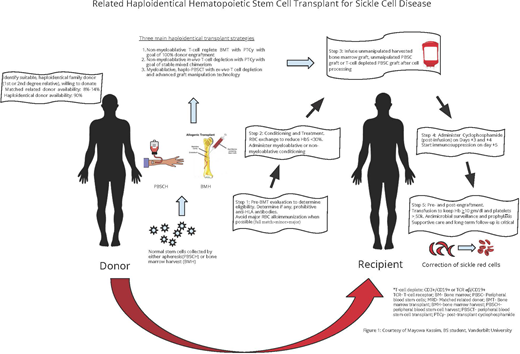
Abstract
The ideal curative therapy for sickle cell disease (SCD) must be applicable across all ages and include individuals with strokes and preexisting heart, lung, and kidney disease. Myeloablative, matched sibling donor hematopoietic stem cell transplant (HCT) for children with SCD has shown excellent outcomes over the past 3 decades but has been restricted due to the limited availability of a human leukocyte antigen–matched sibling donor (10%-15%) and increased treatment-related death in adults with myeloablative conditioning. To overcome these 2 significant barriers to curative therapy in SCD, related haploidentical HCT has become an active area of research. The use of related haploidentical donors (first- and second-degree relatives) increases the donor pool to at least 90% of those eligible across the life span. Importantly, most adults, even with strokes or significant comorbidities, can tolerate the nonmyeloablative conditioning regimen without treatment-related death. Since 2013, at least 3 related haploidentical HCT strategies have emerged as potential curative therapies for SCD: (1) a nonmyeloablative, T-cell replete, bone marrow transplant with thiotepa and posttransplant cyclophosphamide with a goal of complete donor chimerism; (2) a nonmyeloablative, in vivo T-cell depletion, using peripheral blood stem cells (PBSCs) with a goal of stable mixed donor-recipient chimerism; and (3) a myeloablative, ex vivo T-cell depletion using PBSCs and advanced-technology graft manipulation, with a goal of complete donor chimerism. We review the similarities, differences, outcomes, and gaps in knowledge with these 3 haploidentical HCT approaches for SCD.
Learning Objectives
Understand the evolution of current haploidentical stem cell transplant as a curative modality for sickle cell disease
Determine the indication for haploidentical stem cell transplant for sickle cell disease
Distinguish the different types of haploidentical stem cell transplants and evaluate their outcomes for sickle cell disease
Describe pros and cons of the 2 prominent haploidentical stem cell transplant platforms to cure individuals with sickle cell disease
Introduction
Sickle cell disease (SCD) is no longer associated with early mortality for children living in high-income countries, with more than 98% living to 18 years of age.1,2 Despite the dramatically increased survival in children with sickle cell anemia (SCA), overt strokes and silent cerebral infarcts remain the most common cause of progressive neurologic injury, even after starting regular blood transfusion therapy with a goal of keeping the hemoglobin >9.0 g/dL and HbS <30%.3,4
In contrast, adults with SCD have a significant risk of earlier death with no meaningful increase in median survival over 25 years. In a recent national cohort of adults with SCD receiving care based on Medicare and Medicaid claims data (2008-2016) in all 50 states, including 94,616 individuals, life expectancy at birth was only 52.6 years (95% CI, 51.9-53.4).5 Further, a 2-center cohort of adults with SCD receiving medical care at tertiary care centers demonstrated median survival for HbSS/HbSβ0/HbSD and HbSC/HbSβ+, based on the pooled Kaplan-Meier estimates and adjusted for entry age, was 48.0 years (95% CI, 44.4-58.4) and 54.7 years (95% CI, 38.6-62.9), respectively.6
Major causes of death in adults with SCD include progressive heart, lung, and kidney disease.7 Pulmonary hypertension occurs in 20% to 40% of adults with SCD, with a 10-fold increase in the risk of premature mortality.8 In a prospective cohort study of adults with SCA followed for a median of 5.5 years, low forced expiratory volume at 1 second was associated with earlier death.9 Among adults with SCD, end-stage renal disease occurs in 4.2% with SCA and 2.4% with HbSC disease.10 However, when present, end-stage renal disease when compared to a reference population is associated with higher mortality risk (hazard ratio, 1.66; 95% CI, 1.36-2.03) and higher hospitalization rates (incidence rate ratio, 2.12; 95% CI: 1.88-2.38), and it is associated with a 26% death risk within 1 year.11,12 Unfortunately, no US Food and Drug Administration–approved therapy for adults with SCD has been developed to attenuate the progression of heart, lung, and kidney disease in this medically fragile population.
Related haploidentical, hematopoietic stem cell transplant (HCT), initially developed in adults with cancer,13,14 has been adapted for SCD to overcome the barrier of a limited donor pool with matched sibling donor HCT for SCD. Earlier haploidentical approaches for SCD that used related donors were associated with high graft failure rates of greater than 30% and unacceptably high death rates.15,16 These SCD haploidentical transplant strategies are evolving and have increased the curative therapy option to at least 90% of those eligible across the life span because of the corresponding increase in the donor pool.
Unfortunately, pooled analyses of haploidentical transplant outcomes in individuals with SCD have provided an outdated perception that this strategy is an unfavorable approach for curative therapy in SCD.17 This pooled analysis included only 137 participants with SCD who underwent haploidentical-related donor transplantation between 2008 and 2017. During this period, most recipients received different conditioning intensities, graft sources, graft-versus-host disease (GvHD) prophylaxis, and supportive care regimens. While more haploidentical transplants were included in the pooled analysis from 2013 to 2017, 24% (33/137) were done from 2008 to 2012, when haploidentical transplant expertise was limited, and optimal supportive care measures were emerging and not standard care. Also, few participants were enrolled in multicenter trials with rigorous stopping rules and a data safety monitoring board. Importantly, the pooled analysis included early results of the related haploidentical bone marrow transplant (BMT) approaches with posttransplant cyclophosphamide (PTCy) at a stage when the approach was evolving,16 nor did the results include the more recent alternate haploidentical transplant strategy of ex vivo T-cell depletion with CD34+ selection, CD3+/CD19+, or T-cell receptor (TCR) αβ+/CD19+ depletion.18-20 Taken together, these limitations attenuate any reasonable inference about haploidentical trial efficacy in curing SCD in the current era.
We review the similarities, differences, outcomes, and gaps in knowledge of recent haploidentical transplant approaches, namely, (1) T-cell replete haploidentical BMT with PTCy, with a goal of complete donor chimerism; (2) in vivo T-cell deplete haploidentical peripheral blood stem cell (PBSC) HCT approach with a goal of stable mixed donor-recipient chimerism; and (3) ex vivo T-cell deplete haploidentical HCT regimens with advanced-technology graft manipulation for SCD with a goal of complete donor chimerism.
CLINICAL CASE
A 34-year-old African American man with homozygous SCD (HbSS) presents with a history of overt stroke at 5 years of age and moyamoya vasculopathy. He was initially on regular blood transfusion therapy, but this was replaced with hydroxyurea therapy because of excessive iron and poor adherence to iron chelation. During hospitalization, he was found on the floor, combative and disoriented with seizure-like activity. The initial head computed tomography scan showed no acute abnormality. Follow-up magnetic resonance imaging of the brain 4 days later showed new acute infarcts in the left parietal, bilateral temporal, and occipital lobes. He was started on regular blood transfusion therapy with a goal HbS% of 15% and a target hematocrit of 28%. He is interested in curative treatment options but does not have a matched sibling or unrelated donor; he had a half-sibling who was a 6/10 haploidentical match.
Preclinical models of cyclophosphamide in HCT
Santos and Owens discovered in the 1960s the immunosuppressive properties of cyclophosphamide.21 Subsequently, the team developed cyclophosphamide to replace total body irradiation in allogeneic HCT conditioning due to its beneficial effect on GvHD modulation.22 Further studies on the immunobiology of cyclophosphamide showed that hematopoietic and other tissue stem cells, including memory T cells, express high levels of aldehyde dehydrogenase 1, the body's primary means of inactivating cyclophosphamide, conferring resistance to cyclophosphamide. In contrast, maturing lymphocytes generally express low levels.23 Aldehyde dehydrogenase 1 inactivates cyclophosphamide by oxidizing the active metabolic aldehyde intermediate aldophosphamide to the inactive carboxylic acid carboxyphosphamide (Figure 1). More recently, investigators using animal models have tried to evaluate the immunologic underpinnings of PTCy and its effects on alloreactive conventional and regulatory T cells.24-26
High-dose cyclophosphamide given early after transplant effectively prevents alloreactivity (GvHD and graft rejection) and to spare stem cells, allowing successful mismatched donor transplant. Hematopoietic and other tissue stem cells, including memory T cells, express high levels of aldehyde dehydrogenase 1 (ALDH1), the body's primary means of inactivating cyclophosphamide, whereas mature lymphocytes generally express low levels. Figure reproduced by Andrea Sikora, PharmD with permission; modification from Expert Rev Hematol. 2019;12(9):733-752.
High-dose cyclophosphamide given early after transplant effectively prevents alloreactivity (GvHD and graft rejection) and to spare stem cells, allowing successful mismatched donor transplant. Hematopoietic and other tissue stem cells, including memory T cells, express high levels of aldehyde dehydrogenase 1 (ALDH1), the body's primary means of inactivating cyclophosphamide, whereas mature lymphocytes generally express low levels. Figure reproduced by Andrea Sikora, PharmD with permission; modification from Expert Rev Hematol. 2019;12(9):733-752.
Current haploidentical HCT approaches for SCD
The preclinical studies with PTCy led to phase 1 and 2 haploidentical clinical trials for adults with cancer with the addition of tacrolimus and mycophenolate mofetil for GvHD prophylaxis.27-29 The beneficial effects of PTCy on GvHD appear to be independent of donor type, graft source, or conditioning regimen intensity,30 with similar outcomes between non-first- degree and first-degree relatives, increasing the donor pool to an average of 2.7 donors per patient31 and approximately >90% donor availability for children and adults with SCD. Over the past decade, improvements in transplant technology designed to overcome the human leukocyte antigen barrier have resulted in an alternative to T-cell–replete haploidentical transplant, namely, T-cell–deplete haploidentical HCT using PBSC grafts. This strategy uses advanced biomedical technology for graft manipulations for ex vivo T-cell depletion of GvHD mediating T cells (CD3+/CD19+ or TCRαβ/CD19+).18-20 The goal of therapy is complete donor chimerism.
T-cell replete haploidentical transplant approaches for SCD
Investigators at Johns Hopkins published an initial experience using a nonmyeloablative haploidentical BMT with PTCy in adults with severe SCD.16 The initial cohort included 14 participants with a median age of 23.5 years, receiving a conditioning regimen with antithymocyte globulin, fludarabine, cyclophosphamide, and total body irradiation (TBI), using unmanipulated bone marrow grafts from related haploidentical donors, and mycophenolate mofetil, sirolimus, and PTCy for GvHD prophylaxis. At a median follow-up of 711 days, only 57% (8/14) of the recipients successfully engrafted, with a graft failure rate of 43% (6/14) and no deaths. All participants with graft failure had autologous reconstitution. Among engrafted participants, 14% (2/14) had mixed donor-recipient chimerism at the study conclusion with no incidence of acute or chronic GvHD and 100% survival. All engrafted participants had amelioration of SCD-related symptoms, which provided the momentum for further investigating this platform for SCD. To reduce the graft rejection rate, investigators at Johns Hopkins added granulocyte colony-stimulating factor bone marrow priming to improve T-cell content in the graft because T cells are essential for engraftment. However, T cells may also drive the pathogenesis of acute and chronic GvHD, resulting potentially in a net negative effect and no impact on engraftment. The Johns Hopkins team also avoided prohibitive donor-specific anti- human leukocyte antigen antibodies associated with high graft rejection rates in haploidentical HCT recipients.32 To improve donor engraftment in participants, the Johns Hopkins team increased TBI from 200 cGy to 400 cGy in their related haploidentical BMT platform.33 Among 17 participants with severe hemoglobinopathies transplanted with the modified protocol, full-donor myeloid engraftment was observed in 76% (13/17), 18% (3/17) had mixed donor-recipient chimerism, and 6% (1/17) had primary graft failure.
In 2013, the Vanderbilt international, multi-institutional learning collaborative with participants in both middle- and high- income countries sought to improve donor engraftment using the Johns Hopkins platform in a phase 2 trial of nonmyeloablative haploidentical BMT with PTCy for participants with SCD.34 In the initial 3 participants with SCD, 2 participants experienced graft rejection. The investigators elected to add thiotepa to the conditioning regimen at 10 mg/kg (Figure 2). A near-final update of the trial results with thiotepa added was presented at the American Society of Hematology meeting in December 2022.35 Among 80 evaluable participants who underwent haploidentical BMT with thiotepa and PTCy, the median age was 17.6 years, 97.5% had HbSS/HbSβ0, and graft failure occurred in 12.5% (10/80) of the participants (4 primary and 6 secondary). Unexpectantly, all graft failures occurred in participants <18 years of age (P = .001), and all had autologous reconstitution. The Kaplan-Meier–based analysis for survival found no difference by age group <18 years or >18 years (P = .760). Kaplan-Meier–based overall survival probability was 96.7% (95% CI, 87.1%-99.2%) at 1 year and 94.3% (95% CI, 83.0%-98.2%) at 2 years. All engrafted participants had median whole-blood donor-recipient chimerism values at D + 180 and D + 365 posttransplant of 100.0%, respectively, and 97.7% (43/44) were off immunosuppression therapy at 1-year posttransplant. The prevalence of grades 3 to 4 acute and moderate to severe chronic GvHD was 8.8% (7/80) each. Mortality was 5.0% (4/80), attributable primarily to viral infections. The trial closed, and the final results are expected to be reported before the end of 2023.
Conditioning schema for haploidentical BMT with thiotepa and PTCy used in the Vanderbilt Global Haploidentical Transplant Consortium.
Conditioning schema for haploidentical BMT with thiotepa and PTCy used in the Vanderbilt Global Haploidentical Transplant Consortium.
In 2009, investigators at the National Institutes of Health designed a prospective phase 1/2 study, a non-chemotherapy-based, nonmyeloablative haploidentical protocol with PTCy, for participants with severe SCD, with the goal of stable mixed donor-recipient chimerism. The stem cell source was granulocyte colony-stimulating factor–mobilized PBSC; conditioning included low-dose TBI and in vivo T-cell depletion with alemtuzumab.36 The trial was designed with 3 dosing cohorts for PTCy. Patients in cohort 1 (n = 3) did not receive PTCy, patients in cohort 2 received a single dose of 50 mg/kg (n = 8), and those in cohort 3 (n = 12) received 100 mg/kg in divided doses. The engraftment rate was 33% (1/3), 63% (5/8), and 83% (10/12) in cohorts 1, 2, and 3, respectively; 0% of patients in cohort 1 remained free of SCD, while 25% of patients in cohort 2 remained free of SCD compared to 50% in cohort 3, reinforcing the importance of the higher doses of PTCy. Three patients died, all of whom had graft rejection; 86% survived; 2 had grade 1 acute GvHD, and 1 had limited ocular GvHD, which resolved with systemic and topical steroids, respectively. However, 50% (6/12) of the participants in cohort 3 had graft rejection, an undesirable outcome. As anticipated, no participant achieved complete donor chimerism based on the primary trial's goal of stable mixed donor-recipient chimerism. All engrafted participants continued immunosuppression. The trial was closed early due to stopping rules related to graft rejection. Subsequently, 2 participants developed myeloid neoplasm after graft rejection, raising the investigator's concern about the association between stable mixed donor-recipient chimerism, graft failure, and an increased incidence rate of acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS). Recent evidence indicates AML/MDS rarely occurs after myeloablative matched-related donor transplants, where the goal is complete donor chimerism.37,38 To replace the prior protocol with the goal of mixed donor-recipient chimerism, National Institutes of Health investigators have opened a new single-center haploidentical protocol with a goal of complete donor chimerism (NCT03077542).
Other investigators have attempted to improve the engraftment rate for SCD using the Johns Hopkins–based haploidentical BMT platform with PTCy using different conditioning regimens and stem cell sources with mixed results.39-41 Most trials are small, single-institution studies that do not provide sufficient evidence to be considered standard therapy or replicated as a therapeutic option in a clinical trial. The absence of a SCD-specific BMT consortium that allows multiple phase 1, 2, and 3 trials to be open is a significant barrier to hastening curative therapy options for SCD. Table 1 reviews transplant outcomes from published studies using haploidentical HCT with PTCy for SCD.
Transplant outcomes from published studies using haploidentical HCT with PTCy for SCD
| Characteristic . | Bolanos-Meade et al16 . | De la Fuente et al33 . | Fitzhugh et al35 . | Saraf et al39 . | Pawlowska et al41 . |
|---|---|---|---|---|---|
| Protocol | Phase 1/2, single center | Phase 2, multicenter | Phase 1/2, single center | Phase 2 single center | Phase 2 single center |
| Goal | Full donor chimerism | Full donor chimerism | Stable mixed donor-recipient chimerism | Full donor chimerism | Full donor chimerism (PTIS-HCT approach) |
| Stem cell source | Bone marrow* | Bone marrow* | Peripheral blood | Peripheral blood | Bone marrow (3) Peripheral blood (1) |
| Donor type | Haploidentical | Haploidentical | Haploidentical | Haploidentical | Haploidentical |
| Donor availability† | 100% | >90% | 100% | 90% | 100% |
| Number of patients | 14 | 18 | 23 (21-SCD) | 8 | 4 |
| Age, median (range), y | 30 (15-46) | 20.9 (12.1-26) | 36 (20-56) | 29 (20-38) | 18.5 (13 to 23) |
| Type of conditioning | Nonmyeloablative | Nonmyeloablative | Nonmyeloablative | Nonmyeloablative | Reduced toxicity |
| Conditioning | ATG, fludarabine, cyclophosphamide, TBI (200 cGy) | ATG, fludarabine, cyclophosphamide, thiotepa, TBI (200 cGy) | Alemtuzumab, TBI (400 cGy) | ATG, fludarabine, cyclophosphamide, TBI (300 cGy) | PTIS: fludarabine and dexamethasone × 2 courses; then ATG, busulfan, fludarabine |
| GvHD prophylaxis | PTCy, tacrolimus, sirolimus, MMF | PTCy, sirolimus, MMF | PTCy,‡ sirolimus | PTCy, sirolimus, MMF | PTCy, tacrolimus, MMF |
| Median follow-up, mo | 23.4 (minimal 6.9) | 13.3 (IQR, 3.8-23.1) | 38 (range, 8-74) | 17 (range, 12-30) | 5-11 |
| Engraftment rate | 57% (8/14) | 83% (15/18) | Cohort 1: 33% (1/3) Cohort 2: 63% (5/8) Cohort 3: 83% (10/12) | ≥95% (7/8) | 100% |
| Mixed chimerism | 25% (2/8) at 6 months | None | 100% (16/16) | 13% (1/8) | None |
| EFS | NA | 93% | NA | 75% (6/8) | NA |
| OS | 100% | 100% | 87% (11/12) | 88% (7/8) | 100% |
| Graft failure | 43% (1 primary; 5 secondary) | 6% (1/15)# | 65% (7 primary; 8 secondary); 50% in Cohort 3 | 12.5% (1/8) | 0% |
| TRM | 0% | 0% | 9.5% (2/21) | 13% (1/8) | 0% |
| Acute GvHD >2 | 0% | 13% (2/16) | 0% | 25% (2/8) | 25% (1/4) |
| Chronic GvHD (moderate-severe) | 0% | 6% (1/16) | 0% | 13% (1/8) | 75% (3/4) |
| Immunosuppression duration (IST) | 14.2% (2/14) still on at publication | 85% (6/7) off at 1 year | Continuing | 3/6 on IST | 1/4 on IST |
| Complications | PRES (3), viral reactivations | PRES (1), viral reactivations, VOD (1) with second transplant | Viral reactivation, CMV colitis, PTLD (1), MDS after graft failure (2) | SAH (2), viral reactivations, | Viral reactivations |
| Protocol status | Completed | Completed | Abandoned | unknown | unknown |
| Characteristic . | Bolanos-Meade et al16 . | De la Fuente et al33 . | Fitzhugh et al35 . | Saraf et al39 . | Pawlowska et al41 . |
|---|---|---|---|---|---|
| Protocol | Phase 1/2, single center | Phase 2, multicenter | Phase 1/2, single center | Phase 2 single center | Phase 2 single center |
| Goal | Full donor chimerism | Full donor chimerism | Stable mixed donor-recipient chimerism | Full donor chimerism | Full donor chimerism (PTIS-HCT approach) |
| Stem cell source | Bone marrow* | Bone marrow* | Peripheral blood | Peripheral blood | Bone marrow (3) Peripheral blood (1) |
| Donor type | Haploidentical | Haploidentical | Haploidentical | Haploidentical | Haploidentical |
| Donor availability† | 100% | >90% | 100% | 90% | 100% |
| Number of patients | 14 | 18 | 23 (21-SCD) | 8 | 4 |
| Age, median (range), y | 30 (15-46) | 20.9 (12.1-26) | 36 (20-56) | 29 (20-38) | 18.5 (13 to 23) |
| Type of conditioning | Nonmyeloablative | Nonmyeloablative | Nonmyeloablative | Nonmyeloablative | Reduced toxicity |
| Conditioning | ATG, fludarabine, cyclophosphamide, TBI (200 cGy) | ATG, fludarabine, cyclophosphamide, thiotepa, TBI (200 cGy) | Alemtuzumab, TBI (400 cGy) | ATG, fludarabine, cyclophosphamide, TBI (300 cGy) | PTIS: fludarabine and dexamethasone × 2 courses; then ATG, busulfan, fludarabine |
| GvHD prophylaxis | PTCy, tacrolimus, sirolimus, MMF | PTCy, sirolimus, MMF | PTCy,‡ sirolimus | PTCy, sirolimus, MMF | PTCy, tacrolimus, MMF |
| Median follow-up, mo | 23.4 (minimal 6.9) | 13.3 (IQR, 3.8-23.1) | 38 (range, 8-74) | 17 (range, 12-30) | 5-11 |
| Engraftment rate | 57% (8/14) | 83% (15/18) | Cohort 1: 33% (1/3) Cohort 2: 63% (5/8) Cohort 3: 83% (10/12) | ≥95% (7/8) | 100% |
| Mixed chimerism | 25% (2/8) at 6 months | None | 100% (16/16) | 13% (1/8) | None |
| EFS | NA | 93% | NA | 75% (6/8) | NA |
| OS | 100% | 100% | 87% (11/12) | 88% (7/8) | 100% |
| Graft failure | 43% (1 primary; 5 secondary) | 6% (1/15)# | 65% (7 primary; 8 secondary); 50% in Cohort 3 | 12.5% (1/8) | 0% |
| TRM | 0% | 0% | 9.5% (2/21) | 13% (1/8) | 0% |
| Acute GvHD >2 | 0% | 13% (2/16) | 0% | 25% (2/8) | 25% (1/4) |
| Chronic GvHD (moderate-severe) | 0% | 6% (1/16) | 0% | 13% (1/8) | 75% (3/4) |
| Immunosuppression duration (IST) | 14.2% (2/14) still on at publication | 85% (6/7) off at 1 year | Continuing | 3/6 on IST | 1/4 on IST |
| Complications | PRES (3), viral reactivations | PRES (1), viral reactivations, VOD (1) with second transplant | Viral reactivation, CMV colitis, PTLD (1), MDS after graft failure (2) | SAH (2), viral reactivations, | Viral reactivations |
| Protocol status | Completed | Completed | Abandoned | unknown | unknown |
ATG, antithymocyte globulin; CMV, cytomegalovirus; IQR, interquartile range; IST, immunosuppression therapy; MMF, mycophenolate mofetil; NA, not available; PRES, posterior reversible encephalopathy/neurological complications (means peripheral neuropathy, neuralgia); PTIS, pretransplant immunosuppressive therapy; PTLD, posttransplant lymphoproliferative disorder; SAH, sub-arachnoid hemorrhage; TRM, transplant-related mortality; VOD, veno-occlusive disease.
Three patients received granulocyte colony-stimulating factor–primed bone marrow.
Patient who had IST stopped due to severe reaction and PRES.
Escalating doses of PTCy: 0 mg/kg in cohort 1, 50 mg/kg in cohort 2, and 100 mg/kg in cohort 3.
T-cell–deplete haploidentical HCT approaches for SCD
Outcomes of initial attempts at graft manipulation before related haploidentical HCT for SCD were associated with high graft failure rate and significant GvHD.13-15 Foell and colleagues19 in Germany are conducting a phase 2 trial to assess α/β T-cell depleted haploidentical HCT in children and adults with SCD who lack sibling donors and have failed at least 1 year of hydroxyurea therapy (NCT04201210). In their pilot trial, investigators used a treosulfan (L-treitol-1,4-bis-methanesulfonate)–based myeloablative conditioning regimen with CD3+/CD19+ or αβ/CD19+-depleted PBSC grafts and tacrolimus and mycophenolate mofetil for GvHD prophylaxis. A total of 17 participants were initially recruited, and the median age was 13 years. After a median follow-up of 22 months, event-free survival (EFS) and overall survival (OS) were 88% (22/25) each, transplant-related mortality was 12% (3/25), grade 1 to 2 GvHD was 28% (7/25), and mild to moderate GvHD was 16% (4/25). The main complications were early viral reactivation (52%), pain (72%), posterior reversible encephalopathy (20%), and 1 case each of veno-occlusive disease of the liver and tumor-associated macrophages/macrophage activation syndrome. An update, including trial data, was shared at the 49th annual European Society for Blood and Marrow Transplantation (EBMT) meeting in 2023 Paris, France. Of 37 participants with SCD enrolled, all were engrafted with OS 89% and EFS 85%, but 11% of participants maintained mixed donor-recipient chimerism. The incidence of grade 1 to 2 GvHD was 32% (12/37), and mild to moderate GvHD was 14% (5/37), all resolved 18 months posttransplant. The intensity of this conditioning may be too toxic for patients with SCD and significant organ dysfunction.
In August 2009, Gilman and colleagues42 started a single- institution phase 2 study in children and young adults with severe SCD using a reduced-intensity conditioning regimen with CD34+-selected, T-cell–depleted PBSC grafts, evaluating engraftment and GvHD. The median age was 14 years (range, 5-23), and 8 patients underwent haploidentical HCT, all engrafted, with an OS and EFS of 90% and 80%, respectively. The incidence of grade 2 to 4 acute GvHD was 20% and 1 chronic GvHD in a patient who received a donor lymphocyte infusion for a refractory posttransplant lymphoproliferative disorder.
In November 2017, Gaziev and colleagues20 published a single-center retrospective study using myeloablative conditioning (busulfan, thiotepa, cyclophosphamide, and antithymocyte globulin preceded by fludarabine, hydroxyurea, and azathioprine), followed by TCRαβ+/CD19+-depleted grafts in 14 children with hemoglobinopathies. The median age was 7 years (range, 3-15.2), 3 had SCD, and 11 had thalassemia. The investigators showed improved outcomes in this cohort compared to 40 patients with hemoglobinopathies and similar baseline characteristics who received CD34+-selected PBSC and bone marrow grafts. Table 2 reviews outcomes from published studies using T-cell–deplete haploidentical HCT approaches for SCD. These small single-center clinical trials, myeloablative conditioning platform, and requirements for ex vivo stem cell manipulations with complex technical equipment limit this approach for widespread use in middle- and high-income countries and are inaccessible for most adults with heart, lung, and kidney disease.18-20
Transplant outcomes from competing strategies using T-cell deplete haploidentical HCT for SCD
| Characteristic . | Foell et al19 . | Gilman et al42 . | Gaziev et al20 . | Cairo et al18 . |
|---|---|---|---|---|
| Protocol | Phase 2 trial (single center) | Phase 2 trial (single center) | Phase 2 trial (single center) | Phase 2 trial (multicenter) |
| Goal | Full-donor chimerism | Full-donor chimerism | Full-donor chimerism | Full-donor chimerism |
| Stem cell source | Peripheral blood | Peripheral blood | Peripheral blood | Peripheral blood |
| Donor type | Haploidentical | Haploidentical | Haploidentical | Haploidentical |
| Donor availability | 100% | 100% | 100% | 100% |
| Number of patients | 25 | 10 | 14 (3 SCD; 11 TDT) | 19 |
| Graft manipulation (T-cell depletion strategies) | CD3/CD19 T-cell depletion (19) or TCRαβ+/CD19+ depletion (6) | CD34+ cell-selected, T-cell–depleted (8 Haplo; 2 MUD) | TCR αβ+/CD19+– depleted grafts | CD34+ enrichment and mononuclear cell add-back |
| Age, median (range), mo | 13 (3-31) | 49 (14-60) | 47 (6-62) | 46 (1.9-76) |
| Type of conditioning | Myeloablative | Reduced intensity | Myeloablative | Myeloimmunoablative |
| Conditioning | ATG, fludarabine, thiotepa, treosulfan | Melphalan, thiotepa, fludarabine, and rabbit ATG + rituximab | PTIS: fludarabine, hydroxyurea, and azathioprine; then busulfan, thiotepa, cyclophosphamide, and ATG | PTIS: hydroxyurea and azathioprine; then fludarabine, busulfan, thiotepa, cyclophosphamide, total lymphocyte irradiation, and rabbit ATG |
| GvHD prophylaxis | Cyclosporine, MMF | DLI + methotrexate | Cyclosporine and methylprednisolone or MMF | None |
| Follow-up, median (range) | 22 mo | 49 (14-60) mo | 3.9 (0.5-5.2) y | 1409 (59-2330) d |
| Stable engraftment | 84% | 70% | 93% | 97.1% |
| Mixed chimerism | 16% (4/25) | 30%* | None | None |
| EFS | 88% (22/28) | 80% | 69% | 84% |
| OS | 88% (22/25) | 90% | 84% | 84% |
| Graft failure | 0% | 10% | 14% | 0% |
| TRM | 12% (3/25) | 10% (1/10) | 14.2% (2/14) | 15.7% (3/19) |
| Acute GvHD | Grades 1-2: 28% (7/25) Grades 3-4: 0% | Grades 2-4: 20% (2/10) | Grades 2-4: 28% | Grades 2-4: 6.2% |
| Chronic GvHD | Mild/moderate 14% Severe 0% (4/25) | Extensive skin (1/10) | Extensive 21% | Moderate-severe 6.7% |
| Immunosuppression duration | Off by 18 mo in patients with GvHD | Unknown | Unknown | Unknown |
| Complications | PRES (5), seizures (1), VOD (1), TAM/MAS (1). Viral reactivations 13 (52%) | PTLD (3), engraftment syndrome (2), PRES (2), viral reactivations | PTLD (3), DLBCL, viral reactivations (64%), AIHA (3), ITP (1); delayed immune reconstitution | Death from VOD (1), 1 each of acute and chronic GvHD |
| Protocol status | Ongoing | Ongoing | Ongoing | Completed |
| Characteristic . | Foell et al19 . | Gilman et al42 . | Gaziev et al20 . | Cairo et al18 . |
|---|---|---|---|---|
| Protocol | Phase 2 trial (single center) | Phase 2 trial (single center) | Phase 2 trial (single center) | Phase 2 trial (multicenter) |
| Goal | Full-donor chimerism | Full-donor chimerism | Full-donor chimerism | Full-donor chimerism |
| Stem cell source | Peripheral blood | Peripheral blood | Peripheral blood | Peripheral blood |
| Donor type | Haploidentical | Haploidentical | Haploidentical | Haploidentical |
| Donor availability | 100% | 100% | 100% | 100% |
| Number of patients | 25 | 10 | 14 (3 SCD; 11 TDT) | 19 |
| Graft manipulation (T-cell depletion strategies) | CD3/CD19 T-cell depletion (19) or TCRαβ+/CD19+ depletion (6) | CD34+ cell-selected, T-cell–depleted (8 Haplo; 2 MUD) | TCR αβ+/CD19+– depleted grafts | CD34+ enrichment and mononuclear cell add-back |
| Age, median (range), mo | 13 (3-31) | 49 (14-60) | 47 (6-62) | 46 (1.9-76) |
| Type of conditioning | Myeloablative | Reduced intensity | Myeloablative | Myeloimmunoablative |
| Conditioning | ATG, fludarabine, thiotepa, treosulfan | Melphalan, thiotepa, fludarabine, and rabbit ATG + rituximab | PTIS: fludarabine, hydroxyurea, and azathioprine; then busulfan, thiotepa, cyclophosphamide, and ATG | PTIS: hydroxyurea and azathioprine; then fludarabine, busulfan, thiotepa, cyclophosphamide, total lymphocyte irradiation, and rabbit ATG |
| GvHD prophylaxis | Cyclosporine, MMF | DLI + methotrexate | Cyclosporine and methylprednisolone or MMF | None |
| Follow-up, median (range) | 22 mo | 49 (14-60) mo | 3.9 (0.5-5.2) y | 1409 (59-2330) d |
| Stable engraftment | 84% | 70% | 93% | 97.1% |
| Mixed chimerism | 16% (4/25) | 30%* | None | None |
| EFS | 88% (22/28) | 80% | 69% | 84% |
| OS | 88% (22/25) | 90% | 84% | 84% |
| Graft failure | 0% | 10% | 14% | 0% |
| TRM | 12% (3/25) | 10% (1/10) | 14.2% (2/14) | 15.7% (3/19) |
| Acute GvHD | Grades 1-2: 28% (7/25) Grades 3-4: 0% | Grades 2-4: 20% (2/10) | Grades 2-4: 28% | Grades 2-4: 6.2% |
| Chronic GvHD | Mild/moderate 14% Severe 0% (4/25) | Extensive skin (1/10) | Extensive 21% | Moderate-severe 6.7% |
| Immunosuppression duration | Off by 18 mo in patients with GvHD | Unknown | Unknown | Unknown |
| Complications | PRES (5), seizures (1), VOD (1), TAM/MAS (1). Viral reactivations 13 (52%) | PTLD (3), engraftment syndrome (2), PRES (2), viral reactivations | PTLD (3), DLBCL, viral reactivations (64%), AIHA (3), ITP (1); delayed immune reconstitution | Death from VOD (1), 1 each of acute and chronic GvHD |
| Protocol status | Ongoing | Ongoing | Ongoing | Completed |
Viral reactivation indicates patients with reactivation of cytomegalovirus, herpes virus, Epstein-Barr virus, rotavirus, or polyomavirus.
AIHA, auto-immune hemolytic anemia; DLBCL, diffuse large B-cell lymphoma; DLI, donor lymphocyte infusion; ITP, immune thrombocytopenia; MAS, macrophage activation syndrome; MUD, matched unrelated donor; TAM, tumor-associated macrophages; TDT, transfusion dependent thalassemia.
Received prophylactic DLI or second transplant.
CLINICAL CASE (continued)
The participant underwent a haploidentical BMT with thiotepa and PTCy from his half-sibling. His transplant was complicated by pure red cell aplasia that resolved after treatment with daratumumab. He remains 100% engrafted 2 years posttransplant, is on antiseizure therapy, and has had no recurrent strokes and improved cerebral hemodynamics (Figure 3).
Changes in cerebral blood flow before and after blood transfusions and haplo-BMT with thiotepa and PTCy in a patient with SCD. Quantitative cerebral blood flow (CBF) maps show that CBF increases to maintain sufficient oxygen and glucose supply in people with anemia. The healthy image depicts the brain of an African American woman (aged 32 years, HbAA) with a hemoglobin concentration of 12.6 g/dL and a cortical CBF (assessed by arterial spin labeling magnetic resonance imaging) of 40 to 60 mL per 100 g per min. The SCD images are from an African American man with SCD (aged 34 years, hemoglobin SS); pretransfusion, his CBF was elevated to offset reduced oxygen content. Following blood transfusion, which increased the total hemoglobin and reduced the proportion of hemoglobin S, the CBF decreased but remained elevated relative to that in nonanemic individuals. After haploidentical BMT (haplo-BMT) with thiotepa and PTCy, both anemia and HbS were eliminated, and CBF approached that of a healthy control (HbAA). CBF heterogeneity was still present in this patient due to underlying moyamoya vasculopathy. Hb, hemoglobin concentration. (From Lancet Neurol. 2021 May;20(5):398-408.)
Changes in cerebral blood flow before and after blood transfusions and haplo-BMT with thiotepa and PTCy in a patient with SCD. Quantitative cerebral blood flow (CBF) maps show that CBF increases to maintain sufficient oxygen and glucose supply in people with anemia. The healthy image depicts the brain of an African American woman (aged 32 years, HbAA) with a hemoglobin concentration of 12.6 g/dL and a cortical CBF (assessed by arterial spin labeling magnetic resonance imaging) of 40 to 60 mL per 100 g per min. The SCD images are from an African American man with SCD (aged 34 years, hemoglobin SS); pretransfusion, his CBF was elevated to offset reduced oxygen content. Following blood transfusion, which increased the total hemoglobin and reduced the proportion of hemoglobin S, the CBF decreased but remained elevated relative to that in nonanemic individuals. After haploidentical BMT (haplo-BMT) with thiotepa and PTCy, both anemia and HbS were eliminated, and CBF approached that of a healthy control (HbAA). CBF heterogeneity was still present in this patient due to underlying moyamoya vasculopathy. Hb, hemoglobin concentration. (From Lancet Neurol. 2021 May;20(5):398-408.)
Conclusion
For individuals with SCD, selecting the optimal nonmyeloablative haploidentical conditioning regimen that minimizes toxicity but ensures complete donor engraftment has become increasingly important in the past decade. The recent recognition that haploidentical trials with a goal of stable mixed donor-recipient chimerism are associated with increased risk of AML/MDS has resulted in at least 1 trial closure and careful consideration for future trials with a similar therapeutic goal of stable mixed chimerism (Table 3). Among the 3 haploidentical platforms, the most promising approach is the nonmyeloablative BMT with thiotepa and PTCy, an approach proven to be effective across all ages and transferable to almost all transplant programs in middle- and high-income countries.43
Incidence of hematologic malignancies is highest in adults with mixed chimerism following HCT for SCD
| . | NHLBI HLA matched . | NHLBI haploidentical . | Gene therapy . | French group . | CIBMTR . | |||
|---|---|---|---|---|---|---|---|---|
| Conditioning . | Alemtuzumab 300 cGy TBI . | Pentostatin/Cy alemtuzumab 300 cGy TBI . | (Chicago, Riyadh) alemtuzumab 300 cGy TBI . | Alemtuzumab 400 cGy TBI ± PTCy . | Pentostatin/Cy alemtuzumab 400 cGy TBI PTCy . | Busulfan . | Cy ± ATG busulfan . | Cy ± ATG busulfan (mostly) . |
| Number enrolled in the study | 57 | 24 | 64 | 21 | 19 | 44 | 234 | 908 |
| At-risk time (person-years) | 518.7 | 96 | Chicago: 123 Riyadh: 87.5 | 176.4 | 49.4 | 111.9 | 1848.6 | HLA-matched sibling: 1674 |
| Hematologic malignancies/ 100 person-years* | 0.57 | 2.08 | Chicago: 0.8 Riyadh: 0 | 1.70 | 0 | 1.79 | 0.05 | HLA-matched sibling: 0 |
| Median follow-up, y | 9.1 | 4.0 | 4 | 8.4 | 2.6 | 2.5 | 7.9 | 2.1-3.9 |
| . | NHLBI HLA matched . | NHLBI haploidentical . | Gene therapy . | French group . | CIBMTR . | |||
|---|---|---|---|---|---|---|---|---|
| Conditioning . | Alemtuzumab 300 cGy TBI . | Pentostatin/Cy alemtuzumab 300 cGy TBI . | (Chicago, Riyadh) alemtuzumab 300 cGy TBI . | Alemtuzumab 400 cGy TBI ± PTCy . | Pentostatin/Cy alemtuzumab 400 cGy TBI PTCy . | Busulfan . | Cy ± ATG busulfan . | Cy ± ATG busulfan (mostly) . |
| Number enrolled in the study | 57 | 24 | 64 | 21 | 19 | 44 | 234 | 908 |
| At-risk time (person-years) | 518.7 | 96 | Chicago: 123 Riyadh: 87.5 | 176.4 | 49.4 | 111.9 | 1848.6 | HLA-matched sibling: 1674 |
| Hematologic malignancies/ 100 person-years* | 0.57 | 2.08 | Chicago: 0.8 Riyadh: 0 | 1.70 | 0 | 1.79 | 0.05 | HLA-matched sibling: 0 |
| Median follow-up, y | 9.1 | 4.0 | 4 | 8.4 | 2.6 | 2.5 | 7.9 | 2.1-3.9 |
Bolded values represent: Incidence of hematologic malignancies/100 person-years.
Personal communication from C. Fitzhugh and courtesy of C. Fitzhugh CD (Blood. 2022;140(23):2514-2518).
CIBMTR, Center for International Blood and Marrow Transplant Research; Cy, cyclophosphamide; HLA, human leukocyte antigen; NHLBI, National Heart, Lung, and Blood Institute.
Pros and cons of the 2 promising different haploidentical HCT approaches
| . | Haploidentical nonmyeloablative, T-cell–replete, bone marrow transplant with thiotepa and PTCy18,33,34 . | Haploidentical myeloablative, ex vivo T-cell–deplete PBSC transplant using advanced-technology graft manipulation19-21,42 . |
|---|---|---|
| Pros | • Nonmyeloablative • >90% donor availability • Most adults can tolerate the conditioning regimen • Good rates of engraftment • Multicenter trial completed in middle- to high-income settings • Event-free survival: >90% in adults • Overall 2-year survival: >90% • Low rates of acute and chronic GvHD • Typically, discontinuation of immunosuppressive therapy in 1 year • Protocols easily replicable at different institutions and relatively inexpensive | • >90% donor availability • Event-free survival: >80% • Overall survival: 80%-90% • Low rates of acute and chronic GvHD • Prevents EBV-PTLD by removing CD19+ cells ex vivo • Reduced need for posttransplant immune-suppressive medications |
| Cons | • Late health effects are not well established • Graft rejection (~12.5% in <18 years) • Increased risk of viral reactivations • May be prohibitive to patients with significant chronic kidney disease stage 4 or 5 • Limited insurance coverage, donor eligibility, and a high rate of DSAs may limit access • Late health effects on fertility are not well described, expected, or studied systematically | • Limited to single-center experiences • Expensive and labor-intensive • Variability in quality of cells depending on the source • Limited follow-up • Late health effects are not well established • May be prohibitive to patients with significant chronic kidney disease stage 4 or 5 • Specialized expertise required • Delayed immune reconstitution • Increased risk of viral reactivations • Graft rejection (0%-14%) • Fertility in women is likely to be low, not studied systematically |
| . | Haploidentical nonmyeloablative, T-cell–replete, bone marrow transplant with thiotepa and PTCy18,33,34 . | Haploidentical myeloablative, ex vivo T-cell–deplete PBSC transplant using advanced-technology graft manipulation19-21,42 . |
|---|---|---|
| Pros | • Nonmyeloablative • >90% donor availability • Most adults can tolerate the conditioning regimen • Good rates of engraftment • Multicenter trial completed in middle- to high-income settings • Event-free survival: >90% in adults • Overall 2-year survival: >90% • Low rates of acute and chronic GvHD • Typically, discontinuation of immunosuppressive therapy in 1 year • Protocols easily replicable at different institutions and relatively inexpensive | • >90% donor availability • Event-free survival: >80% • Overall survival: 80%-90% • Low rates of acute and chronic GvHD • Prevents EBV-PTLD by removing CD19+ cells ex vivo • Reduced need for posttransplant immune-suppressive medications |
| Cons | • Late health effects are not well established • Graft rejection (~12.5% in <18 years) • Increased risk of viral reactivations • May be prohibitive to patients with significant chronic kidney disease stage 4 or 5 • Limited insurance coverage, donor eligibility, and a high rate of DSAs may limit access • Late health effects on fertility are not well described, expected, or studied systematically | • Limited to single-center experiences • Expensive and labor-intensive • Variability in quality of cells depending on the source • Limited follow-up • Late health effects are not well established • May be prohibitive to patients with significant chronic kidney disease stage 4 or 5 • Specialized expertise required • Delayed immune reconstitution • Increased risk of viral reactivations • Graft rejection (0%-14%) • Fertility in women is likely to be low, not studied systematically |
Preexisting comorbidities in the adult SCD population must be considered before haploidentical HCT, although only individuals with kidney failure are generally excluded. Further, the late health effects of all curative therapy results should be collected prospectively for better-informed decision-making for the full range of options. We predict the optimal curative therapy option among myeloablative matched related donor, nonmyeloablative haploidentical BMT with thiotepa and PTCy, myeloablative gene therapy, or myeloablative gene-editing treatment protocols will depend on each curative therapy's distinct late health effects and personal preferences.
Acknowledgment
This research was supported by the Cooperative Study of Late Effects for SCD Curative Therapies (COALESCE, IU01HL156620- 01, NHLBI).
Conflict-of-interest disclosure
Adetola A. Kassim: no competing financial interests to declare.
Michael R. DeBaun and his institution are the sponsors of 2 externally funded research investigator-initiated projects. Global Blood Therapeutics will provide funding for these clinical studies but will not be a cosponsor of either study; he is not receiving any compensation for the conduct of these 2 investigator-initiated observational studies: he is a member of the Global Blood Therapeutics advisory board for a proposed randomized controlled trial, for which he receives compensation; he is on the steering committee for a Novartis-sponsored phase 2 trial to prevent priapism in men; he was a medical adviser for the development of the CTX001 Early Economic Model; he provided medical input on the economic model as part of an expert reference group for Vertex/CRISPR CTX001 Early Economic Model in 2020; and he provided consultation to the Forma Pharmaceutical company about sickle cell disease from 2021 to 2022.
Off-label drug use
Adetola A. Kassim: Nothing to disclose.
Michael R. DeBaun: Nothing to disclose.