R/R HL | Nivolumab with BV | ||
| AEs: grade 3 or higher TRAEs in 16%, grade 5 in 1.5% | ||||
| ORR of 89%, CR rate of 61% | ||||
| Median PFS not reached, with median follow-up of 2.4 y | ||||
| Pembrolizumab | PD-1 | II23 | R/R HL | Pembrolizumab monotherapy |
| ORR of 69%, CR rate of 22.4% | ||||
| Response lasting 6 mo or longer in 75.6% | ||||
| TRAEs: hypothyroidism (12.4%), pyrexia (10.5%) | ||||
| AEs: immune-mediated AEs and IRRs in 28.6% | ||||
| III25 | R/R HL | Median PFS: 13.2 (pembrolizumab) vs 8.3 mo (BV) | ||
| ORR: 65.6% vs 54.2% | ||||
| CR rate: 24.5% vs 24.2% | ||||
| Median time to response: 2.8 vs 2.8 mo | ||||
| Median DoR: 20.7 vs 13.8 mo | ||||
| Grade 3-5 TRAEs: 19.6% vs 25% | ||||
| II29 | R/R PMBCL | Pembrolizumab monotherapy | ||
| ORR of 45%, CR rate of 13% | ||||
| Median DoR not reached with median follow-up of 12.5 mo | ||||
| Grade 3-4 TRAEs: 23% | ||||
| II31 | R/R MF and SS | Pembrolizumab monotherapy | ||
| ORR of 38% | ||||
| Median DoR not reached, with median follow-up of 58 wk | ||||
| Immune-related AEs leading to treatment discontinuation in 4 patients | ||||
| Nivolumab + BV +/− ipilimumab | PD-1, CD30, CTLA-4 | I/II26 | R/R HL | Ipilimumab with BV |
| AEs: grade 3 or higher TRAEs in 43%, no grade 5 | ||||
| ORR of 76%, CR rate of 57% | ||||
| Median PFS 1.2 y, with median follow-up of 2.6 y | ||||
| I/II26 | R/R HL | Ipilimumab with nivolumab and BV | ||
| AEs: grade 3 or higher TRAEs in 50%, grade 5 in 1.5% | ||||
| ORR of 82%, CR rate of 73% | ||||
| Median PFS not reached, with median follow-up of 1.7 y |
| Checkpoint inhibitor . | Target . | Study phase . | Patient population . | Outcome(s) . |
|---|---|---|---|---|
| Nivolumab | PD-1 | II24 | R/R HL after ASCT failure | Nivolumab monotherapy |
| ORR of 69%, CR rate of 40% CRs | ||||
| Median DoR of 16.6 mo | ||||
| Median PFS of 14.7 mo | ||||
| Grade 3-4 drug-related AEs: lipase increases (5%), neutropenia (3%), ALT increases (3%) | ||||
| I/II27 | R/R HL | Nivolumab with BV | ||
| ORR of 82%, CR rate of 61% | ||||
| AEs: 98%, mostly grades 1-2, IRRs in 44% | ||||
| I/II26 | R/R HL | Nivolumab with BV | ||
| AEs: grade 3 or higher TRAEs in 16%, grade 5 in 1.5% | ||||
| ORR of 89%, CR rate of 61% | ||||
| Median PFS not reached, with median follow-up of 2.4 y | ||||
| Pembrolizumab | PD-1 | II23 | R/R HL | Pembrolizumab monotherapy |
| ORR of 69%, CR rate of 22.4% | ||||
| Response lasting 6 mo or longer in 75.6% | ||||
| TRAEs: hypothyroidism (12.4%), pyrexia (10.5%) | ||||
| AEs: immune-mediated AEs and IRRs in 28.6% | ||||
| III25 | R/R HL | Median PFS: 13.2 (pembrolizumab) vs 8.3 mo (BV) | ||
| ORR: 65.6% vs 54.2% | ||||
| CR rate: 24.5% vs 24.2% | ||||
| Median time to response: 2.8 vs 2.8 mo | ||||
| Median DoR: 20.7 vs 13.8 mo | ||||
| Grade 3-5 TRAEs: 19.6% vs 25% | ||||
| II29 | R/R PMBCL | Pembrolizumab monotherapy | ||
| ORR of 45%, CR rate of 13% | ||||
| Median DoR not reached with median follow-up of 12.5 mo | ||||
| Grade 3-4 TRAEs: 23% | ||||
| II31 | R/R MF and SS | Pembrolizumab monotherapy | ||
| ORR of 38% | ||||
| Median DoR not reached, with median follow-up of 58 wk | ||||
| Immune-related AEs leading to treatment discontinuation in 4 patients | ||||
| Nivolumab + BV +/− ipilimumab | PD-1, CD30, CTLA-4 | I/II26 | R/R HL | Ipilimumab with BV |
| AEs: grade 3 or higher TRAEs in 43%, no grade 5 | ||||
| ORR of 76%, CR rate of 57% | ||||
| Median PFS 1.2 y, with median follow-up of 2.6 y | ||||
| I/II26 | R/R HL | Ipilimumab with nivolumab and BV | ||
| AEs: grade 3 or higher TRAEs in 50%, grade 5 in 1.5% | ||||
| ORR of 82%, CR rate of 73% | ||||
| Median PFS not reached, with median follow-up of 1.7 y |
AEs, adverse events; ALT, alanine aminotransferase; ASCT, autologous stem cell transplantation; BV, brentuximab vedotin; CR, complete response; CRS, cytokine release syndrome; CTLA-4, cytotoxic T-lymphocyte-associated protein-4; DoR, duration of response; IRR, infusion-related reaction; MF, mycosis fungoides; NHL, non-Hodgkin lymphoma; ORR, overall response rate; PFS, progression-free survival; PMBCL, primary mediastinal large B-cell lymphoma; R/R, relapsed or refractory; SS, Sézary syndrome; TRAEs, treatment-related adverse events.
In NHLs, results of checkpoint blockade studies have been less impressive. Pembrolizumab has the sole US Food and Drug Administration approval in R/R primary mediastinal large B-cell lymphoma, a subtype of DLBCL that shares features with nodular sclerosis HL and also overexpresses PD-L1,28 for the treatment of patients who have failed 2 or more prior therapies. The KEYNOTE-170 trial supported the approval with an ORR of 45% including 13% CRs, median DoR not reached after a median follow-up time of 12.5 months, and grade 3 or 4 treatment-related adverse effects occurring in 23% of patients.29 Pembrolizumab has shown durable antitumor activity in R/R MF and SS, subtypes of CTCL also with frequent genomic alterations in PD-L1 and PD-L2,30 with an ORR of 38% and median DoR not reached with a median follow-up time of 58 weeks.31 Although the response rates in NHL have been modest, there are data to suggest that checkpoint blockade therapy may sensitize some R/R patients to subsequent therapy regardless of their response to the checkpoint inhibitor.32
The use of checkpoint inhibitors in lymphoma poses a unique challenge in monitoring treatment response because of pseudo-progression in which imaging findings suggest progression of disease despite clinical improvements likely from increased tumor infiltration by activated immune cells in setting of immunotherapy. In a phase II study of R/R cHL, patients were allowed to continue treatment with nivolumab beyond disease progression, and 61% of such patients had stable or reduced tumor burdens.24 This approach of treating beyond conventional disease progression appears to be safe, and a revised response criteria is needed for immune checkpoint inhibitor therapies.
Small molecule inhibitors
Lenalidomide, an IMiD best known for its activity in multiple myeloma, has pleotropic effects on immune cells of the TME in addition to its direct antitumor mechanism via an activation of cereblon’s E3 ligase. These effects may include repair of defective immune synapses and enhanced T-cell expansion resulting in improved cytotoxicity, enhancement of ADCC by natural killer cells, and increased efficiency of antigen presentation to CD8+ T cells by dendritic cells.33 Lenalidomide plus rituximab is a chemotherapy-free regimen with a PFS benefit over rituximab monotherapy in indolent lymphoma, 39.4 vs 14.1 months,34 and is approved for the treatment of previously treated FL and marginal zone lymphoma patients. Although the pathophysiology of tumor flare reaction, seen in 10% of patients in the study, remains unclear, it likely occurs via an immunomodulatory mechanism. Lenalidomide is also used to treat patients with mantle cell lymphoma after a failure of 2 prior therapies and has shown an ORR of 28% and median DoR of 16.6 months as a monotherapy.35
Although not specifically designed to target immune cells in the TME, a number of small molecule inhibitors appear to have off-target immunomodulatory effects. Ibrutinib, an irreversible inhibitor of Bruton tyrosine kinase, also inhibits interleukin-2–inducible T-cell kinase, thereby tipping the balance of T helper-1 versus T helper-2 (Th1/Th2) immunity in favor of Th1-based immune activation against tumors.36 In the class of phosphoinositide 3-kinase inhibitors, PI3Kα/δ isoform inhibition has been shown to promote CD8+ T-cell activation and enhance effector T-cell function, in addition to directly targeting tumor cells.37
Conclusion
Since the introduction of allo-HCT, optimizing antitumor immunity has taken an expanding role in the treatment of lymphomas with many new classes of drugs such as mABs, ADCs, BiTEs, and checkpoint inhibitors, which have shown impressive efficacy and manageable tolerability. Further studies are needed to improve the understanding of the unique side effects of immunomodulation, to determine the optimal sequence and combinations of these agents, and to identify predictive biomarkers that may drive personalization, yet it is clear that they represent a growing list of treatment options for both HL and NHL patients.
References
Competing Interests
Conflict-of-interest disclosure: C.S.D. receives research funding and/or has served in a consulting role with BMS, Merck, Roche/Genentech, Millenium, Janssen, and Seattle Genetics. Y.C. declares no competing financial interests.
Author notes
Off-label drug use: This article covers off-label and investigational uses of many novel drugs, and drug combinations including: investigational combinations of nivolumab, brentuximab, and nivolumab in first line in for HL, and iplimumab, magrolizumab, mogamulizumab, CD20 TCB, and polatuzumab vedotin in first line for NHL.
CorrespondenceCatherine S. Diefenbach, NYU Ambulatory Cancer Center, 19th Floor, 240 East 38th St, New York, NY 10016; e-mail: catherine.diefenbach@nyulangone.org.