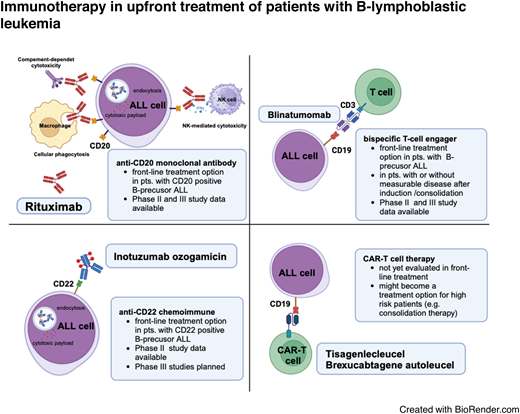
Abstract
Antibody-based and cell-based novel immunotherapies, such as bispecific T-cell engagers (BiTE), antibody-drug conjugates, or chimeric antigen receptor (CAR) T cells are currently standard treatment options for patients with relapsed or refractory (R/R) B-cell precursor acute lymphoblastic leukemia (ALL). To date, CD20-targeting monoclonal antibodies and the CD19-targeting BiTE's blinatumomab have been established elements of frontline therapy, either in patients with CD20+ ALL or in patients with measurable disease (MRD) following conventional chemotherapy. Recently, blinatumomab has also demonstrated a survival benefit in patients with MRD-negative ALL. Based on the observed high response rates and improved survival outcomes in patients with R/R ALL, antibody-based immunotherapies are being prospectively studied in the upfront setting, particularly in older adult patients, where even age-adapted conventional chemotherapies are still associated with significant rates of early death, treatment-related toxicity, and poor prognosis. In these approaches, conventional chemotherapy has been replaced or reduced and supplemented by immunotherapeutic agents, resulting in promising outcomes that form the basis for evaluating and defining new treatment standards.
Learning Objectives
Review antibody-based treatments for patients with B-lymphoblastic leukemia as add-on or replacement in first-line therapy
Understand the clinical implications for patients with or without MRD during frontline induction and consolidation therapy
CLINICAL CASE
A 61-year-old patient with hypertension and sepsis due to a severe perianal abscess, requiring surgical intervention and the use of a protective stoma, is diagnosed with acute lymphoblastic leukemia (ALL) shortly after recovering from surgery. A peripheral blood count shows leukocytes at 1.7/nL, hemoglobin level at 8.2 g/dL, and platelet count at 143/nL, with no circulating lymphoblasts. Bone marrow blasts are at 70%. The immune phenotype of leukemia is as follows: CD34−, TdT+/−, CD19+, CD20+ (84%), CD10+, CD22+ (84%). The karyotype is 46XY, with detection of a biallelic CDKN2A/2B gene deletion by fluorescence in situ hybridization.
What are the treatment options for this patient? Classical chemotherapy-based induction therapy carries a considerable risk of complications given the patient's relevant comorbidities. Age-adapted or dose-reduced chemotherapy induction might minimize toxicities but is less effective. Therefore, exploring innovative new treatment approaches may help to avoid toxicity and improve treatment response.
Introduction
Continuous refinement of polychemotherapy protocols and the introduction of antibody-based targeted therapies, such as anti-CD20 antibody treatment, have contributed to steadily improving outcomes for adult patients with ALL.1,2 Despite the high rates of complete remission (CR) achieved with induction chemotherapy, relapsed or refractory ALL (R/R ALL) remains a major therapeutic challenge and is associated with poor overall survival (OS).3 Dose-adapted pediatric-derived chemotherapy regimens have improved outcomes, but in patients older than 55 years the tolerability of intensified treatment is often limited, particularly in patients with poor performance status or significant comorbidities.4 Currently, the reported 2- and 5-year OS rates in older adult patients with ALL range from 16% to 52% and 5% to 20%, respectively.5,6 Novel treatment strategies are needed not only for this vulnerable patient population but also for young and older adult patients to reduce toxicity, early mortality, and hospitalization and to improve long-term outcomes and quality of life. In B-cell precursor (BCP)-ALL, targeted therapies including the CD19/CD3 bispecific T-cell engager blinatumomab and the CD22-targeting antibody-drug conjugate inotuzumab-ozogamicin (InO) have been successfully introduced as salvage regimens in patients with RR ALL and measurable residual disease (MRD).7-9
CD20 and rituximab
CD20 expression on the surface of all B cells begins in the early phase of B-cell development and increases until maturity. This expression affects cell cycle progression and differentiation via downstream pathways, resulting in the overexpression of antiapoptotic proteins. Consequently, CD20 likely confers drug resistance through these mechanisms.4,10
Although the majority of B cells express the CD20 antigen, it is present on only 30% to 50% of BCP-ALL blasts. In the prerituximab era, CD20 expression of 20% or greater was associated with a higher incidence of relapse and poorer OS.11 This observation prompted the addition of rituximab to frontline chemotherapy regimens. The inclusion of rituximab at 375 mg/m2 for 8 doses over the first 4 courses, during early and late hyper-CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone with high-dose methotrexate and cytarabine) intensifications, and throughout maintenance resulted in superior rates of CR duration and OS compared to standard hyper-CVAD chemotherapy. Of note, older patients with CD20+ ALL did not benefit from rituximab-based chemoimmunotherapy.12
In their randomized trial, the Group for Research on Adult Acute Lymphoblastic Leukemia demonstrated that adding rituximab (16-18 applications at a standard dose of 375 mg/m2) to standard chemotherapy significantly improved event-free survival (EFS) in adults with CD20+ ALL. This benefit was attributed to a reduction in the cumulative incidence of relapse, with no significant increase in toxicity.13
The addition of only 4 doses of rituximab to standard induction chemotherapy in adult patients was evaluated in the randomized UKALL14 trial. Patients aged 25 to 65 years were randomized to receive the standard of care with or without rituximab, regardless of baseline CD20 expression or BCR-ABL1 status. This study failed to demonstrate a benefit for the addition of rituximab. Given that rituximab was administered for only 4 doses during induction, the full benefit of rituximab in ALL most likely requires administration throughout the entire course of therapy.14
With this body of evidence, the addition of rituximab to intensive chemotherapy has become the standard of care for adults with CD20+ BCP-ALL. Of note, in protocols that include L-asparaginase, rituximab treatment has been shown to be associated with fewer hypersensitivity reactions and less development of antiL- asparaginase antibodies.15 In older patients, the evidence for adding rituximab to conventional chemotherapy is less clear, probably due to the increased risk of infection during induction and in remission. Current treatment recommendations—eg, from the German Multicenter Study Group for ALL (GMALL)—include the addition of an anti-CD20 monoclonal antibody to conventional chemotherapy in all patients with CD20+ Philadelphia chromosome (Ph)-negative BCP-ALL, regardless of age.16 Given that CD20 is occasionally upregulated during treatment, the 20% cut-off defining an ALL as CD20+ is somewhat arbitrary and needs to be further evaluated, particularly in the context of novel antibody-based protocols.
CD19 and blinatumomab
CD19, a transmembrane glycoprotein part of the immunoglobulin superfamily, is considered B-cell specific. Its expression begins in pre-B cells and increases with maturation. In BCP-ALL, more than 90% of cases are CD19 positive, with these positive blasts accounting for approximately 80% of all blasts.17
Blinatumomab is a bispecific monoclonal antibody construct targeting CD3/CD19 that recruits CD3+ effector T cells to eradicate CD19+ ALL blasts. It is a fusion protein combining a CD19-targeting single-chain variable fragment (scFv) with a CD3-targeting scFv, linked by a short, nonimmunogenic linker. This allows simultaneous binding of CD19 to leukemia cells and CD3 to T cells. Due to its toxicity profile and pharmacokinetics, blinatumomab must be administered as a continuous infusion for 4 weeks, which can continue on an outpatient basis after 3 to 9 days of initial hospitalization.
Blinatumomab was first evaluated as monotherapy in adult BCP-ALL patients in CR with detectable MRD.18 Despite intensive induction/consolidation chemotherapy, 30% to 50% of adult patients in hematologic CR have MRD. The persistence or recurrence of MRD indicates resistance to standard chemotherapy and is the major risk factor for hematologic relapse. In adult patients, 5-year hematologic relapse rates range from 56% to 100% for MRD positivity, compared to 18% to 33% for MRD negativity.19,20
The results of a phase 2 trial evaluating blinatumomab in patients with BCP-ALL who were MRD positive after conventional chemotherapy were published in 2011. The observed MRD response rate was 80%, and the hematologic relapse-free survival rate was 61%.18 This led to a subsequent study in adults with BCP-ALL in hematologic CR with MRD (≥10−3). Blinatumomab was administered at a dose of 15 µg/m2/d by continuous intravenous infusion for up to 4 cycles. Each cycle consisted of 4 weeks of blinatumomab infusion followed by a 2-week treatment-free period. A complete MRD response was achieved in 78% of treated patients. In the subset of 110 patients with Ph-negative ALL, the estimated 18-month relapse-free survival rate was 54%.9
Blinatumomab was further evaluated in patients with R/R BCP-ALL in the randomized TOWER trial. In this study, the rate of CR or CR with incomplete hematologic recovery (CRi) was significantly higher in patients treated with blinatumomab compared to those who received standard chemotherapy (44% vs 25%).7
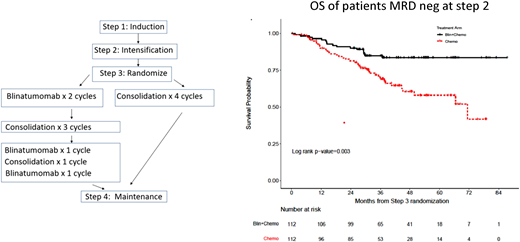
These data formed the basis for the randomized phase III ECOG-ACRIN E1910 study from the National Cooperative Clinical Trials Network (Figure 1). In this study, patients between the ages of 30 and 70 years with newly diagnosed BCP-ALL were treated with conventional induction chemotherapy and, if in CR, with an intensification course of high-dose methotrexate with pegaspargase. All patients were then randomized to receive additional conventional consolidation chemotherapy (4 cycles) or a combination of 4 cycles of blinatumomab at a dose of 28 µg/m2/d for 28 days and 4 cycles of conventional consolidation chemotherapy (same dose of chemotherapy in both arms).21,22
Consolidation therapy with blinatumomab improves OS in newly diagnosed adult patients with B-lineage acute lymphoblastic leukemia in MRD-negative remission (ECOG-ACRIN E1910 trial). Treatment chart and OS of randomized patients becoming MRD-negative after induction treatment. Blin, blinatumomab. Reproduced from Litzow et al21 with permission.
Consolidation therapy with blinatumomab improves OS in newly diagnosed adult patients with B-lineage acute lymphoblastic leukemia in MRD-negative remission (ECOG-ACRIN E1910 trial). Treatment chart and OS of randomized patients becoming MRD-negative after induction treatment. Blin, blinatumomab. Reproduced from Litzow et al21 with permission.
In all, 224 MRD-negative and 62 MRD-positive patients were randomized. Following the approval of blinatumomab for MRD-positive ALL in March 2018, no additional MRD-positive patients were randomized. The addition of blinatumomab to consolidation chemotherapy resulted in a significantly lower relapse rate and better OS (median follow-up, 43 months; median OS, not reached vs 71.4 months). Interestingly, the superior survival was observed only in patients younger than 55 years.
Together with other phase 2 studies in patients with Ph-negative and Ph-positive ALL,23-25 we now have a growing body of evidence indicating that blinatumomab will become a standard treatment option as part of frontline therapy. Despite the lack of formal approval for patients with MRD negativity (as of April 2024), blinatumomab consolidation in MRD-positive and -negative patients is now recommended as a treatment option (eg, within the GMALL treatment recommendation).
For patients aged 55 years and older with newly diagnosed Ph-negative BCP-ALL, blinatumomab alternating with low-intensity chemotherapy is currently being evaluated in a prospective phase 3 trial. The first published results from the run-in phase of this study showed promising outcomes (9 out of 10 patients with MRD-negative CR) and an acceptable safety profile.26
So far, results are available from one study evaluating blinatumomab as frontline induction therapy in older adult patients (aged 65 years and older) with Ph-negative ALL. The study treatment consisted of induction and consolidation therapy with blinatumomab monotherapy, plus intrathecal prophylaxis with methotrexate, followed by POMP maintenance. This strategy resulted in a CR rate of 66% and an estimated 3-year OS rate of 37%. The observed lower CR rate, compared to other novel approaches, may be explained by the lower efficacy of blinatumomab monotherapy in patients with high tumor burden, as seen in R/R ALL.27
CD22 and InO
CD22 is a glycoprotein that inhibits B-cell signaling, expressed on the surface of mature and activated B cells and lost during plasma cell differentiation. Nearly all leukemic cells from patients with BCP-ALL are CD22+, with an average of approximately 70% of blasts being CD22+.17 Rapid antigen internalization following anti-CD22 antibody binding has spurred the development of CD22-targeted antibody-drug conjugates.28
InO consists of a humanized, CD22-targeting immunoglobulin G4 monoclonal antibody linked covalently to calicheamicin, a potent cytotoxic agent.29,30 InO is approved for adults with R/R BCP-ALL based on results from the phase 3 Inotuzumab Ozogamicin Trial to Investigate Tolerability and Efficacy (INO-VATE).8 The approved starting dose of InO is 0.8 mg/m2 on day 1, followed by 0.5 mg/m2 on days 8 and 15 of a 21-day cycle. For patients who achieve a CR/CRi, the dose for subsequent cycles is 0.5 mg/m2 on days 1, 8, and 15 of a 28-day cycle. InO is administered as a 1-hour intravenous infusion and can be given on an outpatient basis. The INO-VATE study demonstrated InO's superior efficacy compared to standard chemotherapy, resulting in higher CR/CRi rates (80.7% vs 29.4%) and higher rates of MRD-negative remission (78.4% vs 28.1%).
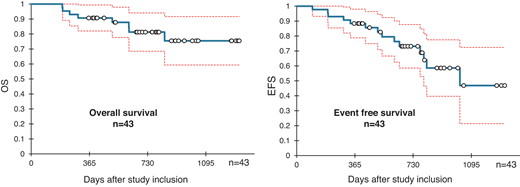
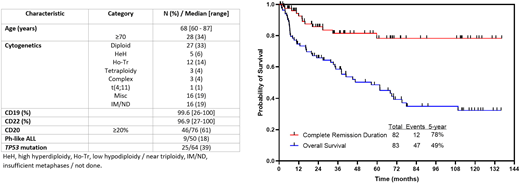
These favorable remission results in patients with R/R ALL led to the prospective evaluation of InO as a frontline treatment. Considering the poor outcomes of older adult patients with ALL treated with conventional chemotherapy, these trials aimed to replace induction chemotherapy with InO, either as monotherapy in combination with dexamethasone or in combination with low-dose chemotherapy and dexamethasone. In the INITIAL-1 study by the GMALL, 45 patients with a median age of 64 years received InO-based induction (up to 3 cycles, dose and schedule similar to the treatment for patients with R/R ALL) followed by age-adapted chemotherapy. After InO induction, all evaluable patients were in CR or CRi, with 73% achieving MRD-negative remission. With a median follow-up of 2.7 years, EFS was 88% at 1 year, decreasing to 55% at 3 years. OS was 91% at 1 year and 73% at 3 years (Figure 2).31
InO induction followed by standard chemotherapy yields high remission rates and promising survival in older (>55 years) patients with de novo B-lymphoblastic leukemia (GMALL-Initial-1 trial). Survival outcome with confidence intervals. Reproduced from Stelljes et al40 with permission.
InO induction followed by standard chemotherapy yields high remission rates and promising survival in older (>55 years) patients with de novo B-lymphoblastic leukemia (GMALL-Initial-1 trial). Survival outcome with confidence intervals. Reproduced from Stelljes et al40 with permission.
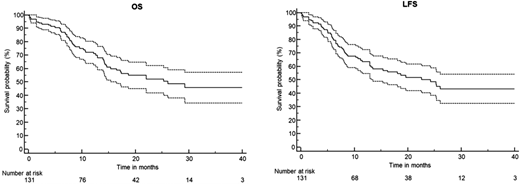
The European Working Group on Adult ALL (EWALL)-InO study included 131 patients with Ph-negative BCP-ALL (median age, 68 years). For induction therapy, patients received fractionated InO at a reduced dose (0.8 mg/m2 on day 1 followed by 0.5 mg/m2 on days 8 and 15 in cycle 1 and 0.5 mg/m2 twice in cycle 2) in combination with low-intensity chemotherapy. Patients who achieved CR/CRi after induction received (the observed CR/CRi rate was 90%) 6 cycles of conventional consolidation and POMP maintenance. At a median follow-up of 15 months, the 1-year OS rate was 73%, and the 2-year OS rate was 54%, while the 1- and 2-year leukemia-free survival rates were 65% and 50%, respectively (Figure 3).32
Fractionated InO combined with low-intensity chemotherapy in older patients with newly diagnosed CD22+ Ph-negative BCP-ALL: Results of the EWALL-INO study. Survival outcomes with confidence intervals. Reproduced from Chevallier et al32 with permission.
Fractionated InO combined with low-intensity chemotherapy in older patients with newly diagnosed CD22+ Ph-negative BCP-ALL: Results of the EWALL-INO study. Survival outcomes with confidence intervals. Reproduced from Chevallier et al32 with permission.
One of the first studies to evaluate InO in frontline therapy was a phase 1/2 study conducted at MD Anderson Cancer Center (MDACC).33 Induction chemotherapy was mini-hyper-CVD with InO administered on day 3 of the first 4 cycles at a dose of 1.3 to 1.8 mg/m2 in cycle 1 followed by 1.0 to 1.3 mg/m2 in subsequent cycles, resulting in a cumulative dose between 4.3 and 5.7 mg/m2. Following a protocol amendment after the first 50 patients, doses of InO were further reduced, and 4 cycles of blinatumomab were added for consolidation. Fifty patients were enrolled in the first part and 31 additional patients after the protocol amendment (median age, 68 years; range, 63-72). All but 1 patient responded and achieved remission (CR/CRi). MRD negativity was seen in 94% of these patients. With a median follow-up of 88 months, the 5-year OS rate was 49% (Figure 4). Notably, 33 patients (40%) died in remission, including 9 deaths due to infectious complications and 9 due to complications related to secondary myeloid malignancies.34
Phase 2 trial of mini–hyper-CVD plus InO, with or without blinatumomab, in older patients with newly diagnosed Ph- negative B-cell acute lymphoblastic leukemia. Baseline patient's characteristic, CR duration, and OS. Reproduced from Jen et al34 with permission.
Phase 2 trial of mini–hyper-CVD plus InO, with or without blinatumomab, in older patients with newly diagnosed Ph- negative B-cell acute lymphoblastic leukemia. Baseline patient's characteristic, CR duration, and OS. Reproduced from Jen et al34 with permission.
In general, the study treatment was well tolerated in all 3 studies. Veno-occlusive disease/sinusoidal occlusive syndrome, as an adverse event of special interest related to InO,35 was documented in 1 patient in the INITIAL-1 study, 1 patient in the EWALL study, and 6 patients in the MDACC study.
As of April 2024, data are available from a study (Alliance A041703) evaluating a chemotherapy-free regimen of InO and blinatumomab in 33 patients (median age, 71 years; range, 60-84) with newly diagnosed, Ph-negative, BCP-ALL.36 The cumulative rate of CR was up to 97%, and after a median follow-up of 22 months, the 1-year EFS and OS were 75% and 84%, respectively.
Summary and outlook
Antibody-based immunotherapies for patients with BCP-ALL have clearly become a part of frontline therapy (Table 1). The available study data suggest that the addition of these therapeutics not only improves patient outcomes but also, when replacing parts of conventional chemotherapies, most likely enhances patient quality of life. Further trials are necessary to define treatment sequences and numbers of treatment cycles and dosing for both younger and older patients and are required for formal approval, which will allow for broader use of these novel treatments.
First-line studies containing blinatumomab and/or inotuzumab for the treatment of Ph-negative BCP-ALL
| Study . | Patients number . | Treatment . | Patient age (range) . | CR rate . | MRD-negative rate . | OS . | EFS/RFS/DFS . |
|---|---|---|---|---|---|---|---|
| ALLG ALL08 | 30 | Alternating cycles of blinatumomab/hyper-CVAD-like regimen (4 cycles) | 52 y (40-67) | 100% | Up to 83% | 69% (2 y) | EFS 62% (2 y) |
| ECOG ACRIN E1910 | 488 | Randomized study: conventional induction chemo followed by chemo or chemo + blinatumomab consolidation therapy | 51 y (30-70) | 81% | NA | Median OS: not reached vs 71.4 | NA |
| EWALL-InO | 131 | Inotuzumab + low-dose chemo induction followed by age-adapted chemotherapy | 68 y (55-84) | 90% | 90% | 54% (2 y) | LFS 50% (2 y) |
| GINEMA LAL2317 | 146 | Sequential chemotherapy (pediatric inspired chemotherapy), 2 cycles of blinatumomab consolidation | 41 y (30-70) | 90% | Up to 96% | 84% (12 mo) | DFS 72% (12 mo) |
| GMALL Bold | 33 | Alternating cycles of age-adapted chemo + blinatumomab as induction therapy | 65 y (56-76) | 76% | NA | 100% (1 y) | DFS 89% (1 y) |
| GRAALL-2014/B | 95 | Blinatumomab during consolidation and maintenance or as bridge to allogeneic SCT | 35 y (18-60) | NA | 74% | 92% (18 mo) | DFS 69% (18 mo) |
| INITIAL-1 | 45 | 3 cycles of inotuzumab induction therapy followed by age-adapted chemotherapy | 64 y (56-80) | 100% | up to 71% | 73% (3 y) | EFS 55% (3 y) |
| MDACC study NCT02877303 | 38 | Hyper-CVAD chemo followed by 4 cycles blinatumomab consolidation | 37 y (17-59) | 100% | up to 95% | 85% (3 y) | RFS 84% (3 y) |
| 20 | Addition of inotuzumab (amendment) | 24 y (18-47) | |||||
| MDACC study NCT01371630 | 50 | Inotuzumab + mini-CVAD | 68 y (60-81) | 98% | up to 94% | 54% (3 y) | PFS 58% (2 y) |
| 30 | With blinatumomab consolidation and maintenance | ||||||
| SWOG 1318 | 29 | Blinatumomab induction and consolidation followed by POMP maintenance | 75 y | 66% | NA | 37% (3 y) | DFS 37% (3 y) |
| Study . | Patients number . | Treatment . | Patient age (range) . | CR rate . | MRD-negative rate . | OS . | EFS/RFS/DFS . |
|---|---|---|---|---|---|---|---|
| ALLG ALL08 | 30 | Alternating cycles of blinatumomab/hyper-CVAD-like regimen (4 cycles) | 52 y (40-67) | 100% | Up to 83% | 69% (2 y) | EFS 62% (2 y) |
| ECOG ACRIN E1910 | 488 | Randomized study: conventional induction chemo followed by chemo or chemo + blinatumomab consolidation therapy | 51 y (30-70) | 81% | NA | Median OS: not reached vs 71.4 | NA |
| EWALL-InO | 131 | Inotuzumab + low-dose chemo induction followed by age-adapted chemotherapy | 68 y (55-84) | 90% | 90% | 54% (2 y) | LFS 50% (2 y) |
| GINEMA LAL2317 | 146 | Sequential chemotherapy (pediatric inspired chemotherapy), 2 cycles of blinatumomab consolidation | 41 y (30-70) | 90% | Up to 96% | 84% (12 mo) | DFS 72% (12 mo) |
| GMALL Bold | 33 | Alternating cycles of age-adapted chemo + blinatumomab as induction therapy | 65 y (56-76) | 76% | NA | 100% (1 y) | DFS 89% (1 y) |
| GRAALL-2014/B | 95 | Blinatumomab during consolidation and maintenance or as bridge to allogeneic SCT | 35 y (18-60) | NA | 74% | 92% (18 mo) | DFS 69% (18 mo) |
| INITIAL-1 | 45 | 3 cycles of inotuzumab induction therapy followed by age-adapted chemotherapy | 64 y (56-80) | 100% | up to 71% | 73% (3 y) | EFS 55% (3 y) |
| MDACC study NCT02877303 | 38 | Hyper-CVAD chemo followed by 4 cycles blinatumomab consolidation | 37 y (17-59) | 100% | up to 95% | 85% (3 y) | RFS 84% (3 y) |
| 20 | Addition of inotuzumab (amendment) | 24 y (18-47) | |||||
| MDACC study NCT01371630 | 50 | Inotuzumab + mini-CVAD | 68 y (60-81) | 98% | up to 94% | 54% (3 y) | PFS 58% (2 y) |
| 30 | With blinatumomab consolidation and maintenance | ||||||
| SWOG 1318 | 29 | Blinatumomab induction and consolidation followed by POMP maintenance | 75 y | 66% | NA | 37% (3 y) | DFS 37% (3 y) |
DFS, disease-free survival; NA, not available; PFS, progression-free survival; RFS, relapse-free survival.
To date, CAR T-cell therapy has become a valuable treatment option for patients with R/R ALL.37 The first studies have started to evaluate CAR T-cell therapy in the frontline setting, especially in high-risk patients as consolidation therapy. Comparative studies are needed to show that this is an alternative to allogeneic stem cell transplantation (SCT), especially in patients who have already received other immunotherapeutics as induction and consolidation therapy.
In view of the high CR rates with less detection of MRD in patients treated with these novel approaches, the classical risk factors defined in the past (eg, based on genetic findings, initial hyperleukocytosis, or delayed response to treatment) need to be redefined, including the indication for allogeneic SCT in CR1. The replacement/reduction of conventional chemotherapies significantly reduces toxicities. As these “old” drugs are important for targeting ALL blasts in the central nervous system, conventional chemotherapeutics might not be fully replaced by antibody-based immunotherapies, which are less able to penetrate the central nervous system.38 In addition, treating patients with B-cell–directed immunotherapies will result in prolonged humoral immunosuppression and an additional risk of infectious complications. Finally, the introduction of novel immunotherapies will significantly increase the financial burden on health care systems,39 which may limit access in low- and middle-income countries.
Acknowledgments
Thanks to Jörn Albring for proofreading the article and to Julian Ronnacker for designing the visual abstract (created with BioRender.com).
Conflict-of-interest disclosure
Matthias Stelljes: consultancy: Pfizer, MSD, Bristol Myers Squibb, Incyte, Takeda, Pierre Fabre, Astellas and Amgen; honoraria: Pfizer, Medac, MSD, Astellas, Jazz Pharmaceuticals, Amgen, Novartis, Kite/Gilead, Pierre Fabre, Celgene, Bristol Myers Squibb, AbbVie, Incyte; research funding: Pfizer; travel support: Medac, Pierre Fabre, Kite/Gilead, Pfizer.
Off-label drug use
Matthias Stelljes: Rituximab, blinatumomab and inotuzumab-ozogamicin are not approved for the front-line therapy setting for patients with BCP-ALL, with the exception of blinatumomab for patients with measurable residual disease.