Abstract
Chemotherapy-induced thrombocytopenia (CIT) is common, resulting in increased bleeding risk and chemotherapy delays, dose reduction, and treatment discontinuation, which can negatively affect oncologic outcomes. The only agent approved by the US Food and Drug Administration to manage CIT (oprelvekin) was voluntarily withdrawn from the market by the manufacturer, leaving few options for patients. Therefore, patients experiencing CIT present a significant clinical challenge in daily practice. The availability of thrombopoietin receptor agonists has led to formal clinical trials describing efficacy in CIT as well as a rather extensive body of published observational data from off-label use in this setting but no formal regulatory indications for CIT to date. The accumulated data, however, have affected National Comprehensive Cancer Network guidelines, which now recommend consideration of TPO-RA clinical trials as well as off-label use of romiplostim. This review article details the evidence to date for the management of CIT with thrombopoietin receptor agonists (TPO-RAs), discussing the efficacy data, the specific circumstances when treatment is warranted (and when it is generally unnecessary), and safety considerations. Specific recommendations regarding patient selection, initiation, dosing, titration, and discontinuation for TPO-RA therapy in CIT are given, based on published data and expert opinion where evidence is lacking.
Learning Objectives
Recognize when thrombopoietin receptor agonists are likely to help (and unlikely to help) in managing chemotherapy-induced thrombocytopenia
Understand proper patient selection, initiation, dosing, titration, and discontinuation for use of romiplostim to treat chemotherapy-induced thrombocytopenia
CLINICAL CASE 1
A 44-year-old man with oligometastatic colorectal cancer receiving neoadjuvant chemotherapy with folinic acid, oxaliplatin, and fluorouracil (FOLFOX) for a total of 12 cycles prior to a planned curative resection of a solitary liver metastasis develops significant thrombocytopenia after his fourth cycle of chemotherapy. He had a normal platelet count (201 × 109/L) prior to his first chemotherapy cycle. On day 1 of cycle 5 of treatment, his platelet count is 61 × 109/L, so chemotherapy administration is delayed. One week later, his platelet count is 72 × 109/L. The patient is anxious about further delays, worried that suboptimal treatment could jeopardize his chance of cure. Cycle 5 is administered, albeit with dose-reduced oxaliplatin and without the initial bolus of fluorouracil. Two weeks later, when the patient presents for cycle 6, his platelet count is 51 × 109/L; chemotherapy is again delayed. One week later, his platelet count is 57 × 109/L. De-escalation of his regimen to fluorouracil and folinic acid only is discussed; the patient, discouraged by the lower chance of cure with this option, asks if there are any ways to raise his platelet count to continue receiving FOLFOX. Regular platelet transfusion is briefly considered but felt to be impractical and unlikely to be approved by the institutional blood bank due to the large number of transfusions that would be needed to get him through his remaining planned treatment cycles.
Diagnosis and clinical consequences of chemotherapy-induced thrombocytopenia
Chemotherapy-induced thrombocytopenia (CIT) is a common complication of systemic treatment for cancer.1,2 Although historically associated only with cytotoxic chemotherapy, thrombocytopenia as a complication of treatment with oral targeted therapies is now common as well and also considered under the umbrella of CIT. CIT commonly poses a formidable barrier to receipt of potentially curative or life-prolonging systemic cancer therapy, resulting in suboptimal treatment, patient and provider anxiety, and, when severe, potentially life-threatening bleeding complications.1,3
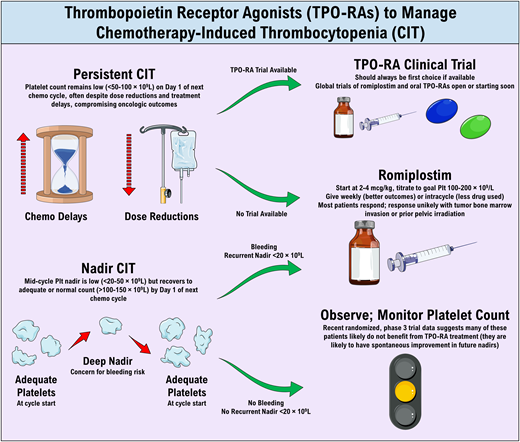
Most thrombocytopenia in patients with cancer receiving chemotherapy is caused by the myelosuppressive or cytotoxic effects of the chemotherapy (so CIT diagnosis is generally straightforward); however, it is important to consider other causes depending on the clinical presentation of the patient (Table 1). There is no universally accepted definition or threshold for diagnosing CIT. In general, CIT is diagnosed when thrombocytopenia presents a barrier to continued chemotherapy at full dose and on schedule, results in clinically significant bleeding, or precludes administration of indicated antithrombotic treatment (ie, treatment of cancer-associated venous thromboembolism [VTE]); studies of CIT usually use a platelet threshold between 50 and 100 × 109/L for diagnosis.4-6 Just as important as the platelet count itself, however, is the timing of the thrombocytopenia. As such, 2 subtypes of CIT are recognized: persistent CIT (demonstrated in Clinical Case 1), characterized by chronic, mild to moderate thrombocytopenia (platelet count 50-100 × 109/L) that does not resolve despite delays in chemotherapy, and nadir CIT, characterized by more severe thrombocytopenia (platelet count <50 × 109/L or even <20 × 109/L) in the middle of a cycle, with recovery to normal or near-normal platelet counts by the start of the following cycle.7
Causes of thrombocytopenia in patients with cancer from a modern cohorta
| Cause . | % of patients . |
|---|---|
| Chemotherapy | 78.6 |
| Multiple causes | 9.3 |
| Infection | 7.9 |
| Myelophthisis | 2.9 |
| Graft-vs-host disease | 0.7 |
| Liver disease | 0.7 |
| Cause . | % of patients . |
|---|---|
| Chemotherapy | 78.6 |
| Multiple causes | 9.3 |
| Infection | 7.9 |
| Myelophthisis | 2.9 |
| Graft-vs-host disease | 0.7 |
| Liver disease | 0.7 |
Adapted with permission from Mantha et al.2
As Clinical Case 1 exemplifies, in most of the world, there are no available regulatory agency–approved therapies for CIT. Recombinant human interleukin 11 (oprelvekin), an agent approved by the US Food and Drug Administration (FDA) to manage CIT, was voluntarily withdrawn from the market by its manufacturer several years ago. This agent, while efficacious in raising the platelet count, was relatively poorly tolerated and resulted in a high burden of constitutional side effects.8 The first-generation thrombopoietic agents, the recombinant human thrombopoietins (recombinant human thrombopoietin and pegylated recombinant human megakaryocyte growth and development factor) were initially under development for CIT over 2 decades ago and were quite promising for this indication.9,10 However, the development of these agents was halted in the West after the occurrence of neutralizing antibodies cross-reactive with native thrombopoietin (TPO) in a small number of patients receiving pegylated recombinant human megakaryocyte growth and development factor.11,12
As a result, management of CIT has relied on reducing relative dose intensity (RDI) of therapy by reducing chemotherapy doses and/or delaying treatments. Unfortunately, RDI reduction has been shown across numerous studies to reduce progression-free and overall survival in patients with cancer.3,13-16 As illustrated in Clinical Case 1, for many cases of persistent CIT, even significant and repeated RDI reductions do not allow for recovery of the platelet count to an acceptable level. When this occurs, a switch to a nonpreferred regimen or even treatment discontinuation is often necessary. Platelet transfusion has a minimal role in the management of CIT given the ephemeral nature of transfused platelets, the limited availability of platelets for transfusion, and the high degree of resource utilization necessary for recurrent, regular transfusions in patients who are not actively bleeding.17
TPO receptor agonists (TPO-RAs), the second generation of thrombopoietic agents, do not have the immunologic risks of the first-generation agents and include agents currently approved by regulatory agencies for the treatment of immune thrombocytopenia,18,19 aplastic anemia,20 periprocedural thrombocytopenia in patients with chronic liver disease,21-23 and thrombocytopenia associated with antiviral therapy for hepatitis C. With broad efficacy in many causes of thrombocytopenia, the TPO-RAs are a promising treatment modality in CIT. Indeed, multiple early- phase clinical trials and observational studies have been published describing the utility and safety of these agents to treat CIT in patients with solid tumor malignancies.4-6 While maintenance of RDI is expected to improve oncologic outcomes, it is important to state that there has not yet been a specific examination of the impact of TPO-RAs on overall survival or quality of life in patients with CIT.
CLINICAL CASE 1 (Continued)
Given no appreciable recovery of the patient's platelet count despite chemotherapy treatment delays, TPO-RA therapy of the patient's CIT is considered. Because the patient does not qualify for a clinical trial of TPO-RA therapy, off-label treatment is pursued. The patient is deemed to be safe to receive TPO-RA therapy and is begun on weekly romiplostim at a starting dose of 3 µg/kg.
Initiation and dosing of romiplostim in persistent CIT
There are 4 FDA-approved TPO-RAs at present: the subcutaneous injectable drug romiplostim and the oral small-molecule agents eltrombopag, avatrombopag, and lusutrombopag; a fifth TPO-RA, hetrombopag, is a newer agent currently only approved in China (Table 2).24,25 While none yet have an indication for CIT, of these agents, romiplostim has the most well-developed body of evidence for use in CIT4,5,26,27 (Table 3) and so is the only TPO-RA recommended by the National Comprehensive Cancer Network guidelines for consideration for off-label use.28 Clinical trials of eltrombopag in CIT have all been early phase and limited to CIT prevention29-31 (ie, incorporating the drug into chemotherapy regimens in unselected populations to reduce the incidence of developing CIT), an approach that has been abandoned as it results in unnecessary TPO-RA use in many patients. Conversely, trials and observational studies of romiplostim have evaluated this agent in the exact clinical circumstances described in Figure 1: persistent CIT precluding further chemotherapy administration, demonstrating the ability of this agent to improve the platelet count in most patients with persistent CIT.4,5,26,27 Of note, all of the aforementioned studies have been in adult patients, and so off-label use of romiplostim should generally be limited to adult patients at this time. Data in pediatric patients are limited to small case series.32-34
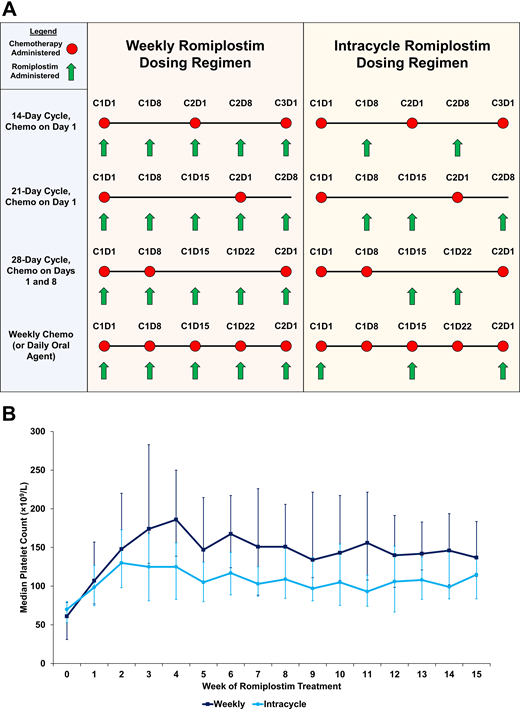
Weekly vs intracycle dosing of romiplostim for CIT. (A) A graphical representation comparing weekly and intracycle dosing over multiple different types of chemotherapy regimens. (B) Median weekly platelet counts in a cohort of patients with solid tumors receiving standard weekly romiplostim dosing (n = 65, dark blue) vs intracycle romiplostim dosing (n = 57, light blue). Error bars represent interquartile ranges. Adapted with permission from Al-Samkari et al.5
Weekly vs intracycle dosing of romiplostim for CIT. (A) A graphical representation comparing weekly and intracycle dosing over multiple different types of chemotherapy regimens. (B) Median weekly platelet counts in a cohort of patients with solid tumors receiving standard weekly romiplostim dosing (n = 65, dark blue) vs intracycle romiplostim dosing (n = 57, light blue). Error bars represent interquartile ranges. Adapted with permission from Al-Samkari et al.5
Comparison of the thrombopoietin receptor agonists
| Characteristic . | Romiplostim . | Eltrombopag . | Avatrombopag . | Lusutrombopag . | Hetrombopag . |
|---|---|---|---|---|---|
| Molecular structure | Peptide | Small molecule | Small molecule | Small molecule | Small molecule |
| TPO receptor site of action | Extracellular domain | Transmembrane domain | Transmembrane domain | Transmembrane domain | Transmembrane domain |
| Route of administration | Subcutaneous | Oral | Oral | Oral | Oral |
| Dosing frequencya | Weekly | Daily | Daily or less frequently than daily | Daily | Daily |
| Relevant food interactions | NA | Yes | No | No | Yes |
| Liver function test monitoring required | No | Yes | No | No | No |
| Current FDA-approved indications | Immune thrombocytopenia (adults and children) | Immune thrombocytopenia (adults and children) Hepatitis C–associated thrombocytopenia Severe aplastic anemia | Periprocedural thrombocytopenia in patients with CLD Immune thrombocytopenia (adults) | Periprocedural thrombocytopenia in patients with CLD | None (approved in China for management of immune thrombocytopenia and severe aplastic anemia) |
| Characteristic . | Romiplostim . | Eltrombopag . | Avatrombopag . | Lusutrombopag . | Hetrombopag . |
|---|---|---|---|---|---|
| Molecular structure | Peptide | Small molecule | Small molecule | Small molecule | Small molecule |
| TPO receptor site of action | Extracellular domain | Transmembrane domain | Transmembrane domain | Transmembrane domain | Transmembrane domain |
| Route of administration | Subcutaneous | Oral | Oral | Oral | Oral |
| Dosing frequencya | Weekly | Daily | Daily or less frequently than daily | Daily | Daily |
| Relevant food interactions | NA | Yes | No | No | Yes |
| Liver function test monitoring required | No | Yes | No | No | No |
| Current FDA-approved indications | Immune thrombocytopenia (adults and children) | Immune thrombocytopenia (adults and children) Hepatitis C–associated thrombocytopenia Severe aplastic anemia | Periprocedural thrombocytopenia in patients with CLD Immune thrombocytopenia (adults) | Periprocedural thrombocytopenia in patients with CLD | None (approved in China for management of immune thrombocytopenia and severe aplastic anemia) |
CLD, chronic liver disease. NA, not applicable.
Per drug label. Like avatrombopag, eltrombopag can be dosed less frequently than once daily,38 although this is not in the drug label.
Findings of representative studies (including 20 or more patients) of thrombopoietin receptor agonists for the treatment of CIT in adults
| Study . | Patient population . | Chemotherapy regimen . | Principal findings . |
|---|---|---|---|
| Romiplostim | |||
| Parameswaran et al27 (CIT treatment) | 20 patients with various solid tumors who developed CIT (platelets <100 × 109/L for ≥6 weeks) Observational cohort | Various regimens | 95% of patients achieved platelets >100 × 109/L 75% resumed cytotoxic chemotherapy and all but 1 of these patients tolerated at least 2 additional chemotherapy cycles on romiplostim support without recurrence of dose-limiting CIT 3 patients developed VTE; bleeding not reported |
| Al-Samkari and Kuter26 (CIT treatment) | 22 patients with various solid tumors who developed CIT (as defined by treating physician) Observational cohort | Various regimens | 95% of patients achieved platelets >100 × 109/L 100% resumed cytotoxic chemotherapy, receiving 2 or more cycles (range, 2-18) on romiplostim Significant reduction in dose reductions and treatment delays on romiplostim, with some patients able to dose-escalate No patients developed VTE; 3 developed bleeding |
| Soff et al4 (CIT treatment) | 60 patients with various solid tumors who developed persistent CIT (platelets <100 × 109/L for ≥4 weeks without chemotherapy treatment) randomized to romiplostim or untreated observation; ultimately, 52 received romiplostim Randomized phase 2 trial | Various regimens | 85% of patients treated with romiplostim achieved platelets >100 × 109/L compared with 12.5% untreated observation Only 7% of patients of patients who achieved platelets >100 × 109/L experienced recurrent chemotherapy dose reduction or treatment delay 10.2% of patients had VTE over 12 months; bleeding not reported |
| Al-Samkari et al5 (CIT treatment) | 173 patients with various malignancies (153 solid tumor, 20 lymphoma/myeloma) who developed persistent CIT (platelets <100 × 109/L for ≥3 weeks or chemotherapy delay of ≥1 week due to thrombocytopenia) Observational cohort | Various regimens | 85% achieved platelets >100 × 109/L (95% without predictors of nonresponse) 71% achieved platelets >75 × 109/L and ≥30 × 109/L higher than pretreatment baseline (82% without predictors of nonresponse) 79% avoided chemotherapy dose reduction or treatment delay; 89% avoided platelet transfusion Bone marrow tumor invasion, prior pelvic irradiation, and prior temozolomide predicted romiplostim nonresponse VTE rate 14 per 100 patient-years; bleeding rate 23 per 100 patient-years (1% of 1063 cycles supported with romiplostim) |
| Eltrombopag | |||
| Kellum et al29 (CIT prevention) | 183 chemotherapy-naive patients with various advanced solid tumors randomized to placebo or eltrombopag 50, 75, or 100 mg; 134 patients completed at least 2 cycles and could be evaluated Randomized phase 2 trial | Carboplatin and paclitaxel | Primary end point (significant difference in the change in platelet count from day 1 in cycle 2 to the platelet nadir in cycle 2 between eltrombopag and placebo-treated patients) not met Eltrombopag-treated patients had higher platelet counts at start of subsequent treatment cycles (higher counts with higher eltrombopag dose) |
| Winer et al31 (CIT prevention) | 26 patients with pancreatic cancer randomized to receive eltrombopag 100 mg or placebo Randomized phase 1 trial | Gemcitabine or gemcitabine plus cisplatin or carboplatin | Mean platelet nadirs significantly higher in eltrombopag-treated patients Chemotherapy dose reduction or treatment delay occurred in 50% of placebo-treated patients vs 14% of eltrombopag-treated patients |
| Winer et al30 (CIT prevention) | 75 patients with various solid tumors randomized to receive eltrombopag 100 mg or placebo; only 26 of the enrolled patients completed the planned number of cycles Randomized phase 2 trial | Gemcitabine or gemcitabine plus cisplatin or carboplatin | Eltrombopag-treated patients had higher platelet counts, lower frequencies of grade 3 or 4 CIT, more rapid platelet count recovery, and fewer dose reductions/treatment delays or missed doses due to thrombocytopenia Rates of grade 3 or 4 CIT remained high overall in both arms |
| Avatrombopag | |||
| Al-Samkari et al5 (CIT treatment) | 122 patients with lung, ovarian, or bladder cancer with primarily nadir CIT randomized to receive avatrombopag 40 mg daily or placebo for 1 chemotherapy cycle Randomized phase 3 trial | Various regimens | Similar proportions of patients achieved the primary end point (of avoidance of chemotherapy treatment delay, dose reduction, bleeding, or platelet transfusion) in avatrombopag (70%) and placebo (73%) groups, due to high rates of unexpected higher platelet count nadirs in the placebo arm during the interventional chemotherapy cycle Avatrombopag-treated patients had higher platelet counts Avatrombopag overall safe and well tolerated in patients with cancer with a safety profile similar to placebo Venous thromboembolism rate 2.4% in avatrombopag arm vs 2.5% in placebo arm |
| Study . | Patient population . | Chemotherapy regimen . | Principal findings . |
|---|---|---|---|
| Romiplostim | |||
| Parameswaran et al27 (CIT treatment) | 20 patients with various solid tumors who developed CIT (platelets <100 × 109/L for ≥6 weeks) Observational cohort | Various regimens | 95% of patients achieved platelets >100 × 109/L 75% resumed cytotoxic chemotherapy and all but 1 of these patients tolerated at least 2 additional chemotherapy cycles on romiplostim support without recurrence of dose-limiting CIT 3 patients developed VTE; bleeding not reported |
| Al-Samkari and Kuter26 (CIT treatment) | 22 patients with various solid tumors who developed CIT (as defined by treating physician) Observational cohort | Various regimens | 95% of patients achieved platelets >100 × 109/L 100% resumed cytotoxic chemotherapy, receiving 2 or more cycles (range, 2-18) on romiplostim Significant reduction in dose reductions and treatment delays on romiplostim, with some patients able to dose-escalate No patients developed VTE; 3 developed bleeding |
| Soff et al4 (CIT treatment) | 60 patients with various solid tumors who developed persistent CIT (platelets <100 × 109/L for ≥4 weeks without chemotherapy treatment) randomized to romiplostim or untreated observation; ultimately, 52 received romiplostim Randomized phase 2 trial | Various regimens | 85% of patients treated with romiplostim achieved platelets >100 × 109/L compared with 12.5% untreated observation Only 7% of patients of patients who achieved platelets >100 × 109/L experienced recurrent chemotherapy dose reduction or treatment delay 10.2% of patients had VTE over 12 months; bleeding not reported |
| Al-Samkari et al5 (CIT treatment) | 173 patients with various malignancies (153 solid tumor, 20 lymphoma/myeloma) who developed persistent CIT (platelets <100 × 109/L for ≥3 weeks or chemotherapy delay of ≥1 week due to thrombocytopenia) Observational cohort | Various regimens | 85% achieved platelets >100 × 109/L (95% without predictors of nonresponse) 71% achieved platelets >75 × 109/L and ≥30 × 109/L higher than pretreatment baseline (82% without predictors of nonresponse) 79% avoided chemotherapy dose reduction or treatment delay; 89% avoided platelet transfusion Bone marrow tumor invasion, prior pelvic irradiation, and prior temozolomide predicted romiplostim nonresponse VTE rate 14 per 100 patient-years; bleeding rate 23 per 100 patient-years (1% of 1063 cycles supported with romiplostim) |
| Eltrombopag | |||
| Kellum et al29 (CIT prevention) | 183 chemotherapy-naive patients with various advanced solid tumors randomized to placebo or eltrombopag 50, 75, or 100 mg; 134 patients completed at least 2 cycles and could be evaluated Randomized phase 2 trial | Carboplatin and paclitaxel | Primary end point (significant difference in the change in platelet count from day 1 in cycle 2 to the platelet nadir in cycle 2 between eltrombopag and placebo-treated patients) not met Eltrombopag-treated patients had higher platelet counts at start of subsequent treatment cycles (higher counts with higher eltrombopag dose) |
| Winer et al31 (CIT prevention) | 26 patients with pancreatic cancer randomized to receive eltrombopag 100 mg or placebo Randomized phase 1 trial | Gemcitabine or gemcitabine plus cisplatin or carboplatin | Mean platelet nadirs significantly higher in eltrombopag-treated patients Chemotherapy dose reduction or treatment delay occurred in 50% of placebo-treated patients vs 14% of eltrombopag-treated patients |
| Winer et al30 (CIT prevention) | 75 patients with various solid tumors randomized to receive eltrombopag 100 mg or placebo; only 26 of the enrolled patients completed the planned number of cycles Randomized phase 2 trial | Gemcitabine or gemcitabine plus cisplatin or carboplatin | Eltrombopag-treated patients had higher platelet counts, lower frequencies of grade 3 or 4 CIT, more rapid platelet count recovery, and fewer dose reductions/treatment delays or missed doses due to thrombocytopenia Rates of grade 3 or 4 CIT remained high overall in both arms |
| Avatrombopag | |||
| Al-Samkari et al5 (CIT treatment) | 122 patients with lung, ovarian, or bladder cancer with primarily nadir CIT randomized to receive avatrombopag 40 mg daily or placebo for 1 chemotherapy cycle Randomized phase 3 trial | Various regimens | Similar proportions of patients achieved the primary end point (of avoidance of chemotherapy treatment delay, dose reduction, bleeding, or platelet transfusion) in avatrombopag (70%) and placebo (73%) groups, due to high rates of unexpected higher platelet count nadirs in the placebo arm during the interventional chemotherapy cycle Avatrombopag-treated patients had higher platelet counts Avatrombopag overall safe and well tolerated in patients with cancer with a safety profile similar to placebo Venous thromboembolism rate 2.4% in avatrombopag arm vs 2.5% in placebo arm |
All patients with clinically significant persistent CIT should first be considered for a clinical trial of TPO-RA therapy, of which several international studies are currently recruiting (phase 3 trials of romiplostim: NCT03937154 and NCT03362177) or set to begin soon (phase 2/3 trial of hetrombopag: NCT05261646). For patients ineligible for a trial or when a trial is not available, off-label use of romiplostim can be considered if payor approval can be obtained.
Based on the existing data, in the off-label setting, romiplostim should be initiated at a dose of 2 to 4 µg/kg.5,28 While romiplostim is dosed weekly in immune thrombocytopenia, studies have compared weekly dosing of romiplostim in CIT with “intracycle” dosing (administration of romiplostim only on chemotherapy off-weeks, resulting in approximately half the dose intensity of usual weekly dosing; Figure 1).5 Intracycle dosing is a reasonable approach (particularly in a resource-limited setting) but unsurprisingly results in higher rates of CIT recurrence and does not appear to result in lower rates of complications (such as thrombocytosis or VTE) as compared with weekly dosing.5 Regardless of whether weekly or intracycle dosing is employed, romiplostim should be titrated based on the platelet count checked immediately prior to its administration, with the dose titrated up or down by 1 to 2 µg/kg at a time to achieve a goal platelet count between 100 and 200 × 109/L in most patients. Thrombocytosis (platelet count >400 × 109/L) is very rare with this approach, but when it occurs, the romiplostim dose can be held for 1 week and restarted at 2 µg/kg lower the following week. Where they are readily available, baseline endogenous TPO levels measured prior to romiplostim initiation may be a useful adjunct to predict likelihood of response to romiplostim treatment in CIT35,36 and potentially guide starting dose, but this is not necessary prior to treatment. TPO-RAs in CIT may be administered alongside granulocyte colony-stimulating factors in situations where the latter are also indicated.
CLINICAL CASE 1 (CONCLUSION)
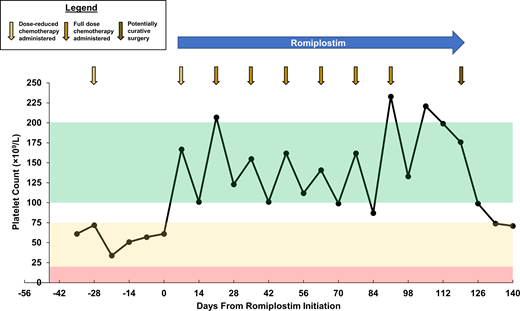
Romiplostim is initiated at a dose of 3 µg/kg with a plan for weekly administration. The following week, the platelet count is 167 × 109/L, and the patient receives cycle 6 of FOLFOX. Weekly romiplostim is titrated to a goal platelet count of 100 to 200 × 109/L without issue or adverse events. After the patient does well during cycle 6 and presents for cycle 7 with a platelet count of 207 × 109/L, the dose intensity of FOLFOX is increased back to baseline (oxaliplatin restored to full dose and fluorouracil bolus added back). The patient receives 6 remaining cycles of FOLFOX on romiplostim support without any recurrence of CIT (Figure 2). Surgery is performed shortly after completion of cycle 12 of FOLFOX, with the final dose of romiplostim just prior to surgery to ensure an adequate operative platelet count. With his platelet count maintained, he receives indicated postoperative venous thromboembolic prophylaxis without issue. Surgery is successful and the patient has no evidence of disease and is followed regularly for surveillance for cancer recurrence.
Platelet count course for Clinical Case 1. Representative of a typical case in which TPO-RA support is necessary for adequate platelet count recovery to support many remaining chemotherapy cycles at full dose and on schedule after development of persistent CIT. Green zone represents goal platelet count on romiplostim, yellow zone represents CIT platelet count range resulting in dose reduction and/or treatment delay of chemotherapy, and red zone represents profound thrombocytopenia resulting in increased risk for bleeding.
Platelet count course for Clinical Case 1. Representative of a typical case in which TPO-RA support is necessary for adequate platelet count recovery to support many remaining chemotherapy cycles at full dose and on schedule after development of persistent CIT. Green zone represents goal platelet count on romiplostim, yellow zone represents CIT platelet count range resulting in dose reduction and/or treatment delay of chemotherapy, and red zone represents profound thrombocytopenia resulting in increased risk for bleeding.
Adverse events and discontinuation of romiplostim support for CIT
Once initiated, if treatment is successful, romiplostim is generally continued until the patient is no longer receiving myelosuppressive or cytotoxic treatments; if surgery is planned shortly after chemotherapy completion as in Clinical Case 1, romiplostim support can be extended to ensure no recurrence of thrombocytopenia resulting in surgical delay. If not, the final dose of romiplostim can be given 1 week prior to the completion of the final chemotherapy cycle (ie, on day 8 of a 14-day cycle).
Treatment is unsuccessful if adequate improvement in the platelet count does not occur despite dose escalation to a maximal weekly dose of 10 µg/kg. In these circumstances, after no significant improvement with 2 to 4 weeks of maximal dosing, romiplostim should be considered futile and discontinued.
As with use of romiplostim in immune thrombocytopenia, adverse events with the use of romiplostim in CIT are uncommon.4,5 Headaches may occur in a minority of patients and are usually transient and readily managed with acetaminophen. Thromboembolic events are generally the most feared potential theoretical complication of TPO-RAs, and patients with cancer are at a baseline elevated risk of thromboembolism. To date, the accumulated data for romiplostim in CIT do not suggest a meaningfully elevated thromboembolic risk, with documented thromboembolic event rates (particularly VTE) similar to comparable cancer patient populations in historical studies.4,5 In addition, randomized data for eltrombopag and avatrombopag in CIT did not demonstrate elevated event rates in TPO-RA–treated patients compared with placebo-treated patients,6,30 but these studies examined short-course treatment only (ie, 1 or 2 chemotherapy cycles). When a thromboembolic event occurs, discontinuation of romiplostim is not necessary in most patients; its continuation will maintain the platelet count at a safe level for both ongoing cancer therapy as well as therapeutic anticoagulation. In the rare scenario of recurrent thromboembolism while on romiplostim despite adequate therapeutic anticoagulation, discontinuation of romiplostim may be considered.
CLINICAL CASE 2
A 67-year-old woman with a history of metastatic pancreatic cancer and cancer-associated VTE receiving treatment with folinic acid, fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) every 2 weeks as the initial regimen to treat her cancer is noted to be significantly thrombocytopenic on her chemotherapy off-weeks. She is on apixaban 5 mg twice daily indefinitely to treat a prior cancer-associated pulmonary embolism, which was hemodynamically significant and resulted in hospitalization. On the off-week of her sixth cycle of FOLFIRINOX, her platelet count is 58 × 109/L. She has experienced no bleeding manifestations, with the exception of a mild nosebleed at home during her fifth treatment cycle (platelet count unknown at the time of the bleed). By day 1 of the following cycle, her platelet count has always reliably recovered to above 100 × 109/L. TPO-RA support to improve her platelet count nadirs is considered given her ongoing need for therapeutic anticoagulation.
Patient selection for TPO-RA treatment of CIT
In contrast to Clinical Case 1, which described a patient with persistent CIT that did not recover despite chemotherapy dose reduction and treatment delay, Clinical Case 2 describes a patient with mild nadir CIT and robust recovery by day 1 of her next treatment cycle. There is anxiety surrounding relatively modest nadirs because she is receiving therapeutic anticoagulation, raising the question of patient selection for TPO-RA support. TPO-RA support is associated with financial cost and risks (as well as a potential modest impact on quality of life with additional clinic visits necessary to administer weekly romiplostim) and should be given only when necessary and to patients likely to benefit.
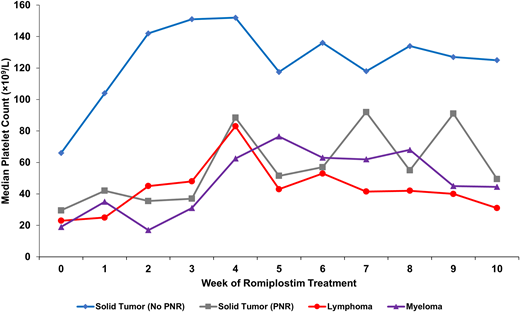
A large observational study evaluating 173 patients receiving romiplostim treatment of persistent CIT found 3 main predictors of nonresponse to romiplostim treatment: known bone marrow involvement by tumor, prior pelvic irradiation, and prior receipt of temozolomide therapy (Figure 3).5 Patients with nonmyeloid hematologic cancers in this study uniformly had involvement of their bone marrow by tumor and had poor outcomes of support as a result. Therefore, data at present primarily support use in patients with solid tumor malignancies without these predictors of nonresponse. There is no role for use of romiplostim to manage CIT in myeloid malignancies, and its use to raise the platelet count to prevent severe thrombocytopenia in myelodysplastic syndrome (recently demonstrated to have no increased risk of leukemic transformation in this population over several years of follow-up37 ) is beyond the scope of this article.
Median weekly platelet counts for various patient populations treated for CIT with romiplostim from a study of 173 patients with CIT. Solid tumor patients with no predictors of romiplostim nonresponse (n = 122, blue); solid tumor patients with predictors of romiplostim nonresponse (n = 31, gray) including bone marrow invasion by tumor, prior pelvic irradiation, or prior temozolomide treatment; patients with aggressive lymphoma (n = 13, red); and patients with myeloma (n = 7, purple). All patients with lymphoma and myeloma had known marrow involvement by tumor. PNR, predictors of nonresponse. Reproduced with permission from Al-Samkari et al.5
Median weekly platelet counts for various patient populations treated for CIT with romiplostim from a study of 173 patients with CIT. Solid tumor patients with no predictors of romiplostim nonresponse (n = 122, blue); solid tumor patients with predictors of romiplostim nonresponse (n = 31, gray) including bone marrow invasion by tumor, prior pelvic irradiation, or prior temozolomide treatment; patients with aggressive lymphoma (n = 13, red); and patients with myeloma (n = 7, purple). All patients with lymphoma and myeloma had known marrow involvement by tumor. PNR, predictors of nonresponse. Reproduced with permission from Al-Samkari et al.5
There are few absolute contraindications to TPO-RA treatment of CIT. Patient functional status must already be adequate for ongoing chemotherapy, so it is not a consideration in CIT management. Patients with a history of thromboembolic events should not be excluded from consideration of TPO-RA treatment of CIT, particularly if they remain on antithrombotic therapy. Even clinical trials of TPO-RAs in CIT allow inclusion of these patients at an interval after the thromboembolic event (usually 1-3 months) so long as they are receiving antithrombotic therapy without complication. Use of TPO-RAs in any patient with severe thrombophilia (eg, multiple recurrent thromboembolic events despite adequate antithrombotic therapy, triple-positive antiphospholipid antibody syndrome) should be approached with caution, and avoidance of TPO-RAs in this setting is often advisable.
Importantly, a recently published phase 3, randomized, placebo-controlled trial of avatrombopag for the treatment of relatively chemotherapy-naive patients with nadir CIT (platelet count <50 × 109/L) demonstrated little utility for the treatment of CIT in patients developing nadir CIT during their first or second chemotherapy regimens.6 This study excluded patients who had received multiple prior chemotherapy regimens and those with any prior incidence of CIT. While avatrombopag was effective to increase the platelet count, the study was negative due to high rates of spontaneous recovery in the placebo arm (ie, on-study nadirs improved substantially in both groups compared with nadirs before initiation of study drug, with greater improvement in avatrombopag-treated patients). Given these results, TPO-RA treatment is probably unnecessary in most patients with mild or moderate nadir CIT, especially those without extensive prior chemotherapy exposure. In those with platelet count nadirs <20 × 109/L demonstrated over multiple treatment cycles or with a history of clinically relevant bleeding during a prior nadir, it is reasonable to consider off-label TPO-RA use to try to prevent the occurrence of spontaneous bleeding events. The patient in Clinical Case 2 is receiving her first chemotherapy regimen, and her platelet count nadir is not impressive (58 × 109/L); therefore, at this time, she is best managed with observation of her subsequent platelet count nadirs rather than initiation of a TPO-RA. If her nadirs worsen significantly, a trial of romiplostim would be reasonable. It is important to note that major bleeding events in CIT are relatively rare (3.4% of cycles in 1 study of 1262 cycles of treatment) but associated with significant reductions in overall survival in patients experiencing them.3 There are no large studies directly comparing major bleeding rates in TPO-RA–treated patients with those not receiving TPO-RAs, but this rate of 3.4% in nonsupported patients compares with 1.0% of cycles in a large observational study including 1063 treatment cycles that were supported with romiplostim.5
CLINICAL CASE 2 (CONCLUSION)
The patient is not initiated on TPO-RA therapy and is observed, without dose reduction or treatment delay of her FOLFIRINOX. Her next 2 platelet count nadirs are 72 × 109/L and 63 × 109/L, and she reports no further bleeding events. She continues chemotherapy and anticoagulation without any need for TPO-RA support.
Conclusions
CIT is common and affects oncologic care. In those patients with persistent CIT compromising their ability to receive optimal cancer therapy, treatment with TPO-RAs, in either the clinical trial or the off-label setting, may facilitate safe administration of cancer-directed therapy on time and at full dose. This may reduce bleeding risks and improve oncologic outcomes, particularly in patients with curable malignancies. By contrast, recent randomized phase 3 data confirm that most patients with nadir CIT are unlikely to derive much benefit from TPO-RA support. Most evidence to date for use of TPO-RAs in CIT is for romiplostim, and outside of a clinical trial setting, it is the only TPO-RA currently recommended for management of CIT by the National Comprehensive Cancer Network guidelines. Phase 2 and 3 clinical trials of romiplostim and oral TPO-RAs for the management of persistent CIT are ongoing and hopefully will result in additional treatment options for these patients, who have an ongoing unmet clinical need.
Acknowledgments
H. Al-Samkari is the recipient of the American Society of Hematology Scholar Award.
Conflict-of-interest disclosure
Hanny Al-Samkari: Consultancy (Agios, Dova/Sobi, Novartis, Rigel, argenx, Forma), Research Funding (Agios, Dova/Sobi, Amgen).
Off-label drug use
Hanny Al-Samkari: Use of romiplostim, eltrombopag, and avatrombopag for chemotherapy-induced thrombocytopenia.