Abstract
Aggressive B-cell lymphoma is a heterogeneous entity with disparate outcomes based on clinical and pathological characteristics. While most tumors in this category are diffuse large B-cell lymphoma (DLBCL), the recognition that some cases have high-grade morphology and frequently harbor MYC and BCL2 and/or BCL6 translocations has led to their separate categorization. These cases are now considered distinct from DLBCL and are named “high-grade B-cell lymphoma” (HGBL). Most are characterized by distinct rearrangements, but others have high-grade morphological features without these and are called HGBL-not otherwise specified. Studies have demonstrated that this group of diseases leads to poor outcomes following standard rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone therapy; retrospective and recent single-arm, multicenter studies suggest they should be approached with dose-intense treatment platforms. As yet, this has not been validated in randomized trial settings due to the rarity of these diseases. In the relapsed and refractory setting, novel approaches such as anti-CD19 chimeric antigen receptor T cells and antibodies against CD19 have demonstrated high efficacy in this subgroup. Recently, genomic studies have made much progress in investigating some of the molecular underpinnings that drive their lymphomagenesis and have paved the way for testing additional novel approaches.
Learning Objectives
Understand recent developments in the elucidation of MYC and BCL2 aberrations that are helpful in the categorization of these lymphomas and the implications for therapeutic approaches
Review recent clinical outcomes using various strategies for HGBL, both in the up-front and relapsed/refractory setting, and understand which novel therapies may be useful in managing these diseases
CLINICAL CASE
A 46-year-old previously well man presented with a 4-week history of bilateral neck swelling, fevers, night sweats, and moderate weight loss. Fluorodeoxyglucose-positron emission tomographic (FDG-PET) imaging demonstrated hypermetabolic diffuse lymphadenopathy (node diameters up to 4 cm: maximum standardized uptake values >25) on both sides of the diaphragm in addition to bone marrow involvement. His lactate dehydrogenase was elevated; his Eastern Cooperative Oncology Group performance status was 1. HIV testing was negative. Following an inconclusive fine-needle aspiration, he underwent an excisional biopsy of a right 3-cm cervical lymph node. This demonstrated aggressive CD20+ B-cell lymphoma; tumor cells were CD10+, BCL2+ (>60%), and MYC+ (>90%), and Ki67 was >90%. Fluorescence in situ hybridization (FISH) studies demonstrated a rearrangement of MYC and BCL2 with no BCL6 rearrangement. Hence, his final diagnosis was stage IVB (International Prognostic Index [IPI] 3), high-grade B-cell lymphoma (HGBL) with MYC and BCL2 translocations. He received treatment with 6 cycles of rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone (R-CHOP) that included intrathecal prophylaxis with methotrexate and achieved a complete remission by end-of-treatment PET scan. Eight weeks following the PET scan, he developed recurrent symptoms and neck lymphadenopathy. Reimaging and subsequent biopsy confirmed recurrent disease.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is now recognized as and continues to evolve as a clinically and molecularly heterogeneous disease.1-5 While the addition of rituximab to anthracycline-based treatment has resulted in an overall survival (OS) benefit, standard R-CHOP therapy remains noncurative for a substantial proportion of people. Among the challenges faced in managing newly diagnosed patients are accurately predicting who will not be cured with R-CHOP and deciding if alternative approaches should be used in certain patient subsets. In several studies over a long time span, the IPI has been a reliable predictor of outcome; the prognostic value of baseline tumor biological factors is much less well established and remains very controversial.6 Different groups have reported outcomes and associations with tumor biological characteristics that vary considerably, particularly when comparing prospective and retrospective data. It is well established that most DLBCL cases are of germinal center B-cell (GCB) or activated B-cell (ABC) origin, and many studies have demonstrated an inferior outcome for the latter group. In attempts to improve outcome in this subset, efforts have been underway to add agents to R-CHOP that have selective activity in ABC-DLBCL. However, 2 recently reported large randomized studies did not show a benefit to adding lenalidomide or ibrutinib in ABC- or non-GCB-DLBCL.7-9 Since the conception of these trials, the categorization of DLBCL has undergone further refinement and is focused on more accurately identifying prognostically meaningful genetic drivers of tumorigenesis, which paves the way for novel and more precise therapeutic approaches.1-3 As well as cell-of-origin-focused studies, the recognition that aberrant MYC and BCL2 expression is associated with inferior outcomes has led to investigations aimed at uncovering the mechanistic basis for MYC and BCL2 overexpression. In parallel with this, following improved outcomes with dose- intense approaches in retrospective comparisons, alternatives to R-CHOP are being investigated in these subsets. Coming back to our patient, based on his high IPI score and “double-hit” status, his predicted curability rate with R-CHOP is low. While this is clear from retrospective data, the negative prognostic impact of double-hit is not as well established in prospective experiences, and the optimal management of cases such as these remains controversial. The goal of this review is to explore this question in the context of emerging biological insights and novel clinical data for this subset of patients.
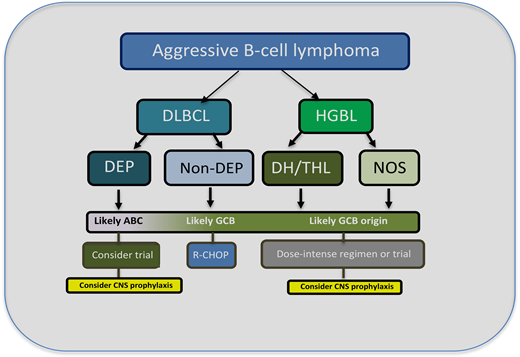
HGBL cases are now considered a distinct entity in the 2016 World Health Organization Lymphoid Tumor Classification.10 They encompass a subset of aggressive cases that, despite overlapping clinical and pathological characteristics, are different from the parent entities of DLBCL and BL (Figure 1). The category includes lymphomas that were previously named “Burkitt-like” and “high grade” as well as “double-hit” or “triple-hit.” In addition to the major category “HGBL with MYC and BCL2 and/or BCL6 translocations,” there is a subset called “HGBL-not otherwise specified” (NOS). Most cases with a single MYC rearrangement fall under DLBCL, as do most cases with high MYC and BCL2 expression but without rearrangements. The updated categorization is helpful in the clinic, as HGBL behaves more aggressively than DLBCL and likely requires distinct therapeutic approaches.11 Though double-hit/triple-hit lymphoma (DHL/THL) and double protein expresser (DPE) cases have MYC and BCL2 aberrations in common, they are distinct in terms of their lymphomagenesis because DLH/THL is mostly GCB derived, while DPE cases are principally of ABC origin (Figure 2).
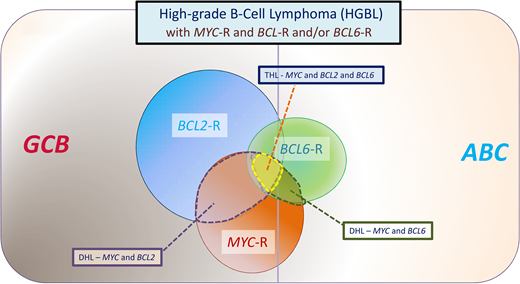
Category of aggressive B-cell lymphomas “HGBLs with MYC and BCL2 and/or BCL6 rearrangements” described in the 2016 revision to the World Health Organization classification of tumors of hematopoietic and lymphoid tumors. Most cases with MYC and BCL2 rearrangements are of GCB origin, whereas most cases with BCL6 rearrangements are of ABC origin. This category includes DH lymphomas, which involve MYC and BCL2 or MYC and BCL6, as well as THLs that involve MYC, BCL2, and BCL6. When translocated, MYC may have an IG or non-IG partner gene, with the former associated with an inferior outcome. In a large study, 7.9% of tumors with DLBCL morphology were assigned to HGBL-DHL/THL, composing 13.3% of GCB and 1.7% of ABC DLBCL.30
Category of aggressive B-cell lymphomas “HGBLs with MYC and BCL2 and/or BCL6 rearrangements” described in the 2016 revision to the World Health Organization classification of tumors of hematopoietic and lymphoid tumors. Most cases with MYC and BCL2 rearrangements are of GCB origin, whereas most cases with BCL6 rearrangements are of ABC origin. This category includes DH lymphomas, which involve MYC and BCL2 or MYC and BCL6, as well as THLs that involve MYC, BCL2, and BCL6. When translocated, MYC may have an IG or non-IG partner gene, with the former associated with an inferior outcome. In a large study, 7.9% of tumors with DLBCL morphology were assigned to HGBL-DHL/THL, composing 13.3% of GCB and 1.7% of ABC DLBCL.30
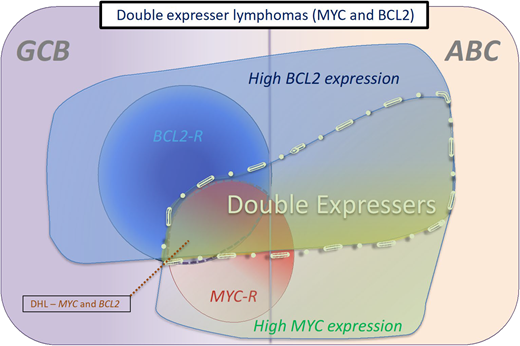
Categories of double-expresser lymphomas. These are typically cases that have a high-protein expression of MYC and BCL2. Most DPE cases that are associated with rearrangements of MYC and BCL2 are of GCB origin, whereas most cases that do not harbor these rearrangements are of ABC origin. The proportion of DLBCL cases that are double expressers has been calculated at between 21% and 44% across various studies.31
Categories of double-expresser lymphomas. These are typically cases that have a high-protein expression of MYC and BCL2. Most DPE cases that are associated with rearrangements of MYC and BCL2 are of GCB origin, whereas most cases that do not harbor these rearrangements are of ABC origin. The proportion of DLBCL cases that are double expressers has been calculated at between 21% and 44% across various studies.31
Recent advances in understanding HGBL cases
Recent studies have set out to better understand the biological underpinnings of HGBL cases and elucidate the specific characteristics associated with inferior outcomes. While FISH testing is currently the gold standard to identify HGBL with MYC and BCL2 and/or BCL6 rearrangements, emerging data from more complex genomic studies suggest that FISH has sensitivity limitations compared to techniques such as whole-exome sequencing.12 Therefore, an important clinically applicable question arises: Does FISH adequately identify—within GCB-DLBCL and HGBL—cases with aberrations of MYC/BCL2 and/or BCL6 that portend an inferior outcome? Probably not, and many cases with critical DHL/THL aberrations are not identified by FISH alone. A double-hit gene expression signature (DHIT-sig) was recently proposed based on the analysis of RNA sequencing data from 157 cases of GCB-DLBCL (including HGBL) treated with R-CHOP.4 This was a 104 gene signature that represented/distinguished 27% of GCB-DLBCLs—and while the majority of GCB-DLBCLs with high-grade morphology had this signature, only half of the DHIT-sig+ cases harbored concurrent MYC and BCL2 rearrangements.4 DHIT-sig+ cases had a significantly inferior time to disease progression compared to DHIT− cases. Another recent study demonstrated that DHIT-sig+ cases with concomitant TP53 abnormalities had a particularly poor outcome.13 It is interesting to consider HGBL cases in the context of newer molecular classifications of DLBCL and propose where they may lie within newly defined, more potentially targetable, subgroups.1-3 In 1 recent study that comprehensively genetically analyzed 304 DLBCL cases and defined 5 distinct subsets (clusters 1-5), tumors with co-occurring BCL2 and MYC structural variants were significantly more frequent in cluster 3, a GCB-defined signature.1
The influence of a rearrangement of MYC is likely affected by the MYC partner gene, whether it is an immunoglobulin (IG) or a non-IG gene.14 In Burkitt lymphoma, the partner of MYC is an IG gene in almost all cases. In contrast, approximately 50% of HGBL-DHL/THLs have a non-IG partner. A recent study demonstrated inferior outcomes for MYC DHL/THL cases with MYC translocated to an IG partner vs a non-IG partner following treatment with R-CHOP.14 In interpreting the results of studies looking at the prognostic impact of DHL/THL and single-hit lymphoma (SHL) MYC aberrations, it is important to consider recent genomic studies that have elucidated novel genetic subtypes of DLBCL.1-4 Within GCB tumors, newly characterized subsets with distinct clinical outcomes exhibit the co-occurrence of specific genetic alterations. There is likely significant overlap between aberrations in these subsets and DHIT-sig+ cases that require future investigation.
Approach to HGBL with MYC and BCL2 and/or BCL6 rearrangements
Currently, no widely accepted standard approach exists to the initial management of these cases. Clinical presentation differences between DLBCL and HGBL have not been well defined, but early studies demonstrated frequent extranodal involvement and a higher rate of central nervous system (CNS) disease in the latter entity. Multiple retrospective and observational studies have demonstrated that following R-CHOP treatment, survival is significantly inferior compared to patients who do not harbor these aberrations. Early retrospective studies demonstrated a particularly adverse outcome for this subset that is less striking in some recent studies. This may be partly explained by a historical selection bias in applying FISH testing to select patients with more aggressive clinical presentations vs the more recent standard of applying it to the majority of new cases. A recent large-scale retrospective analysis of DLBCL patient outcomes following R-CHOP—from prospective trials and patient registries—reported interesting findings.14 While the analysis showed that patients with a MYC rearrangement had a significantly shorter progression-free survival (PFS) and OS, the adverse impact of the rearrangement was confined to those harboring a concurrent BCL2 and/or BCL6 rearrangement and an IG vs non-IG partner with MYC. It is important to note that this study only included cases with DLBCL morphology, and this may have contributed to better outcomes compared to historical experiences in which blastoid and Burkitt morphologies were included.
Should approaches beyond R-CHOP be considered for HGBCL? Two recent prospective studies of DLBCL patients harboring a MYC rearrangement (with a high proportion having DHL) were reported. Based on retrospective comparisons showing that DHL cases did better with more dose-intensive approaches compared to R-CHOP, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, hydroxydaunorubicin, and rituximab (DA-EPOCH-R) was tested in a prospective, multicenter study of 53 patients with MYC rearrangement (MYC-R) aggressive B-cell lymphoma.15 More cases were DHL than SHL-MYC-R. The majority of patients had advanced-stage disease (81%), and 48-month event-free survival (EFS) and OS were 71% and 77%, respectively. A phase 2 HOVON trial added lenalidomide to R-CHOP in 82 patients, hypothesizing that because lenalidomide downregulates MYC and its target genes it may be therapeutically advantageous in MYC-R lymphoma.15 Sixty-five percent of cases had a DHL or THL, and 2-year EFS and OS were 63% and 73%, respectively. A recent British study evaluated patients with high-risk DLBCL using cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate, ifosfamide, etoposide, and high-dose cytarabine (R-CODOX-M/R-IVAC).16 All patients had an IPI score of 3 or higher. Most were GCB, and over 10% had DHL, suggesting that a high proportion may have had HGBL. Two-year PFS was 67.9%, and 2-year OS was 76%, and while not directly compared to R-CHOP, the results were superior to historical experiences using R-CHOP in a similar population. Leppa and colleagues investigated dose-dense immunochemotherapy in 139 patients with high-risk DLBCL and demonstrated a 5-year OS rate of 83%.17 The outcome of patients with BCL2/MYC DHL was similar to patients without rearrangements, suggesting a benefit from dose-intense therapy in the DHL group. Also, a large, recently published French retrospective study evaluated 160 patients with HGBL (with MYC and BCL2 and/or BCL6) and demonstrated a significantly longer PFS for intensive therapy vs R-CHOP.18 HGBL-NOS cases are much rarer, and hence, there are a paucity of data to inform on optimal treatment.
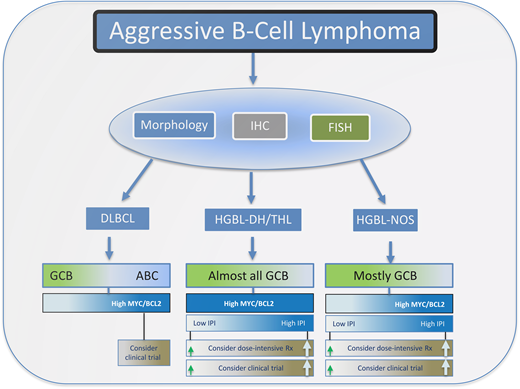
In selecting therapy for patients with HGBL, the stage of disease and IPI characteristics may be important (Figure 3). Retrospective experiences have demonstrated good outcomes for patients with limited-stage aggressive B-cell lymphoma despite high-risk cytogenetics.19 It may be reasonable to approach these early-stage patients with standard R-CHOP. For higher- stage and high-IPI patients, more intensive immunochemotherapy approaches are reasonable to consider, understanding that there are a paucity of robust comparison data (Table 1). HGBL tumors are not uncommonly encountered in the setting of HIV infection, and based on equivalent outcomes to HIV− cases in recent prospective studies (where up to 25% of patients accrued were HIV+), they should not be approached differently.
Outline for the workup and management of aggressive B-cell lymphomas. Typically, morphological, immunohistochemistry, and FISH analysis are performed to differentiate DLBCL from HGBL. HGBL cases are divided into those that are DHL/THL or NOS. For DLBCL cases that have a high-protein expression of MYC and BCL2, which are usually of ABC origin, dose-intensive therapy or enrollment in a clinical trial should be considered, particularly for patients with a high IPI score.
Outline for the workup and management of aggressive B-cell lymphomas. Typically, morphological, immunohistochemistry, and FISH analysis are performed to differentiate DLBCL from HGBL. HGBL cases are divided into those that are DHL/THL or NOS. For DLBCL cases that have a high-protein expression of MYC and BCL2, which are usually of ABC origin, dose-intensive therapy or enrollment in a clinical trial should be considered, particularly for patients with a high IPI score.
Select recent studies in aggressive B-cell lymphoma looking at (differential) outcomes of patients with HGBL and DHL/THL
| Study . | N . | Patient population/study . | DHL/THL % . | Treatment . | Outcome . |
|---|---|---|---|---|---|
| Rosenwald et al14 | 2383: (MYC-R in 11%) | DLBCL and HGBL/retrospective analysis of prospective and patient registry studies | 5.8% | R-CHOP | MYC-R was associated with shorted PFS and OS; neg. prognostic impact of MYC-R only with BCL2 and/or BCL6 and an IG partner. |
| Dunleavy et al15 | 53 | MYC-R and aggressive B-cell lymphoma/prospective, single-arm, multicenter trial | Approx 44%* had MYC-R (SH); 56% had DHL/THL | DA-EPOCH-R | 4-year EFS and OS were 71% and 77%. No difference for SH vs DHL/THL. |
| Chamuleau et al29 | 82 | MYC-R DLBCL/prospective, single-arm multicenter trial | Approx 27%* had MYC-R (SH); 73% had DHL/THL | R-CHOP + lenalidomide | 2-year EFS and OS were 63% and 73%. |
| Leppä et al17 | 139 | DLBCL and high-IPI score/high-risk cohort/prospective, single-arm, multicenter trial | 12% had DHL | Dose-dense chemo (MTX/R-CHOEP-14, ARA C | 5-year FFS and OS were 74% and 83%. No significant worse outcome for DHL group. |
| McMillan et al16 | 111 | DLBCL and IPI 3-5; 12% had HGBL/prospective study. | 12% had DHL; FISH performed in approx. 50% | R-CODOX-M/R-IVAC | 2-year PFS and OS were 68% and 76%. No worse outcome for DHL. |
| Laude et al18 | 160 | All patients had HGBL/retrospective study | 81% had DHL; 19% had THL | R-CHOP vs intensive chemotherapy | At 32 months, 2 and 4-year PFS were 40% and 28% for R-CHOP; 57% and 52% for intensive therapy. |
| Study . | N . | Patient population/study . | DHL/THL % . | Treatment . | Outcome . |
|---|---|---|---|---|---|
| Rosenwald et al14 | 2383: (MYC-R in 11%) | DLBCL and HGBL/retrospective analysis of prospective and patient registry studies | 5.8% | R-CHOP | MYC-R was associated with shorted PFS and OS; neg. prognostic impact of MYC-R only with BCL2 and/or BCL6 and an IG partner. |
| Dunleavy et al15 | 53 | MYC-R and aggressive B-cell lymphoma/prospective, single-arm, multicenter trial | Approx 44%* had MYC-R (SH); 56% had DHL/THL | DA-EPOCH-R | 4-year EFS and OS were 71% and 77%. No difference for SH vs DHL/THL. |
| Chamuleau et al29 | 82 | MYC-R DLBCL/prospective, single-arm multicenter trial | Approx 27%* had MYC-R (SH); 73% had DHL/THL | R-CHOP + lenalidomide | 2-year EFS and OS were 63% and 73%. |
| Leppä et al17 | 139 | DLBCL and high-IPI score/high-risk cohort/prospective, single-arm, multicenter trial | 12% had DHL | Dose-dense chemo (MTX/R-CHOEP-14, ARA C | 5-year FFS and OS were 74% and 83%. No significant worse outcome for DHL group. |
| McMillan et al16 | 111 | DLBCL and IPI 3-5; 12% had HGBL/prospective study. | 12% had DHL; FISH performed in approx. 50% | R-CODOX-M/R-IVAC | 2-year PFS and OS were 68% and 76%. No worse outcome for DHL. |
| Laude et al18 | 160 | All patients had HGBL/retrospective study | 81% had DHL; 19% had THL | R-CHOP vs intensive chemotherapy | At 32 months, 2 and 4-year PFS were 40% and 28% for R-CHOP; 57% and 52% for intensive therapy. |
Of cases tested.
ARA C, cytarabine; CHOEP, CHOP with etoposide; FFS, failure-free survival; MTX, methotrexate.
Approach to DPE lymphomas
MYC, BCL2, and BCL6 are overexpressed by several mechanisms other than gene rearrangements, and a high proportion of aggressive B-cell lymphoma cases have high-protein expression of MYC and BCL2 and/or BCL6. These DPE cases are associated with an inferior outcome following standard therapy—when lacking rearrangements, they are typically of ABC origin. How best to approach them therapeutically is unclear. There is no evidence from retrospective studies that intensive therapy approaches are superior. These patients should therefore be considered for enrollment in clinical trials, where agents and approaches that target the key pathogenetic mechanisms underpinning MYC and BCL2 activation are being investigated.
Role of CNS prophylaxis in HGBL
As is the case in DLBCL, the role of CNS prophylaxis in HGBL is controversial, and unfortunately, prospective studies that could potentially clarify this question are lacking.20,21 From retrospective data, the incidence of CNS relapse in early-stage or low-IPI cases is very low, suggesting that CNS prophylaxis may have very minimal potential benefit.19 However, several series have now demonstrated high CNS relapse rates in advanced-stage and high-IPI cases, and some retrospective single-arm comparison experiences suggest that prophylaxis may diminish CNS relapse incidence.17 It is very challenging to conduct a large-scale prospective trial to properly address this question and reliably guide clinical practice. Until that is done, how to approach CNS prophylaxis will remain controversial, with a lack of consensus. Given our patient's stage IV disease with bone marrow infiltration and DHL status and the high potential for CNS spread, we decided to institute intrathecal CNS prophylaxis.
CLINICAL CASE (continued)
Following confirmation of refractory/relapsed disease, the patient went on to receive rituximab, ifosfamide, carboplatin, and etoposide (R-ICE) chemotherapy followed by autologous stem cell transplantation. Unfortunately, 6 weeks following the transplant, he had further progressive disease. He then went on to receive anti-CD19 chimeric antigen receptor (CAR) T-cell therapy (axicabtagene ciloleucel) and had a complete response. He was still in remission 6 months following the completion of CAR T cells.
Approach to relapsed/refractory HGBL
Given the rarity of these tumors, it is unknown if relapsed/refractory HGBL should be approached differently to DLBCL without high-risk cytogenetics. Some retrospective studies have shown that patients with these diseases (compared to other aggressive B-cell lymphomas) fare more poorly following autologous stem cell transplantation.22 This may be partly explained by the fact that HGBL cases are more likely to receive more intensive immunochemotherapy than R-CHOP in the up-front setting. Recently published and ongoing studies looking at approaches such as targeting CD19 in the relapsed/refractory setting have, interestingly, shown that HGBL biology is not associated with a worse outcome.23,24 Considering that this has not been the experience with autologous stem cell transplantation, it suggests a potential benefit to moving these new treatment modalities to the frontline setting.
Promising new approaches
Many promising approaches are in development for this group of diseases. First, strategies that incorporate targeting BCL2 and MYC are under investigation in trials.25,26 Undoubtedly, the concurrent high expression of MYC and BCL2, irrespective of pathogenesis, is associated with a higher risk of treatment failure, but the individual contributions of these pathways, in terms of conferring resistance, are not well understood. Venetoclax, a highly selective inhibitor of BCL2, has activity in several lymphomas and was recently combined with R-CHOP chemotherapy in a frontline phase 2 study for patients with DLBCL (phase 2 CAVALI study).27 While this combination demonstrated good activity and the potential to improve outcome in a BCL2 immunohistochemistry subgroup, it was associated with increased myelotoxicity compared to R-CHOP alone. Currently, a randomized prospective study of immunochemotherapy with or without venetoclax is ongoing (NCT03984448). Inhibitors of MYC are also in development; small-molecule inhibitors of the bromodomain and extraterminal domain proteins are also interesting with respect to MYC. Epignentic inhibitors such as those targeting histone deacetylase (HDAC)3 and EZH2 inhibitors are under evaluation in pre-clinical studies. Recently, up-front studies have started to incorporate novel strategies such as anti-CD19 CAR T cells in treatments for high-risk DLBCL and HGBCL patients who do not have early complete responses.28
Conclusions
HGBLs are a huge therapeutic challenge, and their optimal management remains undefined at this time. It is critical to continue to make inroads in understanding their biology and how that interacts and overlaps with other key genetic and functional drivers of lymphomagenesis. In that regard, recent work such as the identification of new prognostic signatures such as the DHIT-sig is welcomed; additionally, the definition of novel, potentially more actionable, DLBCL subgroups is helpful in the quest to better understand the molecular basis of MYC and BCL2 dysregulation. It is clear that standard R-CHOP treatment remains inadequate for an unacceptably high proportion of cases; a subset of these cases may benefit from dose-intensive approaches, but this needs better investigation in, ideally, randomized prospective trials. The challenge is that these diseases are rare, and until these trial results are available, standards can only be based on retrospective/observational experiences and single-arm prospective studies. In the relapsed and refractory setting, HGBL cases do not fare worse than DLBCL cases following novel strategies, as discussed earlier. This suggests a role for these approaches in earlier lines of therapy for HGBL. Additionally, many promising novel agents are under investigation for these diseases. Until we augment cure rates significantly for this patient population with a widely available strategy, it is a priority to consider referral of HGBL patients to promising clinical trials.
Conflict-of-interest disclosure
Kieron Dunleavy: advisory board member: Genmab, Morphosys, Beigene, Daiichi Sankyo, Abbvie, ADC Therapeutics, Incyte, Astra Zeneca, Genetech, Janssen.
Off-label drug use
Kieron Dunleavy: nothing to disclose.