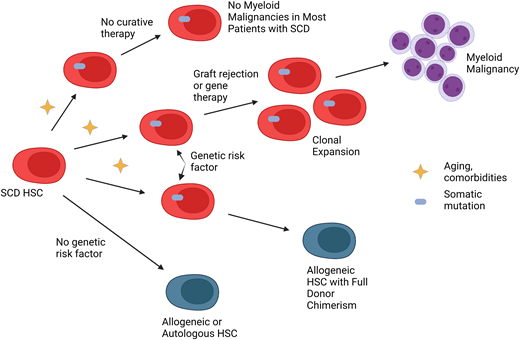
Abstract
Allogeneic hematopoietic cell transplantation, gene therapy, and gene editing offer a potential cure for sickle cell disease (SCD). Unfortunately, myelodysplastic syndrome and acute myeloid leukemia development have been higher than expected after graft rejection following nonmyeloablative conditioning and lentivirus-based gene therapy employing myeloablative busulfan for SCD. Somatic mutations discovered in 2 of 76 patients who rejected their grafts were identified at baseline at much lower levels. While a whole-genome sequencing analysis reported no difference between patients with SCD and controls, a study including whole-exome sequencing revealed a higher prevalence of clonal hematopoiesis in individuals with SCD compared with controls. Genetic risk factors for myeloid malignancy development after curative therapy for SCD are currently being explored. Once discovered, decisions could be made about whether gene therapy may be feasible vs allogeneic hematopoietic cell transplant, which results in full donor chimerism. In the meantime, care should be taken to perform a benefit/risk assessment to help patients identify the best curative approach for them. Long-term follow-up is necessary to monitor for myeloid malignancies and other adverse effects of curative therapies for SCD.
Learning Objectives
Hematopoietic cell transplantation offers a curative option for children and adults with sickle cell disease
Graft rejection after nonmyeloablative transplants and lentivirus-based gene therapy has had a high incidence of myelodysplastic syndrome and acute myeloid leukemia
CLINICAL CASE
The patient, referred to as Mr Johnson, is a 35-year-old African American man with homozygous sickle cell disease. He has infrequent vaso-occlusive crises requiring 1 hospitalization every 1 to 2 years. Mr Johnson has chronic renal insufficiency with a baseline creatinine of 2.5 mg/dL, an estimated glomerular filtration rate (eGFR) of 39 mL/min/1.73 m2 , and a recent kidney biopsy specimen that diagnosed focal segmental glomerulosclerosis. His tricuspid regurgitant velocity (TRV), as diagnosed by a transthoracic echocardiogram, is 2.7 m/s. He has no human leukocyte antigen (HLA)–matched siblings. Mr Johnson is therefore referred for haploidentical hematopoietic cell transplantation due to chronic renal insufficiency and an elevated TRV.
Sickle cell disease (SCD) was initially reported in 1910 when abnormal red blood cells were identified under a microscope in a patient with severe anemia. Later, SCD was found to be caused by a point mutation that changed glutamic acid to valine, leading red blood cells to transform from an easily deformable biconcave disk to a stiff, sickle-shaped cell that occludes the microvasculature. Approximately 100 000 Americans live with SCD.1 However, the estimated number of live births globally was reported to be more than 300 000 in 2010, predominantly in sub- Saharan Africa.2
The most common complication experienced by individuals with SCD is unbearable pain. However, patients may develop acute complications, including acute chest syndrome and stroke, and chronic complications that affect the quality of life. Multiple studies have reported that adults with a TRV of at least 2.5 m/s, obstructive pulmonary disease defined as a forced expiratory volume in 1 second of <70% predicted, and chronic renal insufficiency, defined in part as an eGFR <60 mL/min/1.73 m2 , die early, especially if more than one of the organs is impaired.3 The median survival for patients with SCD has not changed substantially over the past 25 years.4
For about 2 decades, only 1 treatment approved by the US Food and Drug Administration, hydroxyurea, was available to manage patients with SCD. Over the past 5 years, 3 additional drugs have been approved by the Food and Drug Administration: L-glutamine, crizanlizumab, and voxelotor. Red blood cell transfusions can also treat severe complications, including stroke. However, none of those treatments offer a cure. Multiple potentially curative options currently exist. The most extensive experience involves myeloablative HLA-matched sibling hematopoietic cell transplant (HCT) in pediatric patients with SCD. High-dose chemotherapy is given to facilitate the replacement of recipient bone marrow with that of the donor. Efficacy is high, particularly in patients younger than 16 years, with 4- to 5-year overall survival of 95% and event-free survival (EFS) of up to 93%.5,6 However, there is concern that adults such as Mr Johnson cannot tolerate myeloablative conditioning due to organ impairment.
Instead of high-dose chemotherapy, nonmyeloablative conditioning uses immunosuppressive agents to decrease the risk of graft rejection and graft-vs-host disease (GVHD). The latter has no benefit to individuals with SCD. Furthermore, data suggested that full-donor chimerism was unnecessary to reverse SCD. Because mixed chimerism could also decrease GVHD risk, National Institutes of Health investigators developed a regimen that could induce immunologic tolerance with stable mixed chimerism. Alzahrani et al7 recently reported 122 patients with a median age of 29 years who underwent nonmyeloablative HLA-matched sibling HCT at 1 of 3 centers. Five-year overall survival was 93%, and EFS was 85%. Importantly, no one developed grade 3 to 4 acute or chronic GVHD.
Despite excellent outcomes in adults and children with SCD, the lack of HLA-matched sibling donors greatly limits HLA-matched sibling HCT. Because most patients have a haploidentical donor, haploidentical HCT has been increasingly explored. The 2 original adult haploidentical trials were disappointing. While the incidence of GVHD was low and adults with severe organ damage could tolerate nonmyeloablative conditioning, the graft failure rate was at best 50%.8,9 Unfortunately, Mr Johnson was one of the patients who experienced graft rejection.
CLINICAL CASE (Continued)
We discussed with Mr Johnson that his survival was expected to be shortened with standard therapy because of his chronic renal insufficiency and elevated TRV. We also discussed the risks of graft rejection and GVHD and that those same comorbidities may increase his risk for transplant-related mortality. He decided to proceed with HCT and received alemtuzumab, 400 cGy total body irradiation (TBI) in divided doses, peripheral blood stem cells from his 22-year-old half-sister, and 100 mg/kg cyclophosphamide in divided doses on days 3 and 4 post-HCT per protocol. He engrafted on day 22 post-HCT. On day 60 post-HCT, his donor myeloid chimerism was 62% and donor CD3 chimerism 1%. Shortly after that, he presented with fever and pancytopenia. By day 82 post-HCT, he rejected his graft with the eventual return of his SCD.
Due to the high rate of graft rejection with no significant acute or chronic GVHD in the 2 haploidentical studies described above,8,9 additional upfront immunosuppression was explored to increase the efficacy while maintaining a low incidence of GVHD. Fortunately, the efficacy of haploidentical studies for SCD that include increased immunosuppression pre-HCT and posttransplant cyclophosphamide reported since 2018 has been much more encouraging. Nine studies have been reported, including 121 patients (Table 1). Combined EFS is 88%, with a mortality rate of 6%. While 19% of patients experienced grade 2 to 4 acute GVHD, only 7% developed moderate chronic GVHD with no reports of severe chronic GVHD. Therefore, EFS, GVHD, and mortality rates are comparable to the HLA-matched sibling setting.
Outcomes of haploidentical HCT trials with posttransplant cyclophosphamide published since 2018
| Reference . | Graft type . | No. of patients (age, y) . | Alive without SCD, No. . | Acute GVHD (grade 2-4), No. . | Chronic GVHD (moderate to severe), No. . | Death (cause), No. . |
|---|---|---|---|---|---|---|
| Frangoul et al27 | Primed BM or PBSC | 4 (12-23) | 4 | 4 (grade 2) | 0 | 0 |
| Saraf et al28 | PBSC | 8 (20-38) | 6 | 2 | 1 (moderate) | 1 (unknown) |
| Pawlowska et al29 | BM or PBSC | 4 (12-23) | 4 | 0 | 0 | 0 |
| de la Fuente et al30 | Primed BM | 15 (12-26) | 14 | 3 | 1 (moderate) | 0 |
| Bolaños-Meade et al31 | BM | 12 (6-31) | 10 | 29% (grade 2-3) | 1 (moderate) | 0 |
| Kassim et al32 | BM | 41 (1.3-43) | 36 | 4 | 5 (moderate) | 3 (infection) |
| Jaiswal et al33 | PBSC | 5 (8-19) | 5 | 0 | 0 | 0 |
| Oostenbrink et al34 | BM | 7 (1.8-17.5)a | 79%a | 25%a (grade 3-4) | 0 | 0 |
| Kharya et al35 | PBSC | 25 (1-27) | 22 | 5 | 0 | 3 (infection in 2, GVHD in 1) |
| Total | BM or PBSC | 121 | 88% | 19% (of total) | 7% (of total) | 6% |
| Reference . | Graft type . | No. of patients (age, y) . | Alive without SCD, No. . | Acute GVHD (grade 2-4), No. . | Chronic GVHD (moderate to severe), No. . | Death (cause), No. . |
|---|---|---|---|---|---|---|
| Frangoul et al27 | Primed BM or PBSC | 4 (12-23) | 4 | 4 (grade 2) | 0 | 0 |
| Saraf et al28 | PBSC | 8 (20-38) | 6 | 2 | 1 (moderate) | 1 (unknown) |
| Pawlowska et al29 | BM or PBSC | 4 (12-23) | 4 | 0 | 0 | 0 |
| de la Fuente et al30 | Primed BM | 15 (12-26) | 14 | 3 | 1 (moderate) | 0 |
| Bolaños-Meade et al31 | BM | 12 (6-31) | 10 | 29% (grade 2-3) | 1 (moderate) | 0 |
| Kassim et al32 | BM | 41 (1.3-43) | 36 | 4 | 5 (moderate) | 3 (infection) |
| Jaiswal et al33 | PBSC | 5 (8-19) | 5 | 0 | 0 | 0 |
| Oostenbrink et al34 | BM | 7 (1.8-17.5)a | 79%a | 25%a (grade 3-4) | 0 | 0 |
| Kharya et al35 | PBSC | 25 (1-27) | 22 | 5 | 0 | 3 (infection in 2, GVHD in 1) |
| Total | BM or PBSC | 121 | 88% | 19% (of total) | 7% (of total) | 6% |
BM, bone marrow; PBSC, peripheral blood stem cell.
Results reported for the combined group of 14 patients who underwent mismatched HCT: 7 haploidentical and 7 mismatched unrelated donors. Eleven patients had SCD and 3 β-thalassemia major.
In an attempt to decrease the incidence of graft rejection on National Institutes of Health protocols, pentostatin and oral cyclophosphamide preconditioning was added, as the 2 agents have been shown preclinically to synergistically deplete lymphocytes and induce mixed chimerism.10 While the incidence of acute graft rejection on both protocols has been lower than on the historical protocols, enrollment is ongoing (clinicaltrials.gov identifiers NCT02105766 and NCT03077542).
Autologous gene therapy approaches to curative therapy for SCD have also emerged to increase the donor pool and avoid complications such as GVHD. The efficacy of gene addition therapies, where a vector is used to transport a gene into hematopoietic stem and progenitor cells (HSPCs), has been impressive. The largest study was recently reported, using a lentiviral vector that encodes for antisickling hemoglobin (HbAT87Q).11 All 35 patients in the third cohort of the study (group C) engrafted with an increase in median total hemoglobin from 8.5 g/dL at baseline to at least 11 g/dL between 6 and 36 months after infusion. The alternative option is gene editing, where techniques such as clustered regularly interspaced short palindromic repeats-associated protein 9 or zinc finger nuclease are used to edit the abnormal gene and replace it with a normal gene or induce fetal hemoglobin. An early study involving gene editing for SCD has also been promising.12 Because both gene therapy and gene-editing strategies include myeloablative conditioning, however, many adults are not eligible due to preexisting organ damage.
CLINICAL CASE (Continued)
Mr Johnson presented 3 years post-HCT with an absolute neutrophil count of 740/µL, a hemoglobin of 8.2 g/dL, and a platelet count of 135 K/µL. His bone marrow evaluation before the transplant performed per protocol revealed erythroid hyperplasia consistent with SCD. Bone marrow cytogenetics were normal (46,XY). At 3 years post-HCT, bone marrow evaluation showed dysplasia in megakaryocytes and myeloid elements with increased marrow fibrosis and less than 5% myeloid blasts consistent with myelodysplastic syndrome (MDS). The percent donor chimerism on peripheral blood and bone marrow was 0%. Bone marrow cytogenetics were complex. Fluorescence in situ hybridization testing revealed 5q deletion, gain of the mixed-lineage leukemia gene, and a pathogenic TP53 mutation (p.Arg175His (c.524G>A)) with a variant allele frequency (VAF) of 48%. He was treated with 2 cycles of decitabine and venetoclax but did not go into remission. He died 6 months after his diagnosis of MDS.
Interestingly, the first patient with SCD who received curative therapy underwent transplantation for concomitant acute myeloid leukemia (AML), and both diseases were cured.13 Two large population studies have reported that myeloid malignancies (in particular AML) are more prevalent in individuals with SCD than in the general population.14,15 One study, for example, reported 6423 patients with SCD who were identified between 1991 and 2014 and were compared to 118 821 African American controls.15 Individuals with SCD were 3.6 times more likely to develop AML. Multiple risk factors exist for myeloid malignancies in individuals with SCD (Table 2). However, over 23 years, 6 patients with SCD developed AML compared with 1.67 expected when controlled for age, race, ethnicity, and sex.15 Thus, while the relative risk of developing myeloid malignancies is higher in individuals with SCD, the absolute risk is low.
Risk factors for myeloid malignancy development in patients with sickle cell disease
| Risk factor . | Reference . |
|---|---|
| Erythropoietic stress | Ghannam et al21 |
| Inflammation | Platt36 |
| Hypoxia and acidosis | Muz et al37 |
| Transfusion-related immunomodulation | Vamvakas and Blajchman38 |
| Compromised apoptosis | Ribeil39 |
| Endothelial damage | Hebbel et al40 |
| Increased age | Adelman and Figueroa41 |
| Clonal hematopoiesis | Pincez et al25 |
| Risk factor . | Reference . |
|---|---|
| Erythropoietic stress | Ghannam et al21 |
| Inflammation | Platt36 |
| Hypoxia and acidosis | Muz et al37 |
| Transfusion-related immunomodulation | Vamvakas and Blajchman38 |
| Compromised apoptosis | Ribeil39 |
| Endothelial damage | Hebbel et al40 |
| Increased age | Adelman and Figueroa41 |
| Clonal hematopoiesis | Pincez et al25 |
Conversely, the risk of developing myeloid malignancies in certain settings related to curative therapy is higher. The first report of AML developing after curative therapy for SCD was in a pediatric study in which 50 patients received myeloablative conditioning16 (Table 3). The patient had a history of extensive chronic GVHD followed by MDS at 3.5 years post-HCT, which transformed to AML. The AML was donor derived. However, most cases have been reported in 4 studies after adults received nonmyeloablative conditioning and experienced graft failure. The TBI dose reported in 3 studies ranged from 200 to 400 cGy. Eight patients developed MDS or AML from 0.33 to 7 years post-HCT. Age at HCT ranged from 20 to 44 years. Seven patients were deceased, and the eighth patient was alive with refractory AML and 21 months of follow-up.
Summary of reported patients with myeloid malignancies after HCT for SCD
| Reference . | Total No. . | Conditioning . | Graft status . | No. with myeloid malignancies . | Age at HCT, y . | Myeloid malignancy type . | Duration post-HCT, y . | Outcome . |
|---|---|---|---|---|---|---|---|---|
| Vermylen et al16 | 50 | Myeloablative | Engrafted | 1 | NR | AML | 3 | Refractory leukemiaa |
| Janakiram et al42 | NA | ATG, Flu, Cy, 200 TBI, PT-Cy | Graft failure | 1 | 31 | AML | 0.67 | Deceased |
| Li et al43 | NA | Nonmyeloablative + TBI | Graft failure | 1 | 27 | MDS | 7 | Alive |
| Alzahrani et al7 | 65 | Alem, 300 TBI | Graft failure | 1 | NR | MDS | 3 | Deceased |
| Lawal et al19 | 120 | Alem, 300 to 400 cGy TBI, ± PC, ± PT-Cy | Graft failure | 5 | 20 to 44 | AML × 4 MDS × 1 | 0.33 to 5.5 | Deceased |
| Hsieh et al,44 Jones and DeBaun,17 Goyal et al18 | 47 | Busulfan | HGB-206 Group A | 2 | 25 to 42 | AML × 2 | 3 to 5.5 | Deceased |
| Reference . | Total No. . | Conditioning . | Graft status . | No. with myeloid malignancies . | Age at HCT, y . | Myeloid malignancy type . | Duration post-HCT, y . | Outcome . |
|---|---|---|---|---|---|---|---|---|
| Vermylen et al16 | 50 | Myeloablative | Engrafted | 1 | NR | AML | 3 | Refractory leukemiaa |
| Janakiram et al42 | NA | ATG, Flu, Cy, 200 TBI, PT-Cy | Graft failure | 1 | 31 | AML | 0.67 | Deceased |
| Li et al43 | NA | Nonmyeloablative + TBI | Graft failure | 1 | 27 | MDS | 7 | Alive |
| Alzahrani et al7 | 65 | Alem, 300 TBI | Graft failure | 1 | NR | MDS | 3 | Deceased |
| Lawal et al19 | 120 | Alem, 300 to 400 cGy TBI, ± PC, ± PT-Cy | Graft failure | 5 | 20 to 44 | AML × 4 MDS × 1 | 0.33 to 5.5 | Deceased |
| Hsieh et al,44 Jones and DeBaun,17 Goyal et al18 | 47 | Busulfan | HGB-206 Group A | 2 | 25 to 42 | AML × 2 | 3 to 5.5 | Deceased |
Alem, alemtuzumab; ATG, antithymocyte globulin; cGy, centigray; Cy, cyclophosphamide; Flu, fludarabine; NA, not applicable; NR, not reported; PC, pentostatin/oral cyclophosphamide preconditioning; PT-Cy, posttransplant cyclophosphamide.
As opposed to the other patients who underwent allogeneic HCT and have been reported, this patient developed donor-derived leukemia.
Notably, myeloid malignancies after curative therapy for SCD are not limited to the allogeneic setting. Two of 47 individuals were recently reported to develop AML after myeloablative gene therapy for SCD.17 The age at the time of gene therapy was 25 to 42 years. The myeloid malignancy was diagnosed from 3 to 5.5 years after drug product infusion, and both patients are deceased. Both patients were enrolled as part of the study's first cohort (HGB-206 group A). Conditions in the group C cohort have been optimized to include peripheral blood as the stem cell source instead of bone marrow, enabling a higher dose of HSPCs; a precollection red blood cell transfusion regimen; and ensuring myeloablative dosing of busulfan.18 Long-term follow-up of the patients will evaluate whether those modifications will decrease the incidence of myeloid malignancies.
Five of 120 patients (4.2%) developing MDS or AML at a single center19 and 2 of 47 patients (4.3%) after gene therapy for SCD are higher than expected. One study included registry data for patients who underwent transplantation for SCD between 2008 and 2017.5 Of 910 patients who received transplants, 61% had HLA-matched sibling donors, most were children, and patients received myeloablative, reduced-intensity, or nonmyeloablative conditioning. When excluding patients who underwent transplantation at the single center, only 2 of 908 patients (0.22%) developed a myeloid malignancy; the patients with myeloid malignancies all received nonmyeloablative conditioning. Similarly, of 234 patients younger than 30 years who underwent myeloablative HLA-matched sibling HCT for SCD, no one developed a myeloid malignancy, with a median follow-up of 7.9 years.20 Interestingly, 79 of those patients had mixed chimerism long term. Furthermore, of 125 patients aged 15 to 39 years who underwent myeloablative HLA-matched sibling HCT, 47% had mixed chimerism at the most recent follow-up; there was no report of myeloid malignancies.6
Investigators sought to determine whether somatic mutations identified at the time of myeloid malignancy diagnosis were caused by chemotherapy and TBI conditioning or whether, due to a lifetime of erythropoietic stress and inflammation associated with SCD, they were already present at baseline. Two of the first 3 patients who developed myeloid malignancies after nonmyeloablative HLA-matched sibling (n = 1) or haploidentical (n = 2) HCT had next-generation sequencing performed at myeloid malignancy diagnosis. Both patients had different pathogenic TP53 mutations identified, with VAF ranging from 2.9% to 24.3% in the peripheral blood.21 Digital droplet polymerase chain reaction testing to search for the known mutations revealed that the same TP53 mutations were present at baseline but at much lower levels, with VAF levels ranging from 0.03% to 0.35%. The prevalence of somatic mutations, including TP53, in individuals with SCD has been explored more recently.
The sensitivity of clonal hematopoiesis (CH) detection depends on the depth of the sequencing. Whole-genome sequencing can be used to identify somatic mutations at a VAF as low as 5% to 10%. Somatic mutations present at that level are associated with an increased risk of myeloid malignancy development.22,23 Liggett et al24 analyzed whole-genome sequencing data from approximately 3000 individuals with SCD and 71 000 unaffected controls. There was no difference in the prevalence of CH between groups. This finding was not surprising since the absolute risk of myeloid malignancies is low in patients with SCD who do not receive curative therapies.
On the other hand, whole-exome sequencing detects somatic mutations with a VAF as low as 2.5%. Pincez et al25 analyzed data collected from about 1500 patients with SCD and 7000 unaffected controls. The mean age of the SCD group was younger, 23.6 years of age, compared with 50.1 years in the control group. Despite the SCD group being younger, SCD status was independently associated with an increase in CH (odds ratio, 13.5; 95% confidence interval, 3.1-41.9; P = 5.3 × 10−5). Furthermore, of 15 patients identified with somatic mutations, 2 had TP53 mutations, with VAF ranging from 7.0% to 25.8%. The age of the patients was 23 to 36 years. Neither patient was reported to have a myeloid malignancy, but follow-up ranged from 0 to 2 years.
The most widely known risk factor for CH is increased age.22,23 Interestingly, myeloid malignancy development after curative therapies has occurred most commonly in adults. Furthermore, in primarily a pediatric population who, despite myeloablative conditioning, developed mixed chimerism with persistent autologous regenerative hematopoiesis long term, none had a myeloid malignancy with a median follow-up of almost 8 years.20 Evaluation of the prevalence of low-level CH in individuals with SCD across the age spectrum is urgently indicated.
Why is the incidence of myeloid malignancies higher after graft failure and gene therapy for SCD than in patients who do not receive curative therapies and after successful HCT? The reason is likely multifactorial. The prevalence of CH increases with age in individuals with SCD.25 As patients age and develop comorbidities, we hypothesize that due to erythropoietic stress and inflammation, patients with SCD may develop CH. In the absence of curative therapy, the clones do not expand over time, and most do not develop myeloid malignancies. However, with graft failure or gene therapy that requires reconstitution of autologous HSPCs after being exposed to TBI, chemotherapy, or both, preexisting premalignant clones may expand and eventually lead to myeloid malignancy development.17 Thus, older age at the time of attempted curative therapy19 and low HSPC cell dose, which occurred with HGB-206 group A patients,18 would be expected to have a higher incidence of myeloid malignancies. Long-term follow-up will be necessary to evaluate the incidence of myeloid malignancies in various curative therapy settings. Furthermore, decreasing the age of recipients to the pediatric setting to mitigate the risk of myeloid malignancies must be balanced with the risk of transplant-related mortality compared with at least 98% expected survival to age 18 years in developed countries.26
How should patients be counseled about the risk of myeloid malignancies after curative therapies for SCD? If a genetic risk factor for myeloid malignancy development existed, patients who screened positive could decide to undergo allogeneic HCT to decrease the risk. As many allogeneic donors are young and the incidence of donor-derived leukemia is exceedingly low, allogeneic HCT that results in full-donor chimerism would be preferred. Patients who screen negative could receive any type of curative therapy.
Unfortunately, no genetic risk factors currently exist for myeloid malignancy development after curative therapies for SCD, although this is an active area of research. In Mr Johnson's case, not only was his expected survival decreased as a result of SCD but due to his heart and kidney damage, predicted survival was expected to be even shorter.3 Education about the benefits and potential risks should be carefully explained so that the patient, family, and primary provider can make the best decision for that individual patient. Long-term follow-up is crucial to monitor for myeloid malignancy development and other adverse effects of curative therapy in individuals with SCD.
Acknowledgments
This work was funded by the intramural research program of the National Heart, Lung, and Blood Institute (NHLBI) and the Cooperative Study of Late Effects for SCD Curative Therapies (COALESCE, 1U01HL156620-01, NHLBI); it does not necessarily represent the opinions of the National Institutes of Health. The visual abstract was created with Biorender.com.
Conflict-of-interest disclosure
Courtney D. Fitzhugh does not have a relevant conflict of interest.
Off-label drug use
Courtney D. Fitzhugh: nothing to disclose.