Abstract
Smoldering multiple myeloma (SMM) is an asymptomatic precursor condition to multiple myeloma (MM). The prevalence of SMM is 0.5% in persons over 40 years old; it is higher in men than women and increases with age. When SMM is diagnosed, a thorough diagnostic workup is necessary to exclude myeloma-defining events and stratify patients according to risk of progression to MM. While close monitoring for progression remains the best management for most patients with SMM, in this article, we discuss if treatment initiation before myeloma-defining events occur might be relevant in selected high-risk cases. Two randomized clinical trials have shown a clinical benefit of initiating treatment at the SMM stage, whereof 1 showed an overall survival benefit for those receiving treatment. We discuss various risk stratification models in SMM, important treatment trials, and ongoing trials. Finally, we present how to approach the clinical management of patients with SMM.
Learning Objectives
Recognize the current risk stratification models in smoldering multiple myeloma (SMM) and their limitations
Identify the present dilemma about treatment initiation in SMM and the arguments for and against starting antimyeloma treatment at the SMM stage
CLINICAL CASE
A 42-year-old woman was diagnosed with smoldering multiple myeloma (SMM) through the screening study Iceland Screens, Treats, or Prevents Multiple Myeloma (iStopMM).1 She was otherwise healthy, except for a history of anxiety, and she was asymptomatic at the time of SMM diagnosis. Blood tests showed an IgG κ M-protein of 3.5 g/dL, total κ of 53.1 mg/L, total λ of 4.9 mg/L, and free light chain (FLC) ratio of 10.84. Hemoglobin, creatinine, calcium, and total IgA and IgM were normal. Urine protein electrophoresis revealed an IgG κ of 3 g/24 hours and free κ of 2 g/24 hours. Bone marrow biopsy specimen and aspirate showed 20% to 30% clonal plasma cells. Fluorescence in situ hybridization analysis (FISH) showed deletion of 13q. Flow cytometry of the bone marrow revealed a plasma cell population in which 97% expressed an aberrant phenotype. No skeletal lesions were found on whole-body low-dose computed tomography (WBLDCT) or whole-body magnetic resonance imaging (MRI).
Epidemiology and diagnosis of smoldering multiple myeloma
SMM is an asymptomatic precursor condition to multiple myeloma (MM). According to the International Myeloma Working Group (IMWG) diagnostic criteria updated in 2014, SMM is defined by a 10% to 60% plasma cell infiltration in the bone marrow and/or monoclonal (M)–protein concentration in serum over 3 g/dL, in the absence of myeloma-defining events (Table 1).2 SMM is differentiated from monoclonal gammopathy of undetermined significance (MGUS) by a higher risk of progression to MM, with an average risk of progression of 10% annually for the first 5 years following diagnosis.3 Because SMM is asymptomatic, it is rarely diagnosed before progression to MM, and it is estimated that only 3% to 6% of patients with MM are diagnosed at a precursor state.4,5
Diagnostic criteria for smoldering multiple myeloma according to International Myeloma Working Group 2014 criteria2
| Smoldering multiple myeloma diagnosis . |
|---|
| Bone marrow clonal plasma cells 10%-60% |
| M-protein in serum ≥30 g/L or urinary M-protein ≥0.5 g per 24 hours |
| No evidence of other B-cell proliferative disorder |
| No myeloma-related organ or tissue impairment or bone lesions |
| Myeloma-defining events |
| Evidence of end-organ or tissue impairment |
| • Hypercalcemia |
| • Renal insufficiency |
| • Anemia |
| • Osteolytic bone lesions |
| Biomarkers of malignancy |
| • Clonal bone marrow plasma cell percentage ≥60% |
| • Involved: uninvolved serum free light chain ratio ≥100 |
| • >1 focal lesion on MRI studies |
| Smoldering multiple myeloma diagnosis . |
|---|
| Bone marrow clonal plasma cells 10%-60% |
| M-protein in serum ≥30 g/L or urinary M-protein ≥0.5 g per 24 hours |
| No evidence of other B-cell proliferative disorder |
| No myeloma-related organ or tissue impairment or bone lesions |
| Myeloma-defining events |
| Evidence of end-organ or tissue impairment |
| • Hypercalcemia |
| • Renal insufficiency |
| • Anemia |
| • Osteolytic bone lesions |
| Biomarkers of malignancy |
| • Clonal bone marrow plasma cell percentage ≥60% |
| • Involved: uninvolved serum free light chain ratio ≥100 |
| • >1 focal lesion on MRI studies |
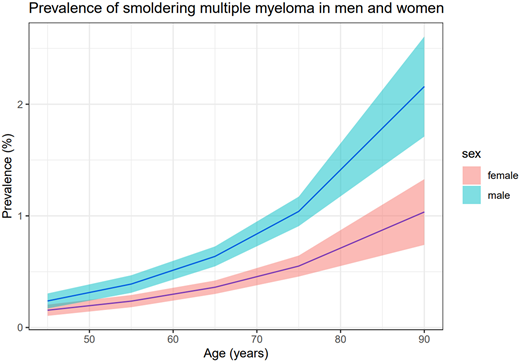
We recently described, for the first time, the prevalence of SMM using the large (>75 000) population-based iStopMM study and found the prevalence of SMM to be 0.5% in persons over 40 years of age. The prevalence of SMM is higher in men than in women (0.7% vs 0.4%) and increases with age (Figure 1).6 In our screened SMM population, the median age was 70 years, but in most clinical cohorts of SMM, the median age of SMM diagnosis is around 65 years.7,8 Both MGUS and MM have been shown to be associated with race and ethnicity, and results from a study in the United States showed that SMM was more common in blacks than other racial groups.9-11 Furthermore, in a recent single-center study from the United States, risk of progression from SMM to MM was shown to be lower in African Americans compared to whites, although this needs to be validated in other cohorts.12
Prevalence of smoldering multiple myeloma according to age with 95% confidence intervals.6
Prevalence of smoldering multiple myeloma according to age with 95% confidence intervals.6
Risk of progression
To identify individuals with SMM at a high risk of progression to MM, several risk stratification models have been suggested based on biomarkers at SMM diagnosis.8,13,14
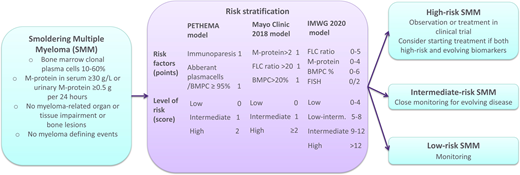
The PETHEMA risk criteria stratify patients with SMM into low-risk, intermediate-risk, and high-risk groups according to whether the uninvolved immunoglobulins are decreased (immunoparesis) and if the percentage of aberrant plasma cells in the bone marrow according to flow cytometry is over 95% (neither factor, 1 factor, or both respectively).13
In 2018, an older Mayo Clinic risk criterion that only included bone marrow plasma cells (BMPCs) ≥10% and M-protein ≥3 g/dL was replaced by a new risk model.3 The new 2/20/20 clinical risk stratification model incorporates 3 factors (serum M-protein >2 g/dL, involved to uninvolved FLC ratio >20, and BMPC infiltration >20%) to stratify patients with SMM diagnosed according to the IMWG 2014 criteria into low-risk, intermediate-risk, and high-risk groups (0 factors, 1 factor, and 2-3 factors, respectively).14 This resulted in an estimated risk of progression of 10%, 26%, and 47% after 2 years for the low-, intermediate-, and high-risk groups, respectively. Subsequently, the IMWG validated the 2/20/20 model in a large international SMM cohort and found similar risk of progression at 2 years as in the initial study.8 Furthermore, they added the presence of high-risk features by FISH (t(4;14), t(14;16), del(13q), or amp(1q)) as an additional predictor of progression and suggested a more detailed risk model resulting in a new risk score with 4 risk groups with a 4%, 26%, 51%, and 73% risk of progression for the low-, low-intermediate-, intermediate-, and high-risk groups, respectively, 2 years from SMM diagnosis (Table 2).8
Clinical risk stratification models for smoldering multiple myeloma
| Characteristic . | PETHEMA model13 . | Mayo Clinic 2018 model14 . | IMWG 2020 model8 . | |||
|---|---|---|---|---|---|---|
| Risk factors (points) | Immunoparesis Aberrant plasma cells/BMPC ≥95% | 1 1 | M-protein >2 FLC ratio >20 BMPC >20% | 1 1 1 | FLC ratio 0-10 10-25 25-40 >40 M-protein 0-1.5 1.5-3 >3 BMPC % 0-15 >15-20 >20-30 >30-40 >40 FISH abnormality | 0 2 3 5 0 3 4 0 2 3 5 6 2 |
| Level of risk (score) | Low Intermediate High | 0 1 2 | Low Intermediate High | 0 1 ≥2 | Low Low-intermediate Intermediate High | 0-4 5-8 9-12 >12 |
| Risk of progression at 2 years (%)* | Low Intermediate High | 5 35 50 | Low Intermediate High | 10 26 47 | Low Low-intermediate Intermediate High | 4 26 51 73 |
| Characteristic . | PETHEMA model13 . | Mayo Clinic 2018 model14 . | IMWG 2020 model8 . | |||
|---|---|---|---|---|---|---|
| Risk factors (points) | Immunoparesis Aberrant plasma cells/BMPC ≥95% | 1 1 | M-protein >2 FLC ratio >20 BMPC >20% | 1 1 1 | FLC ratio 0-10 10-25 25-40 >40 M-protein 0-1.5 1.5-3 >3 BMPC % 0-15 >15-20 >20-30 >30-40 >40 FISH abnormality | 0 2 3 5 0 3 4 0 2 3 5 6 2 |
| Level of risk (score) | Low Intermediate High | 0 1 2 | Low Intermediate High | 0 1 ≥2 | Low Low-intermediate Intermediate High | 0-4 5-8 9-12 >12 |
| Risk of progression at 2 years (%)* | Low Intermediate High | 5 35 50 | Low Intermediate High | 10 26 47 | Low Low-intermediate Intermediate High | 4 26 51 73 |
Estimated from Figure 4B in the original publication for the PETHEMA risk model.
Dynamic risk assessments, taking into account changes in disease status during follow-up of SMM, have been suggested, including evaluations of evolving M-protein, FLC ratio, and hemoglobin.15-19 Fernández de Larrea et al16 defined an evolving pattern of M-protein pattern as a 10% increase in the M-protein size during the first 12 months from diagnosis if baseline M-protein was ≥3 g/dL, or over a period of 3 years in patients with an initial M-protein <3 g/dL, and found that patients with an evolving pattern had a 5-fold risk for progression to MM compared to those without an evolving pattern. Ravi et al18 defined evolving changes of M-protein as a ≥10% increase in M-protein within 6 months or a ≥25% increase within the first year following diagnosis (with a minimum increase of 0.5 g/dL), as well as a decrease in hemoglobin over 0.5 g/dL within 12 months of diagnosis, and found that both factors were independent risk factors for progression. They suggested a risk model for progression from SMM to MM including these 2 evolving factors as well as bone marrow plasma cells of ≥20% within 2 years from SMM diagnosis. Gran et al17 showed that an absolute rise in M-protein >0.5 g/dL, as well as an increase in FLC ratio >4.5 identified patients with SMM at high risk of progression, but could not confirm the prognostic significance of decreasing hemoglobin and stated that using relative increases in FLC ratio and M-protein could lead to overestimates in patients with low levels of these biomarkers at diagnosis.
Recently, Visram et al20 investigated the utility of applying the Mayo 2018 and IMWG 2020 models up to 5 years postdiagnosis and found that both models still stratified patients based on progression risk. Furthermore, according to the Mayo 2018 model, the risk of progression was approximately 3 times higher in patients evolving to the high-risk category during follow-up compared to patients who remained low or intermediate risk.
A disadvantage of the widely used Mayo 2/20/20 risk stratification model, besides only evaluating 1 time point, is that it captures the disease burden without considering the biology of the disease. Other proposed markers of progression that reflect the biology of SMM include genomic alterations, such as chromosomal abnormalities, complex structural chromosomal changes, and mutations.21-23 Besides the high-risk features by FISH included in the IMWG risk model, del(17p) and hyperdiploidy also have been associated with an increased risk of progression in SMM.21 Studies using whole-genome sequencing and next-generation sequencing have furthermore revealed that chromothripsis and mutations in the mitogen-activated protein kinase pathway (KRAS and NRAS), the DNA repair pathway (TP53 and ATM), and MYC are associated with increased risk of progression to MM.22-24 These genomic alterations and other markers such as circulating tumor cells and serum B-cell maturation antigen levels are interesting but need further prospective validation before being implemented in routine clinical care of individuals with SMM.24-26
Low-risk smoldering multiple myeloma
The group of patients with SMM who have stable disease and do not require intensive follow-up is very important to identify to avoid overtreatment and unnecessary concern. Patients with a low risk of progression are likely to have a low score on the IMWG 2020 risk model and have been shown to have a low mutational burden and low or undetectable circulating plasma cells.25,27 Interestingly, an algorithm based on flow cytometry has recently been defined that identifies patients with SMM with an MGUS-like phenotype who have a very low risk of progression.28 This algorithm could be useful if implemented in a clinical context to identify individuals with SMM who do not need close monitoring.
Treatment of smoldering multiple myeloma
Observation until progression to MM is the standard of care in SMM. However, the timing of therapy in MM and precursor diseases has been a subject of debate for some years. In an important study from 2013 on 119 individuals, Mateos et al29,30 found that patients with high-risk SMM (according to the PETHEMA risk criteria or the older Mayo Clinic criteria), randomized to lenalidomide and dexamethasone for nine 4-week cycles followed by lenalidomide maintenance for 2 years, had delayed progression to active MM and, notably, had better overall survival 3 years after the initiation of therapy, compared to the group that did not receive therapy (Table 3). Importantly, no difference was found in overall survival between arms from the time of progression to MM, suggesting that intervention did not lead to development of treatment-resistant clones. However, an increased risk of secondary malignancies was found in the treatment group compared to the observation group (Table 3). Although this was a key study in the management of SMM, it has been criticized that modern imaging techniques were not used to rule out MM, and therefore some of the patients would today be classified as having MM. Furthermore, because reintroduction of low-dose dexamethasone in patients with biological progression was permitted in the treatment arm, the results of the study are complex to interpret.
Selected phase 2 and 3 clinical trials for treatment of SMM with reported outcomes
| Trial . | Phase . | SMM risk inclusion criteria . | Intervention . | Control arm . | Results . | Toxicity . |
|---|---|---|---|---|---|---|
| QuiRedex29,30 | 3 | Both BMPC ≥10% and IgG ≥3 g/dL (or IgA ≥2 g/dL or urine M-protein >1 g/24 h) OR only 1 of the criteria above plus ≥95% aberrant PC and immunoparesis | Lenalidomide (25 mg) and dexamethasone for 9 cycles followed by lenalidomide (10 mg) maintenance for 2 years (n = 57) | Observation (n = 62) | Median TTP (progression defined as end-organ damage) not reached vs 23 months (HR, 0.24), median OS not reached (HR, 0.43) | 16 cases of grade 3 toxicities in intervention arm, 1 treatment-associated death. Second cancers more common in treatment arm (10% vs 2%). |
| ECOG E3A0631 | 3 | BMPC ≥10%-60% and abnormal FLC ratio (<0.26 or >1.65) | Lenalidomide (25 mg per day on days 1-21 every 28-day cycle) until progression (n = 90) | Observation (n = 92) | 3-year PFS (progression defined as biochemical or end-organ damage) 91% vs 66% (HR, 0.28) | Grade 3 or 4 toxicity in 36 (41%) patients; 18 patients discontinued treatment because of toxicities. One treatment-associated death; 3-year cumulative incidence of second cancers 5.2% vs 3.5%. |
| CENTAURUS37 | 2 | BMPC 10%-60% and 1 of the following: IgG ≥3 g/dL, IgA ≥2 g/dL, urine M-protein >500 mg/24 h, FLC ratio <0.126 or >8 (and serum M-protein <3 g/dL but ≥1 g/d) | Daratumumab monotherapy in 3 different regimens: intense (n = 41), intermediate (n = 41), and short (n = 41) | No | ORR of 56%, 53.7%, and 37.5% in the intensive, intermediate, and short arms, respectively | Grade 3 or 4 toxicities in 44% of patients in the intense arm, 26.8% in the intermediate arm, and 15.0% in the short arm |
| GEM-CESAR35 | 2 | Both ≥10% BMPC and M-protein ≥3 g/dL OR 1 of the criteria above plus ≥95% aberrant PC and immunoparesis | Carfilzomib, lenalidomide, and dexamethasone for 6 cycles (induction), HDT and ASCT, carfilzomib, lenalidomide, and dexamethasone for 2 cycles (consolidation) and lenalidomide and dexamethasone maintenance for 2 years (n = 90) | No | ORR of 100%. MRD negativity of 67% at 1 year posttreatment | 46 cases of grade 3 or 4 toxicities have been reported |
| Kazandjian 202134 | 2 | High risk per Mayo Clinic 2018 model or PETHEMA OR both BMPC ≥10% and 1 of the following: M-protein ≥3 g/dL, IgA isotype, immunoparesis, FLC ratio >8, progressive increase in M-protein, BMPC 50%-60%, t(4;14) or del(17p) or 1q gain, increased circulating PC, MRI with diffuse abnormalities or 1 focal lesion, PET/CT with focal lesion with increased uptake | Carfilzomib, lenalidomide, and dexamethasone for 8 cycles followed by 24 cycles of lenalidomide (n = 54) | No | ORR of 100%. MRD negativity was 70% with a median duration of 5.5 years. | Nonhematologic grade 3 toxicities in 21 patients (38.9%) |
| Mailankody 201938 | 2 | Mayo Clinic 2018 model or Spanish PETHEMA criteria | Isatuximab until disease progression or unacceptable toxicity (n = 61) | No | ORR of 62.5% | 5 cases of grade 3 toxicities |
| Trial . | Phase . | SMM risk inclusion criteria . | Intervention . | Control arm . | Results . | Toxicity . |
|---|---|---|---|---|---|---|
| QuiRedex29,30 | 3 | Both BMPC ≥10% and IgG ≥3 g/dL (or IgA ≥2 g/dL or urine M-protein >1 g/24 h) OR only 1 of the criteria above plus ≥95% aberrant PC and immunoparesis | Lenalidomide (25 mg) and dexamethasone for 9 cycles followed by lenalidomide (10 mg) maintenance for 2 years (n = 57) | Observation (n = 62) | Median TTP (progression defined as end-organ damage) not reached vs 23 months (HR, 0.24), median OS not reached (HR, 0.43) | 16 cases of grade 3 toxicities in intervention arm, 1 treatment-associated death. Second cancers more common in treatment arm (10% vs 2%). |
| ECOG E3A0631 | 3 | BMPC ≥10%-60% and abnormal FLC ratio (<0.26 or >1.65) | Lenalidomide (25 mg per day on days 1-21 every 28-day cycle) until progression (n = 90) | Observation (n = 92) | 3-year PFS (progression defined as biochemical or end-organ damage) 91% vs 66% (HR, 0.28) | Grade 3 or 4 toxicity in 36 (41%) patients; 18 patients discontinued treatment because of toxicities. One treatment-associated death; 3-year cumulative incidence of second cancers 5.2% vs 3.5%. |
| CENTAURUS37 | 2 | BMPC 10%-60% and 1 of the following: IgG ≥3 g/dL, IgA ≥2 g/dL, urine M-protein >500 mg/24 h, FLC ratio <0.126 or >8 (and serum M-protein <3 g/dL but ≥1 g/d) | Daratumumab monotherapy in 3 different regimens: intense (n = 41), intermediate (n = 41), and short (n = 41) | No | ORR of 56%, 53.7%, and 37.5% in the intensive, intermediate, and short arms, respectively | Grade 3 or 4 toxicities in 44% of patients in the intense arm, 26.8% in the intermediate arm, and 15.0% in the short arm |
| GEM-CESAR35 | 2 | Both ≥10% BMPC and M-protein ≥3 g/dL OR 1 of the criteria above plus ≥95% aberrant PC and immunoparesis | Carfilzomib, lenalidomide, and dexamethasone for 6 cycles (induction), HDT and ASCT, carfilzomib, lenalidomide, and dexamethasone for 2 cycles (consolidation) and lenalidomide and dexamethasone maintenance for 2 years (n = 90) | No | ORR of 100%. MRD negativity of 67% at 1 year posttreatment | 46 cases of grade 3 or 4 toxicities have been reported |
| Kazandjian 202134 | 2 | High risk per Mayo Clinic 2018 model or PETHEMA OR both BMPC ≥10% and 1 of the following: M-protein ≥3 g/dL, IgA isotype, immunoparesis, FLC ratio >8, progressive increase in M-protein, BMPC 50%-60%, t(4;14) or del(17p) or 1q gain, increased circulating PC, MRI with diffuse abnormalities or 1 focal lesion, PET/CT with focal lesion with increased uptake | Carfilzomib, lenalidomide, and dexamethasone for 8 cycles followed by 24 cycles of lenalidomide (n = 54) | No | ORR of 100%. MRD negativity was 70% with a median duration of 5.5 years. | Nonhematologic grade 3 toxicities in 21 patients (38.9%) |
| Mailankody 201938 | 2 | Mayo Clinic 2018 model or Spanish PETHEMA criteria | Isatuximab until disease progression or unacceptable toxicity (n = 61) | No | ORR of 62.5% | 5 cases of grade 3 toxicities |
ASCT, autologous stem cell transplantation; HDT, high-dose chemotherapy; HR, hazard ratio; ORR, overall response rate; OS, overall survival; PC, plasma cells; PET/CT, positron emission tomography/computed tomography; PFS, progression-free survival; TTP, time to progression.
In another randomized study from 2019 of 182 patients with intermediate- to high-risk SMM (high risk defined as BMPCs ≥10% and abnormal serum FLC ratio), Lonial et al31 showed that lenalidomide monotherapy until disease progression, toxicity, or withdrawal delayed progression to active MM with the highest benefit for the patients who were high risk according to the Mayo Clinic 2018 model. Of note, however, only 29 high-risk patients were included, and the treatment discontinuation rate was 50% in the treatment arm, whereof 40% discontinued due to adverse events. A response was seen in only 50% of patients in the treatment arm, and an effect on overall survival has not been reported. Based on results from this trial where the median duration of therapy was 23 months, as well as the previous Spanish trial, the authors recommended early treatment for high-risk SMM for 2 years.
Treatment regimens with carfilzomib, lenalidomide, and dexamethasone (KRd) have shown good response rates in a number of nonrandomized phase 2 trials in high-risk SMM, as well as inducing high rates of minimal residual disease (MRD) negativity.32-35 Kazandjian et al34 recently reported results from a phase 2 study on 54 individuals with high-risk patients with SMM treated with KRd followed by lenalidomide maintenance that resulted in MRD negativity in 70% of patients, with a median sustained duration of 5.5 years. The GEM-CESAR trial was designed to evade progression from SMM to MM through achievement of MRD negativity with an intensive “curative” strategy. They treated high-risk patients with SMM (defined by PETHEMA criteria and the older Mayo Clinic criteria) with KRd followed by high-dose treatment and autologous stem cell transplantation, consolidation, and lenalidomide maintenance treatment for 2 years. The study is still ongoing, but the authors presented results at the American Hematology Association annual meeting in 2021 showing that 94% of patients were free from progression to MM at 55 months and 67% were MRD negative at 1 year posttreatment.35 The most common adverse events were infections (18% with grade 3-4), and 1 treatment-related death was reported. The combination of ixazomib and lenalidomide and dexamethasone for high-risk SMM was investigated in a phase 2 trial, and the overall response rate in participants who completed at least 2 cycles of treatment was 90.9%.36 Furthermore, monotherapy with both daratumumab and isatuximab has been investigated in phase 2 trials with an overall response rate of 56 (for the intense daratumumab dosing group) and 63%, respectively.37,38
Several phase 2 and 3 studies in high-risk SMM are ongoing, studies that have a curative approach with the aim of a deep response, as well as trials aimed at disease control with less intensive treatment, prolonging time to progression to MM (Table 4).
Ongoing phase 2 and 3 trials for treatment of SMM
| Trial . | Phase . | Intervention . | Control arm . | Outcome . |
|---|---|---|---|---|
| NCT03839459 | 2 | Denosumab monthly every 4 weeks for 12 cycles | No | Proportion of patients with a downgraded risk of progression of smoldering multiple myeloma |
| NCT02916771 | 2 | Ixazomib, lenalidomide, and dexamethasone for 9 cycles, followed by ixazomib and lenalidomide for 15 cycles | No | PFS at 2 years |
| NCT03301220 (AQUILA) | 3 (randomized) | Daratumumab subcutaneously for 39 cycles or until progression or unacceptable toxicity | Observation | PFS |
| NCT03815279 (iStopMM) | 2 | High-risk SMM: carfilzomib, lenalidomide, and dexamethasone for 24 cycles Intermediate-risk SMM: lenalidomide and dexamethasone for 24 cycles | No | MRD negativity at 36 months |
| NCT02279394 | 2 | Elotuzumab, lenalidomide, and dexamethasone for 8 cycles followed by elotzumab and lenalidomide for cycles 9 to 24 | No | PFS at 2 years |
| NCT03236428 | 2 | Daratumumab subcutaneously for 20 cycles (only including low-risk SMM) | No | Proportion of patients in VGPR or better after 20 cycles |
| NCT05014646 | 2 | Leflunomide daily until disease progression or unacceptable toxicity | No | PFS at 2 years and incidence of adverse effects |
| NCT05469893 (Immuno-PRISM) | 2 (randomized) | Teclistamab for 24 months | Lenalidomide and dexamethasone for 24 months | CRR |
| NCT03289299 (ASCENT) | 2 | Daratumumab, lenalidomide, and dexamethasone for 12 cycles followed by daratumumab and lenalidomide for 12 cycles | No | sCR rate |
| NCT04776395 | 2 (randomized) | Iberdomide and dexamethasone for 4 cycles followed by iberdomide until progression or unacceptable toxicity | Iberdomide until disease progression or unacceptable toxicity | ORR |
| NCT04775550 (B-PRISM) | 2 | Daratumumab, bortezomib, lenalidomide, and dexamethasone up to 24 months | No | MRD negativity at 2 years |
| NCT04850846 | 2 | Metformin for 6-12 months (only including low-risk SMM) | Placebo | M-protein concentrations/light chains change from baseline to 6 months |
| NCT03937635 (DETER-SMM) | 3 (randomized) | Daratumumab, lenalidomide, and dexamethasone up to cycles, progression, or unacceptable toxicity | Lenalidomide and dexamethasone until progression or unacceptable toxicity | OS and Functional Assessment of Cancer Therapy–General score |
| NCT03673826 (HO147SMM) | 2 (randomized) | Carfilzomib, lenalidomide, and dexamethasone for 9 cycles followed by lenalidomide up for 24 cycles | Lenalidomide and dexamethasone for 9 cycles followed by lenalidomide up for 24 cycles | PFS |
| NCT04731844 | 2 | Curcumin plus Piperine orally for 12 months (only including low-risk SMM) | No | Response rate from baseline to 12 months |
| NCT04270409 (ITHACA) | 3 (randomized) | Isatuximab, lenalidomide, and dexamethasone for 36 months | Lenalidomide and dexamethasone for 36 months | PFS |
| NCT03792763 | 2 (randomized) | Denosumab every 4 weeks for 6 months, then every 3 months for 3 years | Placebo | Time to progression |
| Trial . | Phase . | Intervention . | Control arm . | Outcome . |
|---|---|---|---|---|
| NCT03839459 | 2 | Denosumab monthly every 4 weeks for 12 cycles | No | Proportion of patients with a downgraded risk of progression of smoldering multiple myeloma |
| NCT02916771 | 2 | Ixazomib, lenalidomide, and dexamethasone for 9 cycles, followed by ixazomib and lenalidomide for 15 cycles | No | PFS at 2 years |
| NCT03301220 (AQUILA) | 3 (randomized) | Daratumumab subcutaneously for 39 cycles or until progression or unacceptable toxicity | Observation | PFS |
| NCT03815279 (iStopMM) | 2 | High-risk SMM: carfilzomib, lenalidomide, and dexamethasone for 24 cycles Intermediate-risk SMM: lenalidomide and dexamethasone for 24 cycles | No | MRD negativity at 36 months |
| NCT02279394 | 2 | Elotuzumab, lenalidomide, and dexamethasone for 8 cycles followed by elotzumab and lenalidomide for cycles 9 to 24 | No | PFS at 2 years |
| NCT03236428 | 2 | Daratumumab subcutaneously for 20 cycles (only including low-risk SMM) | No | Proportion of patients in VGPR or better after 20 cycles |
| NCT05014646 | 2 | Leflunomide daily until disease progression or unacceptable toxicity | No | PFS at 2 years and incidence of adverse effects |
| NCT05469893 (Immuno-PRISM) | 2 (randomized) | Teclistamab for 24 months | Lenalidomide and dexamethasone for 24 months | CRR |
| NCT03289299 (ASCENT) | 2 | Daratumumab, lenalidomide, and dexamethasone for 12 cycles followed by daratumumab and lenalidomide for 12 cycles | No | sCR rate |
| NCT04776395 | 2 (randomized) | Iberdomide and dexamethasone for 4 cycles followed by iberdomide until progression or unacceptable toxicity | Iberdomide until disease progression or unacceptable toxicity | ORR |
| NCT04775550 (B-PRISM) | 2 | Daratumumab, bortezomib, lenalidomide, and dexamethasone up to 24 months | No | MRD negativity at 2 years |
| NCT04850846 | 2 | Metformin for 6-12 months (only including low-risk SMM) | Placebo | M-protein concentrations/light chains change from baseline to 6 months |
| NCT03937635 (DETER-SMM) | 3 (randomized) | Daratumumab, lenalidomide, and dexamethasone up to cycles, progression, or unacceptable toxicity | Lenalidomide and dexamethasone until progression or unacceptable toxicity | OS and Functional Assessment of Cancer Therapy–General score |
| NCT03673826 (HO147SMM) | 2 (randomized) | Carfilzomib, lenalidomide, and dexamethasone for 9 cycles followed by lenalidomide up for 24 cycles | Lenalidomide and dexamethasone for 9 cycles followed by lenalidomide up for 24 cycles | PFS |
| NCT04731844 | 2 | Curcumin plus Piperine orally for 12 months (only including low-risk SMM) | No | Response rate from baseline to 12 months |
| NCT04270409 (ITHACA) | 3 (randomized) | Isatuximab, lenalidomide, and dexamethasone for 36 months | Lenalidomide and dexamethasone for 36 months | PFS |
| NCT03792763 | 2 (randomized) | Denosumab every 4 weeks for 6 months, then every 3 months for 3 years | Placebo | Time to progression |
CRR, complete response rate; sCR, stringent complete response; VGPR, very good partial response.
CLINICAL CASE (Continued)
According to the PETHEMA clinical risk stratification model as well as the IMWG 2020 model, the patient had intermediate-risk SMM, and according to the Mayo Clinic 2018 model, the patient was classified as having high-risk SMM. The patient was offered and accepted early treatment in a nationwide phase 2 clinical trial (NCT03815279), where she received treatment with KRd for 2 years and peripheral autologous blood stem cells were collected. She experienced mild side effects (dry mouth, nausea, constipation, muscle cramps, and fatigue) during the treatment. The patient has now completed the treatment and has been in treatment-free surveillance for 1 year. She is in complete remission and remains MRD negative by next-generation flow. She continues to experience fatigue but is otherwise asymptomatic.
Discussion
Despite being a highly active area of research in recent years, several issues remain unsolved regarding the management of SMM. Risk stratification in SMM is still an area that needs improvement, as none of the existing models is flawless in stratifying patients as having an indolent or aggressive disease. There is significant discordance between clinical risk assessment models that are used at baseline, as demonstrated in the clinical case (and in 2 separate SMM cohorts),6,39 and the dynamic risk scores have not been validated.
Most trials in SMM that are currently active do not have a treatment-free arm and have surrogate end points such as response rates and progression-free survival. This has been criticized, and authors have stated that there is a need to prioritize randomized studies in SMM with end points that are meaningful for patients such as quality of life and overall survival.40,41 However, a treatment-free arm could be problematic considering that in clinical practice, many treating physicians would not wait for a formal myeloma-defining event in patients with SMM who have multiple high-risk features and evolving disease (increasing M-protein and creatinine decreasing hemoglobin) to start treatment.42,43
An unresolved issue regarding treatment initiation at the SMM stage is whether a 1- or 2-drug regimen or a more intensive combination treatment approach should be initiated. Treatment with lenalidomide with or without dexamethasone has been investigated in trials compared to observation and has the benefit of demonstrated improved outcomes.29,31 Problems with this approach include risk of lenalidomide-resistant clones, risk of secondary cancers with prolonged lenalidomide treatment, and low response rates.29,31 The more intensive treatment regimens have more treatment-associated risks (including treatment-associated deaths), and although the reported studies have impressive response rates, it is uncertain how they translate into overall survival and affect quality of life in SMM. Results from ongoing randomized controlled trials comparing 2- and 3-drug combinations as well as from trials with intensive curative strategies, including autologous stem cell transplantation, will provide further data for guidance of which approaches are most beneficial for patients.
Going forward, improved and biology-oriented risk models are needed to stratify patients with SMM to accurately recognize patients with progressive disease who are the right candidates for early treatment and to prevent patients with SMM who have a low risk of progression from being oversurveilled and treated. While treatment initiation at the SMM stage is not yet the standard of care, future trials in SMM will have to answer which patients with SMM benefit from treatment, preferably using updated risk stratification models, with overall survival and quality of life as primary end points. Furthermore, current and future trials will answer if short-term curative intensive approaches, long-term less intensive treatments, or any of the numerous new antimyeloma immunotherapy agents, are best for improving overall survival in SMM while taking into account effects on quality of life.
Recommendations
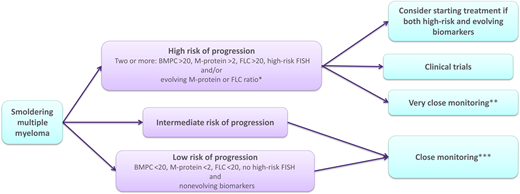
We recommend a thorough workup at diagnosis for individuals with SMM to risk-stratify the patient accurately. The workup of SMM should include complete blood count, serum creatinine, calcium, protein electrophoresis with immunofixation, and FLC analysis, as well as a urine protein electrophoresis and immunofixation (Figure 2). Bone marrow evaluation should include FISH studies and flow cytometry analysis if possible. To exclude bone disease, if WBLDCT is negative, we recommend performing whole-body MRI, MRI of the spine, or fluorodeoxyglucose positron emission tomography/computed tomography.44 We recommend using the Mayo Clinic 2018 risk stratification model or the IMWG 20202 model in the initial workup if FISH analysis is available, as well as evaluation of evolving disease. Furthermore, it is important to consider any comorbidities and to exclude other plasma cell–associated conditions. The individual should be informed of the possibilities of careful follow-up and when treatment could be indicated. Most important, a thorough discussion with the patient about their ideas and goals of potential therapy is important, and for patients with SMM, there is no need to make immediate management decisions. This allows for time to evaluate if there is an evolving pattern in the patient's biomarkers at first reevaluation around 2 to 3 months after initial diagnosis. We recommend considering early treatment in high-risk patients with SMM with evolving disease, preferably in clinical trials, but even off-trial provided an active discussion with the patient regarding risks and benefits, including impact on health-related quality of life. If clinical trials are not available, we consider that the optimal treatment is standard antimyeloma treatment in these selected cases. Patients with low- to intermediate-risk SMM or high-risk patients with stable nonevolving disease should be offered close monitoring, which should include control blood workup and clinical assessment every 2 to 3 months initially, then every 4 to 6 months, possibly increasing to every 6 to 12 months in 2 to 3 years if the condition is stable.42 Imaging evaluation should, according to an expert panel, be repeated yearly for the first 5 years from diagnosis with whole-body MRI if possible (axial MRI and positron emission tomography/computed tomography are acceptable alternatives).44,45 This is costly and can be difficult to follow in the clinic if access to these imaging modalities is limited. In that case, in the authors' opinion, using WBLDCT in patients with stable M-protein and FLC ratio and no clinical suspicion of bone disease is acceptable for repeated imaging, reserving more sensitive imaging modalities for patients with biochemical progression or clinical suspicion of bone disease.
Flowchart for suggested management of patients with smoldering multiple myeloma. *Increasing M-protein or FLC ratio in at least 2 subsequent evaluations. **Clinical control, monoclonal protein studies, complete blood count, creatinine, and calcium every 1 to 3 months. ***Clinical control, monoclonal protein studies, complete blood count, creatinine, and calcium every 4 to 6 months.
Flowchart for suggested management of patients with smoldering multiple myeloma. *Increasing M-protein or FLC ratio in at least 2 subsequent evaluations. **Clinical control, monoclonal protein studies, complete blood count, creatinine, and calcium every 1 to 3 months. ***Clinical control, monoclonal protein studies, complete blood count, creatinine, and calcium every 4 to 6 months.
Conflict-of-interest disclosure
Sigrun Thorsteinsdottir: no competing financial interests to declare.
Sigurdur Yngvi Kristinsson: no competing financial interests to declare.
Off-label drug use
Sigrun Thorsteinsdottir: none of the medications are FDA- approved; all that are discussed are off-label.
Sigurdur Yngvi Kristinsson: none of the medications are FDA- approved; all that are discussed are off-label.