Abstract
The choice of treatment for patients with multiple myeloma (MM) at first relapse/progression is based on many factors: (1) treatment-related factors, which include the regimen used during first induction, the quality and duration of first response achieved, toxicities from the first treatment, whether the patient underwent autologous stem cell transplant, and whether the patient was on maintenance at the time of relapse/progression; (2) disease-related factors, including disease presentation and pace of progression; and (3) patient-related factors, including functional age and performance status. The learning objectives are to present the treatment options for patients with MM upon their first relapse and to learn about various strategies for selecting an optimal treatment regimen.
Learning Objectives
Learn about the treatment options for patients with MM upon their first relapse
Review various strategies for selecting the optimal treatment regimen for patients with first relapse in MM
CLINICAL CASE
Ms. Smith is a 56-year-old woman who was diagnosed with multiple myeloma (MM) 4 years ago when she presented to her primary care physician's office with extreme fatigue. Initial laboratory testing showed a serum hemoglobin level of 8.2 g/dL with a normal mean corpuscular volume. Her hemoglobin 6 months prior had been normal. The rest of the blood work revealed a total serum protein of 10.2 g/dL, serum albumin of 3.2 g/dL, and normal serum creatinine and calcium levels. She was referred to a hematologist, and further evaluation showed a monoclonal (M) protein of 3.2 g/dL, immunoglobulin A (IgA) kappa by immunofixation. Serum-free light chains showed kappa of 560 mg/L and lambda of 26.4 mg/L with a ratio of 21.2. Serum IgA was 3.8 g/dL, with suppressed IgG and IgM. Serum beta-2-microglobulin was 3.3 mg/dL, and lactate dehydrogenase was 347. A 24-hour urine protein electrophoresis showed an IgA kappa M protein of 50 mg. A bone marrow aspirate and biopsy had 80% clonal plasma cells. Cytogenetics showed hyperdiploidy, and fluorescence in situ hybridization showed deletion 13q. Positron emission tomography/computed tomography revealed multiple lytic lesions. She was diagnosed with IgA kappa MM, International Staging System (ISS) stage II and Revised International Staging System (R-ISS) stage II.1 She was treated with 5 cycles of induction chemotherapy consisting of bortezomib (V), lenalidomide (R), and dexamethasone (d) (VRd) and achieved a very good partial response (VGPR). She then underwent high-dose chemotherapy with autologous stem cell transplantation (ASCT) as consolidation, following which her disease went into complete remission. She was started on maintenance therapy with lenalidomide at 10 mg/d. Three years after ASCT, her disease relapsed, with a serum M protein of 1.5 g/dL, a bone marrow biopsy with 50% clonal plasma cells, and imaging showing a new lytic lesion in the right humerus.
Given the findings, what is the next best treatment regimen for Ms. Smith with first relapse of her MM?
Several factors should be considered when choosing a treatment regimen for relapsed MM. These can be categorized into treatment-related, disease-related, and patient-related factors (Table 1).
Factors involved in choosing treatment regimen for relapsed MM
| Disease related | 1. Pace of the disease progression |
| 2. Features of aggressiveness such as extramedullary disease | |
| 3. Circulating plasma cells and/or secondary plasma cell leukemia | |
| 4. Presence or absence of end-organ damage | |
| 5. Bone marrow reserve at the time of relapse | |
| 6. Time to relapse from ASCT | |
| 7. Cytogenetic profile | |
| Treatment related | 1. Induction regimen used |
| 2. Duration and depth of response to prior therapy | |
| 3. ASCT status | |
| 4. Adverse reactions to prior treatment and any residual toxicities | |
| 5. Duration since last effective induction treatment | |
| 6. Availability of novel agents and accessibility to them | |
| Patient related | 1. Functional age of the patient |
| 2. Performance status/frailty | |
| 3. Medical comorbidities | |
| 4. Socioeconomic factors | |
| 5. Patient's health care related goals and preferences |
| Disease related | 1. Pace of the disease progression |
| 2. Features of aggressiveness such as extramedullary disease | |
| 3. Circulating plasma cells and/or secondary plasma cell leukemia | |
| 4. Presence or absence of end-organ damage | |
| 5. Bone marrow reserve at the time of relapse | |
| 6. Time to relapse from ASCT | |
| 7. Cytogenetic profile | |
| Treatment related | 1. Induction regimen used |
| 2. Duration and depth of response to prior therapy | |
| 3. ASCT status | |
| 4. Adverse reactions to prior treatment and any residual toxicities | |
| 5. Duration since last effective induction treatment | |
| 6. Availability of novel agents and accessibility to them | |
| Patient related | 1. Functional age of the patient |
| 2. Performance status/frailty | |
| 3. Medical comorbidities | |
| 4. Socioeconomic factors | |
| 5. Patient's health care related goals and preferences |
Treatment-related factors
These include the regimen used during induction, the quality and duration of response achieved, past and residual toxicities, whether the patient underwent ASCT or not, and the treatment on which the disease progressed. Most patients currently receive triplet regimens, if not quadruplet, as induction therapy for newly diagnosed MM, thus exposing them to a proteasome inhibitor (PI), an immunomodulatory agent (IMiD), and/or an anti-CD38 antibody drug at diagnosis. The use of quadruplet regimens during induction has an impact on chemotherapy options at relapse, as patients are already exposed to drugs from different classes. However, drugs used during induction may be used again at relapse as part of new regimens depending on the mechanism of action of individual drugs and the synergistic effect of the drugs in the combination, the prior response achieved, and the time since last usage. Lenalidomide is a US Food and Drug Administration (FDA)–approved drug for maintenance therapy in MM.2-4 It is also a key component of many of the FDA-approved regimens for the treatment of relapsed MM. Hence, it is helpful to determine if the disease is sensitive or refractory to lenalidomide when choosing the optimal next regimen (Table 2). Bortezomib is often used alone or in combination with lenalidomide for maintenance in patients with high-risk disease, and ixazomib is used for patients who are intolerant to lenalidomide as another FDA-approved drug for maintenance therapy.5-7
Treatment regimens based on disease sensitivity to lenalidomide
| Disease status . | Preferred treatment options . | Alternative treatment options . | Special considerations . |
|---|---|---|---|
| Len sensitive | Dard, KRd, Elo-Rd, DVd | Ixa-Rd, VRd, KCd | Always encourage participation in clinical trial. If newer drugs not available—Rd, Vd, VTd, VCd, or VMP. |
| Len refractory/intolerance | DKd, PVd, DPd, Isa-Kd | DVd, Kd, KPd, SVd, KCd | Always encourage participation in clinical trial. If newer drugs not available—VCd, Vd, or VMP. |
| Plasma cell leukemia | VD-PACE | If patient has CHF, use DCEP |
| Disease status . | Preferred treatment options . | Alternative treatment options . | Special considerations . |
|---|---|---|---|
| Len sensitive | Dard, KRd, Elo-Rd, DVd | Ixa-Rd, VRd, KCd | Always encourage participation in clinical trial. If newer drugs not available—Rd, Vd, VTd, VCd, or VMP. |
| Len refractory/intolerance | DKd, PVd, DPd, Isa-Kd | DVd, Kd, KPd, SVd, KCd | Always encourage participation in clinical trial. If newer drugs not available—VCd, Vd, or VMP. |
| Plasma cell leukemia | VD-PACE | If patient has CHF, use DCEP |
Bort, bortezomib; CHF, congestive heart failure; DCEP, dexamethasone-cyclophosphamide-etoposide-cisplatin; DKd, daratumumab-carfilzomib-dexamethasone; DPd, daratumumab-pomalidomide-dexamethasone; DRd, daratumumab-lenalidomide-dexamethasone; DVd, daratumumab-bortezomib-dexamethasone; Elo-Rd, elotuzumab-lenalidomide-dexamethasone; Isa-Kd, isatuximab-carfilzomib-dexamethasone; Ixa-RD, ixazomib-lenalidomide-dexamethasone; KPd, carfilzomib-pomalidomide-dexamethasone; KRd, carfilzomib-lenalidomide-dexamethasone; Len, lenalidomide; PVd, pomalidomide-bortezomib-dexamethasone; SVd, Selinexor-bortezomib-dexamethasone; VCd, bortezomib-cyclophosphamide-dexamethasone; VD-PACE, bortezomib-dexamethasone-cisplatin-doxorubicin-cyclophosphamide-etoposide; VMP, bortezomib-melphalan-dexamethasone; VRd, bortezomib-lenalidomide-dexamethasone; VTd, bortezomib-thalidomide-dexamethasone.
Lenalidomide-sensitive disease
Daratumumab with lenalidomide-dexamethasone (D-Rd) is an effective regimen when compared with lenalidomide and dexamethasone in MM patients who have progressed after at least 1 prior line of therapy.8,9 Patients in the daratumumab group had improved progression-free survival (PFS; median 44.5 vs 17.5 months; P < .0001) (Table 3) and achieved deeper responses as measured by the rate of measurable residual disease (MRD) negativity (10−5; 30.4% vs 5.3%; P < .0001). The benefit with D-Rd was significantly high at first relapse. Patients in the D-Rd group experienced infusion-related reactions and higher rates of neutropenia compared to the Rd arm.
IMiD-based combination regimens for the treatment of first relapse of MM
| Name of the study . | Regimen trial . | Patient group . | Median prior lines of therapy (range) . | Response ORR ≥ VGPR . | Median PFS (months) . | Median OS (months) . | Toxicity . | |
|---|---|---|---|---|---|---|---|---|
| POLLUX8,9 | Phase 3 randomized | Dara-Rd | 1 (1-11) | 92.9% | 80.4% | 44.5 (34.1-NE) | 65% | Infusion-related reactions, neutropenia |
| Rd | 76.4% (P < .0001) | 49.3%, P < .0001 | 17.5 (13.9-20.8), P < .0001 | 57% | ||||
| ASPIRE10,11 | Phase 3 randomized | KRd | 2 (1-3) | 87.1% | 69.9% | 26.1 (23.3-30.5) | 48.3 (42.4-52.8) | Diarrhea, cough, URTI, fever, hypokalemia, hypertension, cardiac failure, renal failure, ischemic heart disease |
| Rd | 66.7%, P < .001 | 40.4%, P < .001 | 16.6 (15-20.6), P < .001 | 40.4 (33.6-44.4) P = .0045 | ||||
| TOURMALINE-MM112,13 | Phase 3 randomized | Ixa-Rd | (1-3) | 78.3% | 48% | 20.6 | 53.6 (49.2-62.9) | Rash, GI side effects, thrombocytopenia, peripheral neuropathy |
| Rd | 71.5%, P = .04 | 39% P = .01 | 14.7 P = .01 | 51.6, (44.7-59.1) P = .495 | ||||
| ELOQUENT-214,15 | Phase 3 randomized | Elo-Rd | 2 (1-4) | 79% | 33% | 19.4 | 43.7 | Grade 3/4 lymphopenia, GI side effects, fatigue, cough, fever, nasopharyngitis |
| Rd | 66%, P < .001 | 28% | 14.9 P = .0014 | 39.6, P = .025 | ||||
| APOLLO18 | Phase 3 randomized | Dara-Pd | 2 (1-5) | 69% | 51% | 12.4 (8.3-19.3) | NA | Grade 3/4 neutropenia, pneumonia, lower respiratory tract infection |
| Pd | 46%, P < .0001 | 20%, P < .0001 | 6.9 (5.5-9.3) P = .001 | |||||
| OPTIMISSM19 | Phase 3 randomized | PVd | 2 (1-2) | 82.2% | 52.7% | 11.2 (9.66–13.73) | NA | Neutropenia, febrile neutropenia, thrombocytopenia |
| Vd | 50%; P < .0001 | 18.3%; P < .0001 | 7.1 (5.88–8.48); P < .0001 | |||||
| EMN011/HO11432 | Phase 2 | KPd | 1 | 87% | 65% | 18 P = .14 | Febrile neutropenia, infection, cardiovascular, neuropathy, cytopenias | |
| Name of the study . | Regimen trial . | Patient group . | Median prior lines of therapy (range) . | Response ORR ≥ VGPR . | Median PFS (months) . | Median OS (months) . | Toxicity . | |
|---|---|---|---|---|---|---|---|---|
| POLLUX8,9 | Phase 3 randomized | Dara-Rd | 1 (1-11) | 92.9% | 80.4% | 44.5 (34.1-NE) | 65% | Infusion-related reactions, neutropenia |
| Rd | 76.4% (P < .0001) | 49.3%, P < .0001 | 17.5 (13.9-20.8), P < .0001 | 57% | ||||
| ASPIRE10,11 | Phase 3 randomized | KRd | 2 (1-3) | 87.1% | 69.9% | 26.1 (23.3-30.5) | 48.3 (42.4-52.8) | Diarrhea, cough, URTI, fever, hypokalemia, hypertension, cardiac failure, renal failure, ischemic heart disease |
| Rd | 66.7%, P < .001 | 40.4%, P < .001 | 16.6 (15-20.6), P < .001 | 40.4 (33.6-44.4) P = .0045 | ||||
| TOURMALINE-MM112,13 | Phase 3 randomized | Ixa-Rd | (1-3) | 78.3% | 48% | 20.6 | 53.6 (49.2-62.9) | Rash, GI side effects, thrombocytopenia, peripheral neuropathy |
| Rd | 71.5%, P = .04 | 39% P = .01 | 14.7 P = .01 | 51.6, (44.7-59.1) P = .495 | ||||
| ELOQUENT-214,15 | Phase 3 randomized | Elo-Rd | 2 (1-4) | 79% | 33% | 19.4 | 43.7 | Grade 3/4 lymphopenia, GI side effects, fatigue, cough, fever, nasopharyngitis |
| Rd | 66%, P < .001 | 28% | 14.9 P = .0014 | 39.6, P = .025 | ||||
| APOLLO18 | Phase 3 randomized | Dara-Pd | 2 (1-5) | 69% | 51% | 12.4 (8.3-19.3) | NA | Grade 3/4 neutropenia, pneumonia, lower respiratory tract infection |
| Pd | 46%, P < .0001 | 20%, P < .0001 | 6.9 (5.5-9.3) P = .001 | |||||
| OPTIMISSM19 | Phase 3 randomized | PVd | 2 (1-2) | 82.2% | 52.7% | 11.2 (9.66–13.73) | NA | Neutropenia, febrile neutropenia, thrombocytopenia |
| Vd | 50%; P < .0001 | 18.3%; P < .0001 | 7.1 (5.88–8.48); P < .0001 | |||||
| EMN011/HO11432 | Phase 2 | KPd | 1 | 87% | 65% | 18 P = .14 | Febrile neutropenia, infection, cardiovascular, neuropathy, cytopenias | |
Dara, daratumumab; GI, gastrointestinal; Ixa, ixazomib; KPd, carfilzomib-pomalidomide/dexa; KRd, carfilzomib-lenalidomide-dexamethasone; NA, not available; NE, not evaluable; Pd, pomalidomide-dexamethasone; PVd, pomalidomide-bortezomib-dexamethasone; Rd, lenalidomide-dexamethasone; URTI, upper respiratory tract infection; Vd, bortezomib-dexamethasone.
Carfilzomib, a second-generation PI, is approved in combination with lenalidomide and dexamethasone (KRd) for the treatment of first relapse in MM.10,11 Compared with Rd, KRd yielded improved PFS (median 26.1 vs 16.6 months; P = .0001) and overall survival (OS; median 48.3 vs 40.4 months at 2-year follow-up; P = .0045) (Table 3). It is of note that the OS was 11.4 months longer for KRd vs Rd in patients who relapsed after 1 prior line of therapy.
Ixazomib, an oral PI, prolongs PFS (median 20.6 vs 14.7 months; P = .01) (Table 3) when given with Rd (Ixa-Rd) compared to Rd alone in patients with MM after at least 1 prior line of therapy.12 Though OS was no different between the 2 groups, subgroup analysis showed greater OS benefit in stage III disease and high-risk cytogenetics.13 Grade 3 and 4 thrombocytopenia, rash, and gastrointestinal side effects, though low grade, were more frequent in the ixazomib group. That must be kept in mind when treating patients with disease-related cytopenias at relapse. Ixa-Rd is an attractive option since it is all oral and convenient without requiring treatment visits. In patients who have had a long remission after induction with VRd, it can be tried again for relapsed disease if they are not refractory to either drug.
Elotuzumab, an immunostimulatory monoclonal antibody– targeting signaling lymphocytic activation molecule 7, is effective in relapsed MM when combined with lenalidomide and dexamethasone (Elo-Rd) after at least 1 prior line of therapy. Compared to Rd alone, Elo-Rd resulted in a superior overall response rate (ORR; 79% vs 66%; P < .001) and prolonged PFS (median 19.4 vs 14.9 months; P = .0014) (Table 3).14 An updated preplanned final analysis of OS showed a statistically significant improvement of OS compared to Rd (median 43.7 vs 39.6 months; P = .0254) (Table 3), the greatest benefit seen in patients with adverse prognostic factors such as older age, ISS stage III, high-risk cytogenetic abnormalities, and 2 to 3 prior lines of therapy.15 Elo-Rd can be used for the treatment of MM with slow or biochemical progression.
Lenalidomide refractoriness/intolerance
Daratumumab when added to bortezomib and dexamethasone (D-Vd) significantly prolonged PFS (median 16.7 vs 7.1 months; P < .0001) (Table 4) compared to bortezomib and dexamethasone alone (Vd) in patients with relapsed MM who received at least 1 prior line of therapy.16,17 MRD-negativity rates (10−5) were greater with D-Vd vs Vd (14% vs 2%; P < .0001). Neutropenia and thrombocytopenia were more common in the daratumumab group compared to the control group. It is of note that the greatest benefit with DVd was seen at first relapse (median PFS, 44.5 vs 17.5 months; P < .0001), which argues for the use of this regimen at first relapse as opposed to later.
PI-based combination regimen for the treatment of first relapse of MM
| Name of the study . | Study design/regimen . | Patient group . | Median prior lines of therapy (range) . | Response ORR ≥ VGPR . | Median PFS (months) . | Median OS (months) . | Toxicity . | |
|---|---|---|---|---|---|---|---|---|
| CASTOR16,17 | Phase 3 randomized | Dara-Vd | 2 (1-9) | 82.9% | 59.2 | 16.7 | NR | Neutropenia, thrombocytopenia, infusion-related reactions |
| Vd | 63.2%; P < .001 | 29.1%; P < .001 | 7.1 P < .0001 | |||||
| BOSTON22 | Phase 3 randomized | Seli-Vd | 2 (1-3) | 76.4% | 44.6% | 13.9 (11.73-NE) | NR | Thrombocytopenia, anemia, fatigue, pneumonia. Peripheral neuropathy significantly lower in the selinexor group. |
| Vd | 62.3%; P = .0012 | 32.4%; P = .0082 | 9.46 (8.11-10.78) P = .0075 | 25 (23.5-NE) P = .18 | ||||
| CANDOR24,25 | Phase 3 randomized | Dara-Kd | 2 (1-5) | 84% | 69% | 28.6 (22.7-NE) | NA | Thrombocytopenia, diarrhea, URTI, pneumonia, fatigue, viral infection, PN |
| Kd | 75%, P = 0.008 | 49% P = NA | 15.2 (11.1-19.9) P < .0001 | NA | ||||
| IKEMA27 | Phase 3 randomized | Isa-Kd | 2 (1-2) | 87% | 73% | NR | NR | Diarrhea, URTI, neutropenia, thrombocytopenia |
| Kd | 83%, P = .19 | 56%, P = .001 | 19.15 (15.77-NR), P = .0007 | NR | ||||
| MYX.1/MCRN-00343 | Phase 2 study | KCd | 2 (1-3) | 85% | 68% | 17.2 (13.4–21.5) | 27.4 (22.1 – NE) | Anemia, thrombocytopenia, neutropenia, infection, cardiovascular events, TMA |
| Name of the study . | Study design/regimen . | Patient group . | Median prior lines of therapy (range) . | Response ORR ≥ VGPR . | Median PFS (months) . | Median OS (months) . | Toxicity . | |
|---|---|---|---|---|---|---|---|---|
| CASTOR16,17 | Phase 3 randomized | Dara-Vd | 2 (1-9) | 82.9% | 59.2 | 16.7 | NR | Neutropenia, thrombocytopenia, infusion-related reactions |
| Vd | 63.2%; P < .001 | 29.1%; P < .001 | 7.1 P < .0001 | |||||
| BOSTON22 | Phase 3 randomized | Seli-Vd | 2 (1-3) | 76.4% | 44.6% | 13.9 (11.73-NE) | NR | Thrombocytopenia, anemia, fatigue, pneumonia. Peripheral neuropathy significantly lower in the selinexor group. |
| Vd | 62.3%; P = .0012 | 32.4%; P = .0082 | 9.46 (8.11-10.78) P = .0075 | 25 (23.5-NE) P = .18 | ||||
| CANDOR24,25 | Phase 3 randomized | Dara-Kd | 2 (1-5) | 84% | 69% | 28.6 (22.7-NE) | NA | Thrombocytopenia, diarrhea, URTI, pneumonia, fatigue, viral infection, PN |
| Kd | 75%, P = 0.008 | 49% P = NA | 15.2 (11.1-19.9) P < .0001 | NA | ||||
| IKEMA27 | Phase 3 randomized | Isa-Kd | 2 (1-2) | 87% | 73% | NR | NR | Diarrhea, URTI, neutropenia, thrombocytopenia |
| Kd | 83%, P = .19 | 56%, P = .001 | 19.15 (15.77-NR), P = .0007 | NR | ||||
| MYX.1/MCRN-00343 | Phase 2 study | KCd | 2 (1-3) | 85% | 68% | 17.2 (13.4–21.5) | 27.4 (22.1 – NE) | Anemia, thrombocytopenia, neutropenia, infection, cardiovascular events, TMA |
Dara, daratumumab; Isa, isatuximab; KCd, carfilzomib-cyclophosphamide-dexamethasone; Kd, carfilzomib-dexamethasone; NA, not available; NE, not evaluable; Seli, selinexor; Vd, velcade-dexamethasone; TMA, transplant thrombotic microangiopathy; URTI, upper respiratory tract infection.
Daratumumab combined with pomalidomide, a third- generation IMiD, and dexamethasone (D-Pd) is another effective regimen at first relapse in patients who were exposed to both bortezomib and lenalidomide and have lenalidomide-refractory disease. D-Pd was shown to have reduced the risk of disease progression or death compared with pomalidomide and dexamethasone (Pd) alone in a phase 3 randomized trial (median PFS, 12.4 vs 6.9 months; P = .001) (Table 3).18
Pomalidomide when combined with bortezomib and dexamethasone (PVd) showed improved PFS when compared with bortezomib and dexamethasone (Vd) in patients with relapsed MM previously treated with lenalidomide (median 11.2 vs 7.1 months; P < .0001) (Table 3).19 The most common grade 3 or 4 treatment-emergent adverse events (TEAEs) were neutropenia, febrile neutropenia, thrombocytopenia, and infections, which were all more frequent in the PVd arm. A subsequent analysis of the study outcomes by prior treatment at first relapse showed that PVd maintained its efficacy regardless of lenalidomide refractoriness, prior bortezomib exposure, high-risk cytogenetics, or prior ASCT status.20
Selinexor is a potent, orally available, selective inhibitor of nuclear export compound that binds and blocks the function of XPO1, leading to apoptosis of tumor cells.21 Selinexor, given weekly in combination with bortezomib and dexamethasone (SVd), was shown to be more efficacious than bortezomib and dexamethasone (Vd) in patients with relapsed MM after 1 to 3 prior lines of therapy (median PFS, 13.9 vs 9.46 months; P = .0075) (Table 4),22 with activity even in patients with high-risk cytogenetic abnormalities. Apart from more frequent grade 3 to 4 cytopenias, nausea, vomiting, diarrhea, fatigue, weight loss, and asthenia were more common with SVd. Aggressive supportive care that includes a good antiemetic regimen containing 5HT3 receptor agonists, atypical antipsychotics such as olanzapine, thrombopoietin receptor agonists to treat thrombocytopenia, frequent monitoring, and replacement of fluids and electrolytes are essential measures for safe treatment with this regimen.23
Daratumumab in combination with carfilzomib, a second-generation PI, and dexamethasone (DKd) can be used safely in patients with relapsed MM who had prior treatment with bortezomib and lenalidomide and have become refractory to both.24,25 DKd was shown to confer improved PFS over Kd, albeit with higher grade 3 or 4 adverse events (median 28.6 vs 15.2 months; P < .0001) (Table 4). This regimen can be considered for patients with aggressive disease, including plasma cell leukemia, extramedullary disease, and/or high tumor burden.
Isatuximab is another monoclonal antibody that binds to a unique epitope on CD38 that is different from daratumumab and induces antitumor activity through multiple mechanisms, including the direct inhibition of CD38 ectozyme activity.26 Isatuximab added to carfilzomib and dexamethasone (Isa-Kd) is another option for the treatment of relapsed disease that has shown superior efficacy compared with carfilzomib and dexamethasone alone (Kd).27 Isa-Kd significantly prolonged PFS (median not reached [NR] vs 19.15; P = .0007) and improved depth of response (VGPR, 73% vs 56%; P = .001) (Table 4) compared with Kd in patients with relapsed MM. More patients in the isatuximab group experienced grade 3 or worse TEAEs, but rates of treatment discontinuation and deaths due to TEAEs were not higher. It is of note that none of the patients in the study had prior exposure to carfilzomib or refractoriness to prior anti-CD38 therapy.
Carfilzomib-dexamethasone (Kd) is superior to bortezomib-dexamethasone (Vd) in the treatment of relapsed disease with 1 to 3 prior lines of therapy.28 It can be used safely in patients with renal failure due to MM.29 However, it is associated with cardiovascular events, including congestive heart failure, and so screening for cardiovascular risk factors along with close monitoring and treatment of blood pressure is important.30 Carfilzomib given once weekly (70 mg/m2) is as effective as twice-weekly (56 mg/m2) administration and so can be used for more convenience.31
ASCT
ASCT is an effective option even for patients with relapsed MM.33 It should be considered at the time of the first relapse rather than later in the treatment course, especially for those who did not receive it up-front as consolidation therapy.34 Chemo refractoriness of the disease with progression, worsening performance status, and comorbidities with advancing age must be kept in mind when delaying ASCT. Retrospective studies have shown improved PFS with ASCT compared to salvage chemotherapy alone for relapsed MM, especially with longer PFS after the first transplant. A second ASCT can be considered for patients who have had a PFS of at least 18 months from the time of the first transplant.35 A study by the Center for International Blood and Marrow Transplant Research showed that those who relapsed after 36 months from the first transplant did better compared to those whose disease relapsed earlier in terms of PFS and OS.36 The most recent update on the IFM 2009 study on VRd with or without ASCT followed by lenalidomide maintenance showed that close to 77% of patients in the chemotherapy-alone arm received ASCT at relapse, showing that it is feasible even in relapsed disease.37 There was no difference in the PFS2 (time to progression on second-line therapy) or OS in both arms, which may have been due to effective salvage therapy or a lack of continuous maintenance therapy (Table 5). However, the subset of patients in the ASCT arm who achieved MRD negativity at 1 in 106 cells had longer PFS2 and OS. In the DETERMINATION trial, however, only 35.1% of patients who received postprotocol therapy received ASCT.38 This could be explained by the shorter follow-up by the data cutoff date, the physician's perception of the efficacy of ASCT given the lack of OS benefit in the IFM study, and the availability of other effective therapeutic options among other reasons.
Selected studies of efficacy of salvage ASCT in MM
| Trial . | Design . | Patient group . | Reinduction at relapse . | Interval ASCT 1-relapse (months) . | Conditioning regimen . | Median PFS (months) . | Median OS (months) . |
|---|---|---|---|---|---|---|---|
| IFM 2009 – Perrot et al37 (2020) | Prospective, randomized, phase 3 | VRd alone | 40% Pom based | - | Mel 200 | 95 | 60.2% |
| VRd+ASCT | 46.5% Pom based | NR P = .76 | 62.2% P = .81 | ||||
| Cook et al34 (2016) | Prospective, randomized, phase 3 | Second ASCT, (n = 106) | 4 cycles of VAD | >24 | Mel 200 mg/m2 | 19 | 67 |
| Conventional chemotherapy (n = 106) | 30 | Oral cyclophosphamide 400 mg/m2 weekly × 12 weeks | 11 P < .0001 | 52 P = .016 | |||
| Gimsing et al44 (2015) | Prospective, nonrandomized phase 2 (n = 53) | 3 cycles of VD | 25.3 | Mel 200 mg/m2-bortezomib | 19.3 | 44.3 | |
| Ikeda et al45 (2019) | Retrospective (n = 526) | Allo-SCT (n = 192) | VT or R | <9 mo to >30 mo | - | Not done | Intermediate risk 21.5% |
| Re ASCT (n = 334). | 28.2% P < .004 | ||||||
| Gössi et al46 (2018) | Single-institution case-matched | Second ASCT, n = 61 | 28.9 | Age ≤70: Mel 200 mg/m2 Age >70: Mel 140 mg/m2 | 30.2 | 129.6 | |
| Conventional chemotherapy, n = 25 | RD or VD | 14.3 | 13 P = .0262 | 33.5 P = .0003 | |||
| Tremblay et al47 (2017) | Retrospective n = 74 | Conventional chemotherapy (89%); IMiD, 8%; PI, 59.5%; others, 5% | 53 | Mel ≥100 mg/m2 | 6.1 | 19.3 | |
| Singh Abbi et al48 (2015) | Retrospective n = 74 | Most patients received PIs and IMiDs | 37.7 | Mel ≥100 mg/m2 | 10.1 | 22.7 | |
| Grövdal et al49 (2015) | Multi-institution case-matched control analysis | Second ASCT, n = 111 | 28 | Mel 200 mg/m2 | 28 | 48 | |
| Conventional chemotherapy, n = 362 | 25 | 6 | 29 | ||||
| Novel drugs, n = 362 | PI and/or IMiD | 27 | 14 P = .004 | 39 P = .013 |
| Trial . | Design . | Patient group . | Reinduction at relapse . | Interval ASCT 1-relapse (months) . | Conditioning regimen . | Median PFS (months) . | Median OS (months) . |
|---|---|---|---|---|---|---|---|
| IFM 2009 – Perrot et al37 (2020) | Prospective, randomized, phase 3 | VRd alone | 40% Pom based | - | Mel 200 | 95 | 60.2% |
| VRd+ASCT | 46.5% Pom based | NR P = .76 | 62.2% P = .81 | ||||
| Cook et al34 (2016) | Prospective, randomized, phase 3 | Second ASCT, (n = 106) | 4 cycles of VAD | >24 | Mel 200 mg/m2 | 19 | 67 |
| Conventional chemotherapy (n = 106) | 30 | Oral cyclophosphamide 400 mg/m2 weekly × 12 weeks | 11 P < .0001 | 52 P = .016 | |||
| Gimsing et al44 (2015) | Prospective, nonrandomized phase 2 (n = 53) | 3 cycles of VD | 25.3 | Mel 200 mg/m2-bortezomib | 19.3 | 44.3 | |
| Ikeda et al45 (2019) | Retrospective (n = 526) | Allo-SCT (n = 192) | VT or R | <9 mo to >30 mo | - | Not done | Intermediate risk 21.5% |
| Re ASCT (n = 334). | 28.2% P < .004 | ||||||
| Gössi et al46 (2018) | Single-institution case-matched | Second ASCT, n = 61 | 28.9 | Age ≤70: Mel 200 mg/m2 Age >70: Mel 140 mg/m2 | 30.2 | 129.6 | |
| Conventional chemotherapy, n = 25 | RD or VD | 14.3 | 13 P = .0262 | 33.5 P = .0003 | |||
| Tremblay et al47 (2017) | Retrospective n = 74 | Conventional chemotherapy (89%); IMiD, 8%; PI, 59.5%; others, 5% | 53 | Mel ≥100 mg/m2 | 6.1 | 19.3 | |
| Singh Abbi et al48 (2015) | Retrospective n = 74 | Most patients received PIs and IMiDs | 37.7 | Mel ≥100 mg/m2 | 10.1 | 22.7 | |
| Grövdal et al49 (2015) | Multi-institution case-matched control analysis | Second ASCT, n = 111 | 28 | Mel 200 mg/m2 | 28 | 48 | |
| Conventional chemotherapy, n = 362 | 25 | 6 | 29 | ||||
| Novel drugs, n = 362 | PI and/or IMiD | 27 | 14 P = .004 | 39 P = .013 |
allo-SCT, allogenic transplantation; Mel, melphalan; Pom, pomalidomide; RD, revlimid-dexamethasone; TNT, next treatment; VAD; bortezomib-adriamycin-dexamethasone.
Disease-related factors
Various disease-related factors need to be considered while deciding treatment for relapsed MM.39,40 The disease presentation and pace of the progression are the key factors in choosing the regimen. Asymptomatic and/or slow biochemical progression can be monitored closely for symptomatic disease and/or end organ damage. A relapse presenting with end-organ damage such as renal failure warrants the immediate initiation of treatment. Aggressive disease such as secondary plasma cell leukemia often requires treatment with a combination cytotoxic chemotherapy regimen, such as bortezomib, dexamethasone, cisplatin, doxorubicin, cyclophosphamide, and etoposide (VD-PACE) or dexamethasone, cyclophosphamide, etoposide, and cisplatin (DCEP).41
Patient-related factors
Functional age instead of biological age should be used to select a treatment regimen for relapsed disease. An assessment of functional status using a comprehensive assessment tool, such as a frailty score,42 that includes a patient's ability to perform both activities of daily living and instrumental activities along with the Charlson comorbidity index is an effective way to screen for the ability to tolerate a particular regimen. Medical comorbidities such as preexisting hypertension, diabetes mellitus, peripheral neuropathy, chronic kidney disease secondary to diabetes mellitus, or cardiovascular disease play a significant role in the tolerance and toxicity from chemotherapy. An ongoing discussion about the prognosis and the patient's health care–related goals and preferences is of paramount importance in providing optimal care that is both patient centered and effective.
CLINICAL CASE (Continued)
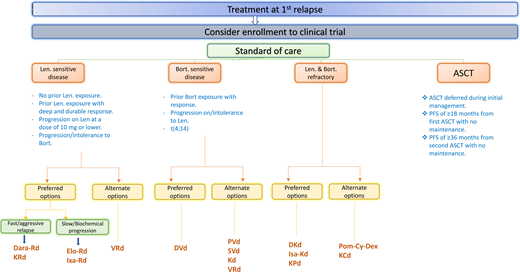
What is the treatment of choice for Ms. Smith? Ms. Smith had extensive rash while receiving the full dose of lenalidomide during induction, necessitating dose reduction. She received bortezomib 3 years ago, and her disease is considered sensitive to it. Therefore, the options at this point include DC, Isa-Kd, DPd, and PVd (Figure 1).
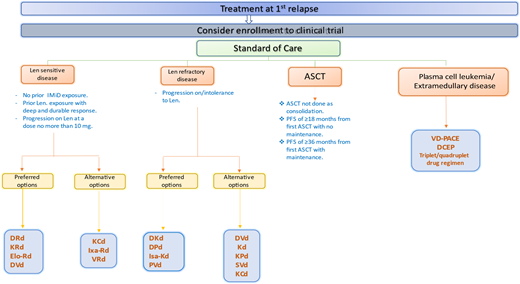
Suggested algorithm for the treatment of multiple myeloma at first relapse. Bort, bortezomib; DRD, daratumumab- lenalidomide-dexamethasone; KCd, carfilzomib-cyclophosphamide-dexamethasone; Len, lenalidomide; VD-PACE, bortezomib- dexamethasone-cisplatin-doxorubicin-cyclophosphamide-etoposide.
Suggested algorithm for the treatment of multiple myeloma at first relapse. Bort, bortezomib; DRD, daratumumab- lenalidomide-dexamethasone; KCd, carfilzomib-cyclophosphamide-dexamethasone; Len, lenalidomide; VD-PACE, bortezomib- dexamethasone-cisplatin-doxorubicin-cyclophosphamide-etoposide.
Since she is 56 now, had a PFS of 3 years from the first ASCT, and still has good performance status, a second ASCT can be considered after achieving the best response with salvage therapy. ASCT has the advantages of having short-lived toxicity for a few weeks and being cost-effective, with the potential to create a bone marrow reset. Prospective studies comparing salvage ASCT with novel drugs, chimeric antigen receptor T cell therapy, and anti–B-cell maturation antigen therapies would shed more light on the role of this modality in the treatment of relapsed disease.
Discussion
Advances in novel therapies have changed the landscape of MM, with a shift in focus from improved ORR to deeper responses such as MRD negativity, translating to longer PFS and event-free survival. However, relapse is inevitable at some point in most patients during the disease. Goals of therapy during the first relapse should be to achieve a rapid reduction of the disease burden and a deep and durable response with an overarching goal of improving PFS and OS. While choosing a treatment regimen, we should consider prior therapies received, including ASCT, the depth and duration of response to prior therapy and any residual toxicity from it, and the patient's general health condition. Increasing attrition rates with each subsequent line of therapy must be kept in mind while choosing a treatment regimen, with special attention to the specific mechanism of action of each drug and the synergistic effect of the drugs in the regimen. Close monitoring for the early detection of symptoms and signs of toxicity and the prompt initiation of mitigation strategies are of paramount importance for successful treatment outcomes.
Conflict-of-interest disclosure
Srinivas Devarakonda: no competing financial interests to declare.
Nidhi Sharma: no competing financial interests to declare.
Yvonne Efebera: no competing financial interests to declare.
Off-label drug use
Srinivas Devarakonda: nothing to disclose.
Nidhi Sharma: nothing to disclose.
Yvonne Efebera: nothing to disclose.