Learning Objectives
Review the evidence to date for the use of autologous hematopoietic cell transplant intensification for relapsed follicular lymphoma (FL)
Identify the role and timing for currently approved chimeric antigen receptor T-cell therapies for relapsed FL
CLINICAL CASE
A 56-year-old woman received bendamustine and rituximab for 6 cycles for untreated follicular lymphoma (FL) grade 1/2 and achieved a complete response. Fourteen months later, she noted an enlarging left axillary lymph node, with biopsy demonstrating FL grade 1/2. She next received lenalidomide and rituximab with a partial response after 3 cycles but after 6 cycles developed a new scalp lesion and imaging-documented disease progression. Biopsy of the scalp lesion again demonstrated FL grade 1/2 without evidence of transformation. What is the role for autologous hematopoietic cell transplant (AHCT) or chimeric antigen receptor T-cell therapy (CAR-T) for this patient?
Introduction
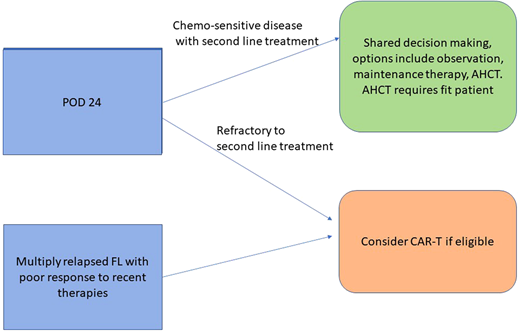
FL is the most common indolent non-Hodgkin lymphoma subtype and has a highly heterogeneous natural history and disease course. Most patients diagnosed with FL today will have survival similar to age-matched controls, but specific clinical scenarios are recognized in which mortality due to FL poses a significantly greater risk. Among patients receiving frontline chemoimmunotherapy treatment for FL, roughly 1 in 5 patients will have their disease fail to achieve remission or relapse within 2 years of starting treatment (POD24). Robust evidence demonstrates that such patients face a significantly higher risk of death due to lymphoma, with a 5-year overall survival (OS) of only 50% following POD24 vs 90% for all other patients after frontline chemoimmunotherapy treatment.1 Significant heterogeneity also exists among patients with POD24. In a recent large cohort study, the 2-year progression-free survival (PFS) among patients receiving treatment for relapsed FL with at least 2 prior lines of treatment with POD24 was 35%, demonstrating that a subset of early progressors still achieve a satisfactory response with subsequent therapies.2 Multiple studies have observed that the expected duration of response decreases with increasing prior lines of FL therapy, including 1 cohort in which the 2-year PFS for third-line therapy in all patients with relapsed FL was less than 50% for all classes of therapy.2 While FL was the leading cause of death among this multiply relapsed FL cohort, the 5-year OS was 75% from time of third-line treatment, underscoring the effectiveness of currently available therapies for most patients with FL and the difficulty in identifying the minority of patients who warrant consideration of intensified treatments due to poor outcomes with less intensive current therapies. Herein we briefly summarize the evidence for the use of 2 intensification approaches: AHCT and CAR-T in the management of relapsed FL.
Evaluating for histologic transformation
It is imperative for clinicians to maintain vigilance for the possibility of transformation of FL to aggressive lymphoma, given high prevalence in early relapses and important differences in the therapeutic management of transformed disease with established roles of AHCT and CAR-T. Biopsies should be performed if feasible at first relapse and in all cases of suspected transformation, and the authors note that clinicians may use intensification strategies in cases of suspected occult transformation in which biopsies are not feasible or not thought to be representative of the more aggressive process.
Autologous hematopoietic cell transplant
Multiple randomized prospective studies have been conducted to assess the benefit of AHCT consolidation in first remission following frontline chemotherapy for FL. With extended follow-up, these studies failed to demonstrate improvement in OS with AHCT and observed higher rates of second primary malignancies in the AHCT arms, predominantly therapy-related myeloid neoplasms.3
Prospective randomized studies of AHCT for relapsed FL have not been performed in era of CD20 monoclonal antibody treatment, and the available data regarding the effectiveness of this approach come primarily from registry and retrospective evaluations. Jurinovic et al4 analyzed patients with FL treated on 2 successive frontline clinical trials who progressed and received subsequent therapies. This study highlighted a key limitation of AHCT, the need for chemosensitive disease, with the worst outcomes seen in those who failed to respond to second-line treatment and thus were deemed AHCT ineligible. Receipt of AHCT was associated with greater 5-year PFS and OS; however, when restricting analysis to patients with chemosensitive disease, the difference in 5-year OS was not significant, suggesting a lack of benefit for AHCT in an unselected population in second remission. Among patients with POD24, AHCT consolidation was associated with improved 5-year PFS (51% vs 19%) and OS (77% vs 59%) and remained associated with superior outcomes even in an intent-to-transplant analysis. Casulo et al. analyzed data from the Center for International Blood and Marrow Transplant Research (CIBMTR) and the National LymphoCare Study (NLCS), comparing 2 cohorts of patients aged ≤70 years with POD24: those undergoing AHCT in the CIMBTR registry and those managed without AHCT in the NLCS. Among the entire CIBMTR cohort, no significance difference in 5-year OS was seen in comparison to the NLCS cohort without AHCT.5 However, a preplanned analysis restricted to patients undergoing AHCT within 12 months of first relapse demonstrated superior 5-year OS in comparison to the NLCS patients. Conclusions are limited due to the observational nature of this study, which, given the requirement of chemosensitive disease for AHCT, results in important differences between the 2 analyzed patient populations. More recently, retrospective data demonstrate an association between complete response (CR) by imaging in patients with FL prior to AHCT and improved PFS, again highlighting the importance of chemosensitivity and the importance of low disease burden and ideally CR prior to AHCT for FL.6 Comparisons of AHCT and CAR-T (discussed below) are highlighted in Table 1.
Comparison of considerations for intensification strategies for relapsed follicular lymphoma—CAR-T vs autologous transplant
| . | Autologous transplant . | CAR-T . |
|---|---|---|
| Requires chemosensitive disease | Yes | No |
| Potential benefit limited to early in relapsed course | Yes | No* |
| Short-term toxicities | Mucositis, infection, sepsis, pneumonitis, poor engraftment | CRS, ICANS, cytopenia, infection |
| Long-term toxicities | Therapy-related myeloid malignancies | B-cell aplasia, hypogammaglobulinemia, cytopenia* |
| Other considerations | Need for adequate marrow reserve for stem cell collection, eligibility limited to fit patients | Need for adequate lymphocyte count, wait time for and requirement of successful T-cell manufacturing, likely lower fitness requirement than autologous transplant |
| . | Autologous transplant . | CAR-T . |
|---|---|---|
| Requires chemosensitive disease | Yes | No |
| Potential benefit limited to early in relapsed course | Yes | No* |
| Short-term toxicities | Mucositis, infection, sepsis, pneumonitis, poor engraftment | CRS, ICANS, cytopenia, infection |
| Long-term toxicities | Therapy-related myeloid malignancies | B-cell aplasia, hypogammaglobulinemia, cytopenia* |
| Other considerations | Need for adequate marrow reserve for stem cell collection, eligibility limited to fit patients | Need for adequate lymphocyte count, wait time for and requirement of successful T-cell manufacturing, likely lower fitness requirement than autologous transplant |
Long-term follow-up evaluating both risk and benefit in large cohorts of patients with FL receiving CAR-T is limited.
ICANS, immune effector cell-associated neurotoxicity syndrome.
Chimeric antigen receptor T-cell therapy
CAR-T has emerged as an effective treatment class for multiply relapsed, high-risk patients with FL, although one must keep in mind that unlike in AHCT, formal comparative studies of CAR-T have not been completed to date, and long-term follow-up regarding safely and efficacy in FL is limited.7,8 Axicabtagene ciloleucel (axi-cel) is a CAR-T granted accelerated approval by the US Food and Drug Administration for FL relapsed after 2 or more lines of treatment based on the phase 2 Zuma-5 study.8 Zuma-5 enrolled 153 patients and treated 148 patients, including 124 patients with FL. Baseline characteristics of the patients with FL included high tumor bulk in 52%, ≥3 prior therapies in 63%, POD24 in 55%, and prior AHCT in 24%. Bridging therapy was administered in 6 patients prior to axi-cel infusion. At a median follow-up of 17.5 months, the overall response rate in analyzed patients with FL was 94% including CR in 79%, with an 18-month estimated PFS and OS of 65% and 87%, respectively. Cytokine release syndrome (CRS) occurred in 78% of patients with FL, including grade ≥3 CRS in 6%, and 50% of the study population received tocilizumab. One death due to CRS occurred. Neurologic events (NEs) occurred in 56% of patients with FL, including grade ≥3 NEs in 15%. Other toxicities included grade ≥3 cytopenia beyond day 30 in 34%, grade ≥3 infections in 18%, and second primary malignancies in 9% of patients. Interestingly the rates of CRS and NEs appeared lower in patients with FL compared with those with marginal zone lymphoma, and lower peak levels of cytokines associated with CRS were observed in patients with FL in correlative analysis.
Tisagenlecleucel (tisa-cel) is a CAR-T therapy granted accelerated Food and Drug Administration approval for the treatment of FL after 2 or more prior lines of treatment based on the phase 2 Elara trial. Elara enrolled 98 patients with relapsed/refractory FL to study treatment with lymphodepletion followed by tisa-cel.7 Baseline patient characteristics included a median of 4 prior lines of treatment, 64% with high tumor bulk, 78% refractory to most recent treatment, 63% with POD24, and 36% with prior AHCT. Bridging therapy was administered while awaiting tisa-cel manufacturing prior to infusion in 44 patients (45%). After a median follow-up of 9.9 months, the observed overall response rate was 86%, including CR in 69% with a 12-month estimated PFS of 67%. Response rates were similar across subgroups, although a lower CR rate was noted in patients with POD24 (59% vs 88% for other patients). CRS of any grade occurred in 49% (0% grade ≥3), with 16 patients (16%) receiving tocilizumab for CRS. NEs (any grade) occurred in 37%, including immune effector cell neurotoxicity syndrome in 4.1% (1% grade ≥3). Other toxicities of note included grade ≥3 infection in 5% and prolonged B-cell aplasia in 16%.
NEs and CRS were numerically greater in Zuma-5 compared with Elara, and a recent matched adjusted indirect comparison analysis demonstrated significantly lower rates of NEs and CRS in patients treated in Elara compared with matched patients treated in Zuma-5.9 The Elara and Zuma-5 studies both demonstrated high response rates with tisa-cel and axi-cel, respectively, in high-risk populations with FL, but given the relatively short follow-up, conclusions cannot be drawn regarding the long-term durability of these treatments in FL. Long-term follow-up of a small single-center study of tisa-cel, which enrolled 14 patients with FL, reported sustained FL remission beyond 5 years in 5 responding patients, suggesting durable remissions may occur in some responding patients with FL.10 Longer follow-up from the larger phase 2 registration studies will better define response durability, and as with other therapies in FL, phase 3 confirmation of survival benefit remains critical.
Conclusions
As the FL treatment landscape continues to change with the development of new classes of therapy, the prioritization of therapies for high-risk patients with FL will continue to evolve. CAR-T represents an important new treatment option for relapsed FL, offering a class of therapy with a high expected response rate for high-risk patients, although comparative data are still needed. Challenges remain as CAR-T requires physiologic reserve to tolerate lymphodepleting chemotherapy as well as expected CAR-T toxicities, notably CRS, and thus requires a level of baseline patient fitness to be eligible. As in other settings where CAR-T is approved, defining the eligible population for CAR-T continues to evolve, but CAR-T may be tolerated by a broader patient population than would be eligible for AHCT. In the current changing FL treatment landscape, the role of intensification to AHCT is less clear. The use of AHCT is limited by the need for chemosensitive disease and the challenge of identifying sufficiently high-risk patients earlier in the disease course; however, AHCT may still be considered in selected, chemosensitive, fit, high-risk patients with relapsed FL.
CLINICAL CASE (Continued)
In a young patient with POD24 and refractory disease or less than 12 months of response to second-line nonchemotherapy treatment who achieves a complete response with third-line chemotherapy-based treatment, we would offer AHCT consolidation and discuss associated risks, including risk for treatment-related myeloid neoplasms. However, in this clinical scenario, we would prioritize clinical trials if available or commercial CAR-T if approved by local regulatory agencies and available over third-line chemoimmunotherapy treatment based on available evidence to date. In the presented case, the patient was treated with third-line axi-cel with clinical course complicated by grade 2 CRS and grade 2 NEs, which resolved with supportive care, including tocilizumab and corticosteroids without ongoing sequelae. The patient experienced rapid improvement in her scalp mass clinically, and day 90 positron emission tomography confirmed CR.
Recommendations
AHCT in first remission does not improve OS in FL and should not be performed—strong recommendation based on moderate certainty to the evidence of effects.
AHCT in second or third remission may be considered for select very high-risk patients with FL with chemosensitive disease, ideally achieving complete metabolic response—conditional recommendation based on low certainty of the evidence of effects.
CAR-T therapy in the third or later line of therapy for FL offers high response rates and should be considered in high-risk patients with a short expected duration of response to alternative available therapies or lack of complete response to chemotherapy—conditional recommendation based on moderate certainty to the evidence of effects.
Conflict-of-interest disclosure
David A. Bond: research funding: Novartis, Nurix Therapeutics. Consultancy/honoraria: SeaGen and Kite/Gilead.
Ajay K. Gopal: research funding: Merck, I-Mab bio, IgM Bio, Takeda, Gilead, Astra-Zeneca, Agios, Janssen, BMS, SeaGen, Teva, Genmab. Consultancy/honoraria: Incyte, Kite, Morphosys/Incyte, ADCT, Acrotech, Merck, Karyopharm, Servier, Beigene, Cellectar, Janssen, SeaGen, Epizyme, I-Mab bio, Gilead, Genentech, Lilly, Caribou.
Off-label drug use
David A. Bond: nothing to disclose.
Ajay K. Gopal: nothing to disclose.