Abstract
Multiple studies have demonstrated that patients with acute myeloid leukemia (AML) who have measurable residual disease (MRD) detected during or after treatment have higher relapse rates and worse survival than similar patients testing negative. Updated response criteria for AML reflect the understanding that achievement of complete remission (CR) with no detectable MRD using high-sensitivity tests represents a superior response over conventional cytomorphological CR alone. Potential use cases for AML MRD testing are diverse and include patient selection for clinical trials and therapeutic assignment, early relapse detection and intervention during sequential monitoring, and drug development, including deep quantification for antileukemia efficacy and as a surrogate endpoint for overall survival in regulatory approvals. Testing for AML MRD has not, however, been harmonized, and many technical and clinical questions remain. The implications of MRD test results for specific therapeutic combinations, molecular subsets, test types, treatment time points, sample types, and patient characteristics remain incompletely defined. No perfect AML MRD test or testing strategy currently exists, and the evidence basis for clinical recommendations in this rare disease is sparse but growing. It is unproven whether conversion of an MRD test result from positive to negative by additional therapeutic intervention improves relapse risk and survival. Several national- and international-level consortia have recently been initiated to advance the generation and collection of evidence to support the use of AML MRD testing in clinical practice, drug development, and regulatory approvals.
Learning Objectives
Explain why the updated AML response criteria now include a best possible response of CRMRD−
Describe how tests validated for diagnostic profiling purposes only are likely insufficient for use in MRD
Understand the objectives of ongoing national and international collaborative efforts to validate AML MRD testing for multiple purposes
CLINICAL CASE
A man in his 70s with significant medical comorbidities presents to your clinic with AML. Flow cytometry demonstrates abnormal CD34+ blasts in both blood (1%) and marrow (~5%). Metaphase cytogenetics are reported as 47XY, +8, inv(16)(p13.1q22), del(20)(q11.2q13.3), and next generation sequencing (NGS) by a “myeloid panel” reported 2 mutations in DNMT3A and 1 in TET2. You are delighted when he achieves a complete remission (CR) by cytomorphology after 1 cycle of treatment until your medical student asks, “But what about measurable residual disease [MRD]?” Your institution has the tests described in the above diagnostic workup available. How, and why, should you measure MRD?
Introduction
Detectable disease after treatment is, by definition, refractory therapy-resistant disease. Patients and their doctors generally strongly prefer no evidence of residual cancer. With a blood cancer like AML, which is typically widely disseminated throughout the body at initial diagnosis, the key issue in assessing posttreatment response is how accurately the evaluation of a small sample of the patient reflects the total remaining leukemic burden in the body with the capacity to lead to a subsequent clinically evident “relapse.” An insufficient sample and/or suboptimal assessment of a sample from a patient after treatment may lead to false reassurance that a patient in “complete remission” has achieved a state of disease clearance, a conceit quickly shattered by the “relapse” that follows. While it has been stated that the most pressing problem in AML is relapse, a very reasonable counterargument is that the most pressing problem is instead that AML therapy is suboptimal and appears adequate only due to insufficiently stringent response criteria. In recognition of this, the European LeukemiaNet (ELN) in 2017 updated the response criteria in AML with the addition of a best possible response category of complete remission without MRD (CRMRD−).1
The case for MRD assessments in AML response criteria is clear, even if the logistics associated with widespread harmonized clinical implementation remain to be defined.2,3 For 40 years the concept that MRD in AML exists and may be detectable and treatable has been well understood.4-6 In the past 5 years, systematic reviews with meta-analyses have demonstrated that, regardless of the MRD methodology used, patients testing positive, either at a specific time point prior to allogeneic hematopoietic cell transplantation (alloHCT) or more generally at any time during treatment,7,8 have worse survival than those who test MRD negative. This stratification at the cohort level can assign patients with AML in CR after treatment into groups with higher and lower risks of subsequent relapse. However, when currently measured at a single time point, MRD testing is suboptimal at an individual patient level for relapse prediction—particularly, perhaps, when baseline patient and disease characteristics are considered.9-12 Nevertheless, given the additional insight provided there is increasing interest in using AML MRD testing results for a variety of potential clinical use cases (Table 1).13-15 Achieving MRD negativity in AML is an important signifier of response to initial treatment, but additional evidence is required to determine if this is the most appropriate goal and, if so, the best ways to get there both in an individual patient and as an international community.
Potential use cases for AML MRD
| • Deep quantification of antileukemia efficacy (eg, log reduction after 2 cycles of therapy) |
| • Early relapse detection and intervention during sequential monitoring |
| • Therapeutic assignment (eg, selection of transplant intensity where otherwise equipoise) |
| • Patient selection for clinical trials (eg, high-risk group of unmet need) |
| • As a surrogate endpoint for overall survival for regulatory approval |
| • Deep quantification of antileukemia efficacy (eg, log reduction after 2 cycles of therapy) |
| • Early relapse detection and intervention during sequential monitoring |
| • Therapeutic assignment (eg, selection of transplant intensity where otherwise equipoise) |
| • Patient selection for clinical trials (eg, high-risk group of unmet need) |
| • As a surrogate endpoint for overall survival for regulatory approval |
When, where, and with what?
Given the strong evidence that testing for MRD in patients with AML in remission can stratify them into groups with higher and lower risks of relapse and survival, there is great appeal in developing a universal “best” single test by which to monitor AML MRD. This potentially unrealistic aspiration may be motivated by the examples of great success in MRD monitoring for other blood cancers, such as chronic myeloid leukemia, acute promyelocytic leukemia (both entities with a single pathognomonic structural variant expressed at the transcript level for tracking by quantitative polymerase chain reaction), and the lymphoid malignancies and multiple myeloma (in which a distinct cell surface immunophenotype and a clonally rearranged immune receptor provide 2 ideal options for MRD monitoring). In contrast, AML is a name given to a genetically heterogeneous collection of myeloid malignancies, which can have changing clonal proportions even within 1 patient over time. While multiple individual targets for the molecular monitoring of AML MRD have been described (Figure 1), there remains great interest in a “one-size-fits-all” approach to MRD monitoring in this diverse set of blood cancers using, for example, multiparameter flow cytometry or NGS. Currently, there is no “one best test” for all cases of AML MRD.
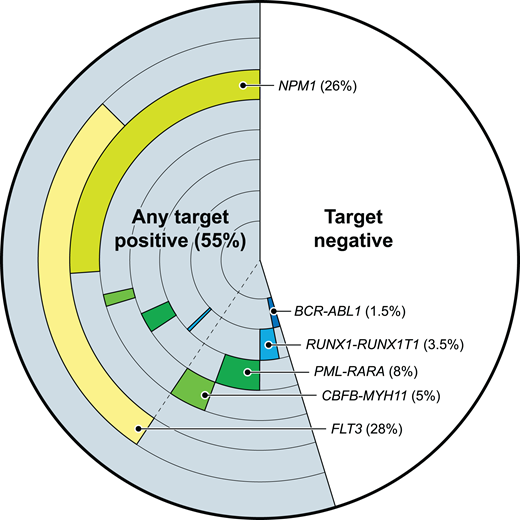
Some validated molecular targets for AML MRD testing. The frequency and co-occurrence of those molecular targets with evidence of utility for AML MRD testing, based on 200 adult AML patients from the Cancer Genome Atlas database.
Some validated molecular targets for AML MRD testing. The frequency and co-occurrence of those molecular targets with evidence of utility for AML MRD testing, based on 200 adult AML patients from the Cancer Genome Atlas database.
Flow cytometry is widely available, is used routinely in the initial diagnostic workup, and has a potentially rapid turnaround time. Limitations include the need for highly specialized expert interpretation for best results, difficulties in test harmonization when not performed and, in particular, analyzed centrally,3,11 and suboptimal relapse risk prediction even in the best centers (a recent study of 743 consecutive adults undergoing their first alloHCT for AML in remission at a single center showed that pretransplant flow cytometry identified just 96 of 230 subsequent relapses).10,16 The ELN guidelines currently state that flow cytometry for AML MRD should be used when a validated molecular test is not available.2,3
NGS of DNA is also widely available and used routinely in the initial diagnostic workup. A longer turnaround time than other methods is balanced by objective output that allows for a decentralized interpretation.17 NGS is not currently ELN recommended for detecting AML MRD as a stand-alone test, however, due to insufficient data on appropriate targets, performance characteristics of optimal testing, and clinical utility.18 It is already clear that the full spectrum of somatic mutations detected at initial AML diagnosis are not all useful as AML MRD targets.2,12,19,20 The concordance of AML MRD testing using flow cytometry and NGS has been observed to be incomplete,19,21 with many potential explanations (Table 2). The remarkable opportunities presented by NGS mean it will almost certainly play a large role in AML MRD as appropriate targets are validated and the performance characteristics of different approaches are better understood and optimized (Figure 2). Other potential forms of NGS, including cell-free DNA, transcript expression, and methylation signatures, do not yet have sufficient evidence for AML MRD monitoring. RNA-based NGS for the ELN-recommended AML MRD molecular targets has been described.22 Single-cell sequencing has the potential to elucidate clonal structure at diagnosis to determine features associated specifically with the malignant clone, including linking genotype with immunophenotype (the differentiation state of the cell with a detected mutation may have implications for both therapy resistance and the ability to lead to relapse).23-25
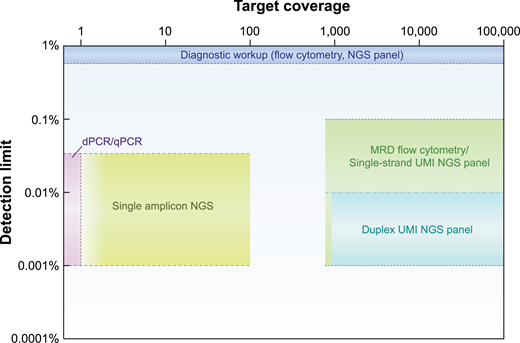
Comparison of performance of tests used for AML diagnosis vs MRD. Cartoon approximation of wide differences in target coverage (ie, number of features tracked, “breadth”) vs detection limit (ie, analytical sensitivity, “depth”) between tests validated for use at initial diagnosis (blue) vs MRD tests in use or development. dPCR, digital PCR; UMI, unique molecular identifier.
Comparison of performance of tests used for AML diagnosis vs MRD. Cartoon approximation of wide differences in target coverage (ie, number of features tracked, “breadth”) vs detection limit (ie, analytical sensitivity, “depth”) between tests validated for use at initial diagnosis (blue) vs MRD tests in use or development. dPCR, digital PCR; UMI, unique molecular identifier.
Potential reasons for discrepancy between AML MRD testing methods
| Flow cytometry true positive, NGS false negative | AML has no appropriate mutation for tracking (rare) NGS test does not include mutation of interest (common) • Core binding factor leukemia without KIT or other RTK mutation • Complex karyotype AML without TP53 mutation • Chromosomal aneuploidy or structural variants NGS test is insufficient sensitivity/not validated for MRD target (common) |
| NGS true positive, flow cytometry false negative | Lack of target (No LAIP/insufficient DfN) (rare) Limit of detection technique mismatch (eg, NPM1-mutated AML) (common) Heterogeneous or unstable immunophenotype, differentiated cells after therapy (common) |
| NGS false positive, flow cytometry true negative | Technical error (NGS test not validated for MRD use) Inappropriate NGS target selection (eg, isolated DNMT3A mutation) Mutation germ line or present only in cells incapable of causing relapse (eg, lymphocytes) |
| Flow cytometry false positive, NGS true negative | Regeneration after chemotherapy or allogeneic transplant |
| Flow cytometry true positive, NGS false negative | AML has no appropriate mutation for tracking (rare) NGS test does not include mutation of interest (common) • Core binding factor leukemia without KIT or other RTK mutation • Complex karyotype AML without TP53 mutation • Chromosomal aneuploidy or structural variants NGS test is insufficient sensitivity/not validated for MRD target (common) |
| NGS true positive, flow cytometry false negative | Lack of target (No LAIP/insufficient DfN) (rare) Limit of detection technique mismatch (eg, NPM1-mutated AML) (common) Heterogeneous or unstable immunophenotype, differentiated cells after therapy (common) |
| NGS false positive, flow cytometry true negative | Technical error (NGS test not validated for MRD use) Inappropriate NGS target selection (eg, isolated DNMT3A mutation) Mutation germ line or present only in cells incapable of causing relapse (eg, lymphocytes) |
| Flow cytometry false positive, NGS true negative | Regeneration after chemotherapy or allogeneic transplant |
DfN, difference from normal; LAIP, leukemia-associated immunophenotype; RTK, receptor tyrosine kinase.
Beyond the specific details of the current and future technology used for this purpose, an optimal AML MRD testing strategy for relapse prediction requires the optimization of multiple factors, including intervals between testing time points, the type (marrow vs blood) and amount of sample tested, and an accounting for AML disease biology and kinetics, as well as patient characteristics that include age, antecedent disorders, time points of treatment, and the intensity and nature of therapy.26-29
Is MRD clinically actionable, or does it just portend fate?
AML patients in a conventional, cytomorphological CR have a higher risk of relapse and worse survival if evidence of residual disease is detected compared to those who test negative—that is, AML MRD test positivity is prognostic. Such patients are high risk, are underserved by the current standard of care, and should be offered a clinical trial where possible. It is, however, important to also say that MRD test negativity does not equal patient MRD negativity. Patients testing MRD negative by flow cytometry prior to alloHCT still have a 20% to 30% relapse rate,30 and deintensification of standard treatment based on an MRD test result should only be attempted cautiously as part of a clinical trial. Patients testing MRD-positive may relapse, may die of a competing risk before relapse, be false positive for technical (assay analytical failure) or clinical (analyte detected but not associated with relapse risk—eg, wrong target or right target but in the wrong cellular context) reasons, or may have their relapse risk reduced by subsequent antileukemic factors (additional therapy, allogeneic or autologous immune responses). Careful systematic study to understand the nature of false-negative and false- positive MRD tests are necessary to allow iterative improvements to MRD tests and MRD testing strategies.
In addition to MRD testing in AML being prognostic, it has been shown to be possible, in some circumstances, to convert a patient testing positive to a negative test status by additional treatment (for example, alloHCT).31,32 The conversion of MRD test result status from positive to negative, however, does not necessarily imply a clinical benefit in terms of increased overall survival (from decreased relapse) or improved quality of life. It is possible to imagine a worst-case scenario in which additional therapy made the biomarker test turn negative but with increased toxicity and no survival benefit. Some patients with MRD in remission are incompletely treated and have chemo-sensitive disease left to treat. Alternatively, MRD may reflect the residual chemo- resistant clone, which may or may not be resistant to novel agents. Both these possibilities are testable.
A large retrospective European Society for Blood and Marrow Transplantation registry suggested that myeloablative (MAC) rather than reduced-intensity conditioning (RIC) in younger patients mitigated some of the risk associated with pretransplant MRD positivity.30 The phase 3 randomized controlled trial (RCT) BMT-CTN 0901 study (NCT01339910) demonstrated reduced relapse and improved overall survival for younger adults in CR randomized to MAC rather than RIC; subsequent analysis showed the greatest benefit for those who were NGS positive before conditioning.20 The poor outcomes of those who were MRD-positive prior to RIC alloHCT were confirmed by the FIGARO trial, an RCT that demonstrated that additional cytoreductive chemotherapy prior to RIC (in patients ineligible for MAC due to age or comorbidity) did not improve outcomes.33 The GIMEMA AML1310 trial (NCT01452646) assigned younger, intermediate-risk patients who were MRD positive to alloHCT, or autologous transplant if MRD negative, and saw no difference between groups, suggesting a benefit to treatment intensification.34 Understanding if an MRD test result is just fate (ie, a prognostic biomarker) or if treatment modification can change outcomes (ie, also a predictive biomarker), and if so in which patients and AML types, is currently one of the most important questions in AML. Observational and registry studies are poorly suited to answer this question, highlighting the need to generate higher-quality evidence for MRD in AML.
Recent initiatives generating AML MRD evidence
AML is a rare disease, further divided into subsets with diverse genetic etiologies and prognoses and, increasingly, with multiple treatment alternatives. In this context, single investigators or institutions can make only limited generalizable contributions to the evidence base supporting AML MRD test uses, motivating the formation of several large national or multinational cooperative efforts (Table 3).
Some recent initiatives generating evidence for MRD testing in AML
| Initiative . | Goal . | Membership . |
|---|---|---|
| ELN AML MRD guidelines: European LeukemiaNet | Evidence-based clinical standard of care consensus guidelines for AML MRD testing | International committee of physicians and scientists with expertise in AML MRD |
| MPAACT: Measurable Residual Disease Partnership and Alliance in Acute Myeloid Leukemia Clinical Treatment | Industry-led research alliance advancing efforts to establish MRD as a surrogate endpoint for overall survival in the treatment of AML | Founded in 2018 by Janssen, Genentech, Novartis, and Celgene (now Bristol Myers Squibb), with recent additions of Amgen, AbbVie, and Kronos Bio.40 |
| FNIH: Foundation of the NIH Biomarkers Consortium for AML MRD | Establish and validate new methods for detecting MRD in AML, including a library of reference standards and evidence of clinical utility. | FDA, NIH, 2 academic partners, and 15-25 private sector industry partners |
| Pre-MEASURE | NIH-led retrospective project on >1000 patients to determine the impact of pre-alloHCT MRD testing in CR1 blood using ultradeep NGS | In collaboration with the CIBMTR |
| MEASURE: Molecular Evaluation of AML Patients After Stem Cell Transplant to Understand Relapse Events | Prospective multicenter protocol to determine clinical utility of MRD testing in up to 1000 AML patients undergoing alloHCT (NCT05224661) | National Marrow Donor Program, CIBMTR, NIH, with initially up to 16 US-based high-volume alloHCT centers |
| NCI MyeloMATCH | Upcoming national precision medicine master protocol for AML. Rapid drug efficacy screening using genetic assignments and MRD testing. | National Cancer Institute, ECOG-ACRIN, SWOG, The Alliance, Canadian Cancer Trials Group Children's Oncology Group |
| Initiative . | Goal . | Membership . |
|---|---|---|
| ELN AML MRD guidelines: European LeukemiaNet | Evidence-based clinical standard of care consensus guidelines for AML MRD testing | International committee of physicians and scientists with expertise in AML MRD |
| MPAACT: Measurable Residual Disease Partnership and Alliance in Acute Myeloid Leukemia Clinical Treatment | Industry-led research alliance advancing efforts to establish MRD as a surrogate endpoint for overall survival in the treatment of AML | Founded in 2018 by Janssen, Genentech, Novartis, and Celgene (now Bristol Myers Squibb), with recent additions of Amgen, AbbVie, and Kronos Bio.40 |
| FNIH: Foundation of the NIH Biomarkers Consortium for AML MRD | Establish and validate new methods for detecting MRD in AML, including a library of reference standards and evidence of clinical utility. | FDA, NIH, 2 academic partners, and 15-25 private sector industry partners |
| Pre-MEASURE | NIH-led retrospective project on >1000 patients to determine the impact of pre-alloHCT MRD testing in CR1 blood using ultradeep NGS | In collaboration with the CIBMTR |
| MEASURE: Molecular Evaluation of AML Patients After Stem Cell Transplant to Understand Relapse Events | Prospective multicenter protocol to determine clinical utility of MRD testing in up to 1000 AML patients undergoing alloHCT (NCT05224661) | National Marrow Donor Program, CIBMTR, NIH, with initially up to 16 US-based high-volume alloHCT centers |
| NCI MyeloMATCH | Upcoming national precision medicine master protocol for AML. Rapid drug efficacy screening using genetic assignments and MRD testing. | National Cancer Institute, ECOG-ACRIN, SWOG, The Alliance, Canadian Cancer Trials Group Children's Oncology Group |
Alongside updating the assessment criteria after AML treatment in 2017 by including a new, best possible response of CRMRD−,1 the ELN in 2016 also established a panel of international experts (initially 24 from 20 countries, including laboratory scientists, pathologists, and leukemia physicians) to recommend laboratory and clinical guidelines for the use of AML MRD testing. The first edition of these consensus standard of care guidelines was published in 2018 and provided technical guidance for performing molecular and flow cytometry–based MRD testing along with clinical recommendations, including that AML with mutated NPM1,35,36 core-binding factor AMLs, acute promyelocytic leukemia, and the rare cases of AML with BCR-ABL1 should be monitored with a validated molecular test, while all others should be monitored by flow cytometry.3 Updated guidelines were published in 2021, with a focus on the available evidence base underlying these expert recommendations and optimized consensus generation using a two-stage Delphi poll approach.2 This group, recently renamed ELN-DAVID, will likely continue to release updated consensus recommendations every 2 to 4 years as new high-quality evidence becomes available.
Two important initiatives in AML MRD from, or in partnership with, the biopharmaceutical industry are worthy of comment. First, following the US Food and Drug Administration (FDA)- convened Duke Margolis Center Public Meeting on Minimal Residual Disease as a Surrogate Endpoint in Hematologic Cancer Trials in 2016, a partnership of 4 pharmaceutical companies was formed (led initially by Sharon McBain, then of Janssen, the partnership was formalized and expanded in 2022 and is now known as MPAACT) to advance efforts in this area, including the planned performance of a meta-analysis of clinical trial data to evaluate the association of MRD with overall survival. Complementary to this, focusing on the generation of new standards, tests, and data, rather than the analysis of existing data sets, in early 2022 the Foundation of the National Institutes of Health (NIH) Biomarkers AML MRD Consortium was launched as a collaboration between public-sector (NIH, FDA), private-sector (~20 pharmaceutical, biotechnology, research, or diagnostic testing companies), and academic (Fred Hutch and Dana Farber) partners.
AlloHCT is a key therapeutic intervention to reduce subsequent relapse risk for many patients with AML in CR, with good evidence that MRD before transplant is prognostic.7,9,16,20,30,33,37 The NIH-funded Pre-MEASURE study evaluated pretransplant blood samples from 1075 patients transplanted in first remission at one of 111 Center for International Blood and Marrow Transplant Research (CIBMTR) sites between 2013 and 2019 to establish the clinical utility of NGS-based AML MRD testing for FLT3-ITD and NPM1 mutations.38 Following this retrospective study, the National Marrow Donor Program and the CIBMTR sponsored a prospective protocol, MEASURE, at 16 major US transplant centers to establish a national framework for introducing MRD testing into the clinical care of AML patients undergoing alloHCT (https://clinicaltrials.gov/ct2/show/NCT05224661).
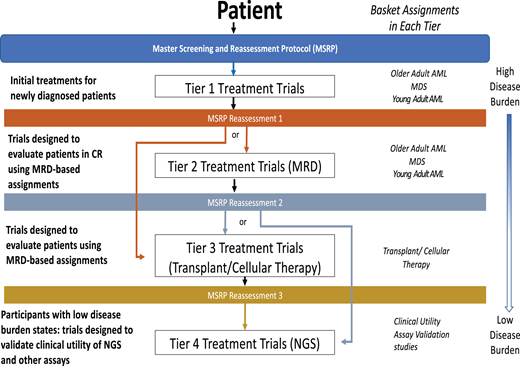
The National Cancer Institute precision medicine initiative for patients with AML, MyeloMATCH, is also launching officially in early 2023. This national umbrella trial will test treatments for AML, typically in randomized phase 2 designs comparing against the current best standard of care therapy, evaluating early endpoint efficacy signals in specific molecular and clinical risk groups. The novel design will assign a unique single patient identifier upon enrollment for initial therapy, allowing subjects to be followed throughout their treatment journey while participating in up to 4 different RCTs based on scheduled reassessments (Figure 3). The intent is to use MRD testing as an efficacy endpoint, as an inclusion criterion for subsequent “tiers” of therapy, while also facilitating the validation of novel, highly sensitive MRD assays such as duplex sequencing.
The upcoming NCI Precision Medicine Initiative MyeloMATCH. Schematic showing potential journey of a patient enrolled in MyeloMATCH through the protocol tiers.
The upcoming NCI Precision Medicine Initiative MyeloMATCH. Schematic showing potential journey of a patient enrolled in MyeloMATCH through the protocol tiers.
Finally, regulatory guidance for the use of MRD, including in AML, for drug development has been published and presumably updated based on evidence resulting from the initiatives above.39
CLINICAL CASE (Continued)
Clinical NGS DNA sequencing “myeloid panels” used for AML diagnostic profiling are 10 to 500 times less sensitive than AML MRD tests and is a poor choice here. Additionally, which somatic mutations detected in remission are most associated with relapse risk remain to be fully described. There is evidence that DNMT3A and TET2 mutations should not be used for MRD testing; this was reinforced by research showing that these mutations were only found in subclones unrelated to the AML of this patient.24 Flow cytometry performed to MRD standards is a reasonable choice but is not optimal given the presence of a defining molecular feature in this inversion-16 AML (CBFB-MYH11), quantifiable using a well-validated test.2,3 The patient achieved MRD negativity by polymerase chain reaction testing and enjoyed a prolonged remission despite being ineligible for consolidative alloHCT.
Conclusion
Testing AML MRD negative is preferable to testing positive, all other factors being equal. The phenomena of higher-sensitivity tools allowing refined, but imperfect, prognostication for patients with AML in remission have been well described in the literature but incompletely translated to the clinic. Because the AML MRD test status reflects only the sample that was tested, not the entire patient, false-positive and false-negative results are expected and have multifactorial causes. On an individual patient level, AML MRD test status can help risk-stratify but is not a guarantee of fate; serial MRD measurement kinetics are likely superior to single landmark assessments. The coming years will see the generation of high-quality evidence for the many potential- use cases for AML MRD testing and collaboration on the first logistical steps toward a harmonized national-level approach for measurable patient benefit.
Acknowledgments
This work was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute of the National Institutes of Health.
The Visual Abstract, Figure 1, and Figure 2 are courtesy of Alan Hoofring and Dr. Laura Dillon. Figure 3 is courtesy of Dr. Rich Little.
Conflict-of-interest disclosure
Christopher S. Hourigan: funding: Sellas, Foundation for the National Institutes of Health Acute Myeloid Leukemia Measurable Residual Disease Biomarkers Consortium.
Off-label drug use
Christopher S. Hourigan: nothing to disclose.