Key Points
Thrombophilia testing does not affect clinical management in the acute setting after a TEE in children and should be avoided.
Potential harms of thrombophilia testing include unnecessary blood draws in children and an estimated cost of $82 000.
Abstract
Routine testing for inherited and acquired thrombophilia defects is frequently performed in pediatric patients with thromboembolic events (TEEs). No consensus guidelines exist regarding the timing of testing or the type of patients to be tested. The primary objective of our study, therefore, was to determine whether thrombophilia testing during the acute TEE setting affected clinical management in pediatric patients. A secondary aim included estimation of potential harm from thrombophilia testing. We retrospectively reviewed data on all pediatric patients diagnosed with a TEE during a 1-year period. Fifty-two (51%) of 102 patients with a TEE underwent thrombophilia testing during the acute phase, with 26 patients (50%) having a positive test result during the acute phase. Only 12% of patients tested were confirmed to have a thrombophilia eventually, yielding a false-positive rate of ∼7% for testing when performed in the acute setting. There were no changes to the acute management, regardless of a positive or negative result. Testing resulted in unnecessary blood loss in 12 patients younger than 1 year and acute testing cost approximately $82 000. Our data show that thrombophilia testing during acute TEEs in pediatric patients did not impact clinical management. There is also a potential for false-positive tests leading to unnecessary long-term anticoagulation. These findings suggest against thrombophilia testing during acute TEE setting in children.
Introduction
The incidence of pediatric venous thromboembolism (VTE) appears to be rising. Recently, a 70% to 109% increase in the rate of pediatric VTE has been reported.1,2 Greater physician awareness, improved diagnostic modalities, increased interventions such as central venous catheters (CVCs), and greater survival of medically complex children with chronic conditions all likely contribute to the rise in pediatric VTE.3 One aspect of VTE management in children includes testing for thrombophilia, often performed to gain insight into the cause of the VTE. The reported prevalence of thrombophilia in pediatric VTE varies greatly across studies and is largely based on variation in the patient population and the tests performed.4
In 2002, the Perinatal/Pediatric Scientific Sub-Committee of the International Society of Thrombosis and Haemostasis proposed that all pediatric patients with VTE or arterial thrombosis be tested for inherited and acquired thrombophilia in a tiered approach.5 These recommendations do not take into account appropriate timing of testing, how testing might affect clinical management, or additional transient prothrombotic risk factors such as CVCs, oral contraceptives, infection, prolonged immobility, trauma, or recent surgery. In contrast, the 2010 British Committee for Standards in Hematology recommended against indiscriminate inherited thrombophilia testing in unselected patients with a first diagnosis of VTE; in patients with a CVC-related thrombosis, upper limb venous thrombosis, pregnancy morbidities, and retinal vein occlusion; and in patients with arterial thrombosis.6 Moreover, in 2012, the American College of Chest Physicians proposed that the duration and intensity of anticoagulation therapy for pediatric venous or arterial thromboembolic events (TEEs) be independent of whether the patient has an inherited thrombophilia, albeit as a weak recommendation with poor evidence to support it. In addition, they recommended that management of VTE in the setting of antiphospholipid antibodies be similar to general VTE management in children.7
In adults, thrombophilia testing rarely affects acute management.8 However, data are lacking for the pediatric population. There is mounting evidence that thrombophilia testing is overused at many centers.2,9 The American Society of Hematology Choosing Wisely Campaign recommends against thrombophilia testing in adults in the setting of transient major thrombotic risk factors because the risk for harm and/or cost likely outweighs the anticipated benefits.10 Given the lack of consensus on thrombophilia testing in children and the fact that more children are likely to undergo testing as the incidence of VTEs continues to rise, we audited thrombophilia testing at our institution to explore whether thrombophilia testing affected clinical management during the acute setting. In addition, we sought to determine whether thrombophilia testing in the acute setting constituted a potential cause of harm in our cohort.
Patients and methods
Study population
Children’s Medical Center, Dallas, is a quaternary care children’s hospital with a dedicated hematology service responsible for primary or consultative care of patients with TEEs and includes an anticoagulation pharmacist. The anticoagulation pharmacist prospectively maintains data on all patients with a TEE in an electronic database. We conducted a retrospective audit of all consecutive patients diagnosed with a venous or arterial TEE during a 1-year period (1 January to 31 December 2015). The University of Texas Southwestern Medical Center Institutional Review Board approved the study and waived the requirement of informed consent.
Inclusion and exclusion criteria
All subjects diagnosed with a venous or arterial TEE during the specified study period were included. Subjects were excluded if they were diagnosed with a stroke, had a history of a TEE before the study period, or already had an established thrombophilia diagnosis.
Study objectives
The primary study aim was to identify a change in clinical management based on the results of thrombophilia testing obtained during the acute TEE setting. We determined a priori that a change in clinical management would encompass decisions regarding acute management of the TEE (either the choice of anticoagulant and/or the intensity of anticoagulation); duration of anticoagulant therapy; informing decisions about thromboprophylaxis during future high-risk situations such as CVCs, pregnancy, surgery, or prolonged travel; and counseling asymptomatic family members of their potential risk. These outcomes did not have to occur within the acute period but had to have been affected by thrombophilia test results obtained during the acute setting of the TEE. Changes in clinical management were determined by reviewing all hematology notes or notes from the primary team if the hematology service was never consulted. If a patient had a positive thrombophilia test and the medication history showed a change in anticoagulant, intensity of anticoagulation, or duration of anticoagulation therapy that differed from the institutional standards, and lack of proper justification within the clinical notes, then it was presumed this change in clinical management was a result of the positive thrombophilia test. A secondary objective was quantifying potential preventable harm caused by thrombophilia testing during the acute TEE setting in our cohort. We defined this as unnecessary blood loss in patients younger than 1 year to obtain thrombophilia testing, misdiagnosis of a thrombophilia defect because of a false-positive test result (as a result of anticoagulation or consumption of natural anticoagulants) resulting in unwarranted long-term anticoagulation, and costs of thrombophilia testing. Unnecessary blood loss was defined as performing thrombophilia testing in clinical situations where a positive or negative test result would not have influenced clinical management. This was determined on the basis of reviewing daily notes by the ordering or consulting provider. We used a conservative estimate of $100 per thrombophilia marker, as previously described.11
Data extraction
Clinical data extracted from the electronic medical record included age, sex, risk factors for thrombosis (provoked vs unprovoked TEE), thrombosis type (venous vs arterial), thrombophilia test results, indication of testing, timing of testing, anticoagulation at time of testing, clinical service ordering the testing, and confirmation of abnormal test results. Tests included were factor V Leiden (FVL), prothrombin gene mutation, antithrombin (AT) activity, protein C (PC) activity, protein S (PS) activity, lupus anticoagulant (LA), anticardiolipin, anti-β2 glycoprotein I (aβ2GPI), and antiphosphatidylserine. Lipoprotein(a), plasminogen activator inhibitor-1, and homocysteine were rarely ordered at our institution and are not included in the present analysis. Patients who had AT tested in view of heparin resistance were not considered as being tested for thrombophilia.
TEE diagnoses were confirmed objectively by imaging studies. The majority of children with TEEs at our institution are seen by the hematology/thrombosis service at the time of initial TEE diagnosis and at least every 3 to 6 months on an outpatient basis.
Clinical variables
A TEE was considered provoked if it occurred in the setting of a venous or arterial catheter, infection, immobilization longer than 72 hours, recent trauma or surgery within 7 days, oral contraceptives initiated in the preceding 12 months, active malignancy, congenital heart disease, nephrotic syndrome, systemic lupus erythematosus, and inflammatory disorders such as inflammatory bowel disease.12 Patients who had a catheter regardless of additional provoking factors were classified as having a catheter-related TEE, whereas patients who only had noncatheter risk factors were considered to have a non–catheter-related TEE. The acute phase of the TEE was defined as the period within 4 weeks of diagnosis.13 Family history of thrombosis was considered positive when a TEE was noted in siblings or parents younger than 50 years.14
Laboratory variables
FVL and prothrombin gene mutation assays were performed by polymerase chain reaction (PCR). For all plasma-based assays, an abnormal test was regarded as a defect only if the level was outside 2 standard deviations of the mean for age-dependent normative values. The hereditary nature of AT, PC, and PS deficiencies consisted of reproducibility of the abnormality confirmed in a second plasma sample (≥12 weeks after initial testing off of anticoagulation) in the absence of a context suggesting acquired deficiency. LA was positive if either the dilute Russell’s viper venom time ratio or hexagonal phospholipid correction was diagnostic. A positive anticardiolipin was at least 40 M phospholipids or G phospholipids, and aβ2GPI and antiphosphatidylserine were positive if above the 99th percentile. Confirmation of a positive antiphospholipid antibody test required 2 separate positive tests at least 12 weeks apart.
Statistical analysis
Continuous variables were expressed as medians (range; interquartile range), and categorical data as counts and percentages. Descriptive characteristics were used to describe details of the TEEs. Relative risks and 95% confidence intervals (CIs) were calculated using previously published formulas.15 Statistical analyses were performed with Medcalc for Windows, version 17.4.4 (MedCalc Software, Ostend, Belgium).
Results
Demographic characteristics
A total of 105 patients were diagnosed with 114 TEEs during the 1-year study period. Three patients with 5 TEEs were excluded because of a prior history of TEEs. Thus, 102 patients were included in the study (Table 1). The median age at diagnosis was 3 years (4 days to 21 years; interquartile range, 5 months to 14 years). Fifty-two patients were female (51%).
TEE categorization
There were a total of 109 TEEs diagnosed in the 102 patients (Table 2). Seven patients were diagnosed with multiple TEEs during the study period; 5 of these patients were diagnosed with multiple TEEs at the same time at different locations. VTEs comprised 87% (n = 95) of the total TEEs. Ninety-three percent of TEEs were provoked (101/109); 62 were catheter-related, and 39 were non-catheter-related provoked. Only 8 patients experienced at least 1 unprovoked TEE.
Thrombophilia testing
During the study period, 51% of patients (52/102) underwent testing during the acute phase of the TEE. An additional 11% (11/102) of patients underwent testing after resolution of the acute phase; 38% (39/102) of subjects did not have any testing performed either during or after resolution of the acute setting. Included in these 39 patients were 6 patients who only had AT tested in the setting of heparin resistance secondary to cardiac surgery or poor liver synthetic function (none was diagnosed with a congenital AT deficiency). Testing during the acute phase was performed in 24 (42%) of 57 patients, 23 (62%) of 37 patients, and 8 (100%) of 8 patients with a catheter-related TEE, non–catheter-related provoked TEE, and unprovoked TEE, respectively. Of the 52 patients who underwent testing during the acute setting, 26 patients had at least 1 positive test (50%). Twenty-eight (54%) of the 52 patients who underwent testing had plasma-based assays performed while receiving anticoagulation. Of the 25 patients with positive nonmolecular test results (1 patient had only an abnormal FVL result and did not undergo repeat testing), 17 (68%) patients underwent repeat testing to confirm or refute the defect.
Type and number of thrombophilia tests
Of the 327 tests ordered during the acute setting, 43 (13%) were positive (Table 3). The most frequently ordered test was anticardiolipin (44 tests), but only 1 test was positive (2%). Of the 40 positive tests (excluding the 3 positive FVL PCR assays), 25 were repeated (63%); however, only 3 remained positive (aβ2GPI, PC, and LA). Including the positive FVL tests, 6 tests from 6 unique patients were confirmed positive. Thus, only 12% of patients (6/52) were diagnosed with a thrombophilia disorder (patients 1-6; Table 4).
Effect on clinical management
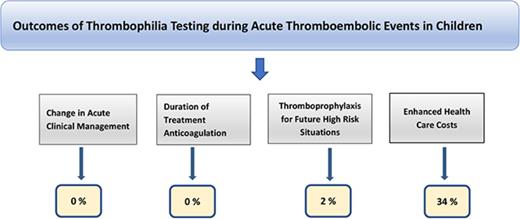
Thrombophilia testing did not affect the acute clinical management in any of the subjects. Acute testing affected duration of anticoagulation in only 2 patients who received extended anticoagulant therapy in view of persistently elevated aβ2GPI (patient #1) and unprovoked TEE in the setting of congenital PS deficiency (patient #3). Thrombophilia testing informed decisions regarding thromboprophylaxis in future high-risk situations in only 1 subject (patient #2). We were not able to ascertain data on counseling of asymptomatic family members from our detailed review.
Potential patient harm
Twelve of 35 patients younger than 1 year (34%), including 1 neonate, had unnecessary blood draws to complete thrombophilia testing; all were classified as having provoked TEEs. None of the patients who had false-positive testing were misdiagnosed with a thrombophilia disorder or received unwarranted anticoagulation because of false-positive test results; however, 38% (15/40) of the initial positive tests were not repeated (Table 3). There was an approximate $82 000 expense because of acute thrombophilia testing.
Discussion
Our retrospective audit of pediatric patients with a TEE found that 51% were tested during the acute phase of the TEE. Although 50% of these patients tested positive during the acute phase, only 12% were ultimately diagnosed with a thrombophilia defect. None of these thrombophilia defects affected management during the acute phase. Patients were anticoagulated regardless of a positive or negative thrombophilia test result.
Interpretation of testing during the acute phase of the TEE is problematic because of ill-defined diagnostic cutoff levels, acute phase effects, and concurrent anticoagulation. For example, 3 patients had combined deficiencies of 2 natural anticoagulants, which would be extremely rare. In 2 patients, repeat testing was negative, and 1 patient never underwent repeat testing. The most common positive tests during the acute phase were a positive LA and decreased PS activity, both of which are subject to acute phase effects.8 Only 1 of these in each category remained positive when tested at 12 weeks or more. By delaying testing until after the resolution of the acute phase, more accurate results can easily be obtained. Results of thrombophilia testing did not affect decisions regarding the intensity or duration of anticoagulation in the acute phase of the TEE.
Inherited thrombophilia testing during the acute setting is only warranted in patients with purpura fulminans, vitamin K antagonist-induced skin necrosis, or heparin resistance without an identifiable cause6 ; however, none of the patients in our cohort met the abovementioned criteria. Although 1 patient received extended anticoagulation because of persistent elevation of high-titer aβ2GPI, one could argue the patient would have received extended anticoagulation even if tested after resolution of the acute phase. Furthermore, although the evidence is weak, American College of Chest Physicians guidelines do suggest management as per general recommendations for VTE management in the setting of antiphospholipid antibodies.7 Thrombophilia testing may have influenced thromboprophylaxis during future high-risk situations in 5 subjects (all with FVL), but was only documented for 1 in our cohort. Despite this finding, deferring thrombophilia testing may have yielded more accurate results. Some contend that every pediatric patient with a history of a TEE should receive prophylactic anticoagulation during high-risk situations independent of a thrombophilia disorder.3
Thrombophilia testing was potentially harmful in 12 patients younger than 1 year (including 1 neonate). With decreased total blood volumes, this population is most at risk for iatrogenic anemia.16 Two patients required repeat testing because of false-positive test results. Fortunately, none of the patients were misdiagnosed with a thrombophilia or received unnecessary long-term anticoagulation. This is likely because our institutional practices dictate that every patient with a TEE follow-up with a pediatric hematologist. This is unlike in the adult population, where many such patients are not followed by hematologists.8 There is also a substantial potential for misdiagnosis based on erroneous laboratory results if the patient follows up with physicians without hemostasis thrombosis expertise.
An estimated $82 000 in annual cost resulting from unnecessary testing is not insignificant. This does not include the costs associated with repeat testing, phlebotomist time, blood loss, and physician time counseling parents. In addition, the FVL PCR test instead of the activated protein C resistance assay further increases costs.17 The activated protein C resistance assay is also more clinically useful for detecting a prothrombotic FV phenotype,17 can be modified to account for the different levels of coagulation factors in children, and can be used for patients with a history of bone marrow or liver transplant.18
Evidence-based indications for thrombophilia testing in pediatric population are lacking.3 The 2002 International Society of Thrombosis and Haemostasis guidelines suggest universal testing of all pediatric patients with a VTE.5 The primary objective of thrombophilia testing is to identify patients at high risk for recurrent thrombosis and possibly determine anticoagulation duration on the basis of the presence of positive thrombophilia markers. However, prior pediatric studies have demonstrated this may not be cost-effective.19 A meta-analysis of inherited thrombophilia markers by Young et al20 showed odds ratios for recurrent pediatric VTE ranging from 0.64 (95% CI, 0.35-1.18) for FVL to 4.46 (95% CI, 2.89-6.89) in patients with combined disorders. However, subgroup analysis for catheter-related VTEs was not performed separately. A more recent meta-analysis by Neshat-Vahid et al21 found only weak associations of FVL, factor VIII activity, and PC deficiency in pediatric patients with catheter-related VTEs. The authors recommend against routine thrombophilia testing in these patients with a catheter-related VTE because of the weak associations, low prevalence of the thrombophilia markers in the meta-analysis, and limited evidence on the use of thrombophilia tests to guide anticoagulation. In a subgroup analysis of our patients with only catheter-related TEEs, the relative risk of developing a recurrent TEE in patients who never had testing (28 patients; median follow-up, 425 days) compared with patients who had some form of testing at any point, whether during or after the acute setting (29 patients; median follow-up, 567 days), was 0.26 (95% CI, 0.03-2.2). There was no difference in the risk of developing a recurrent TEE based on thrombophilia testing. Of the 4 patients who had testing and later developed a recurrent TEE, all recurrent events were also associated with a catheter. None of these patients had a thrombophilia defect, underscoring the fact that thrombophilia testing in children with catheter-related TEEs is likely unwarranted and can be avoided.
Limitations of our study include a lack of comparison with controls. The low rate of follow-up testing of an initial positive result (63%) likely underestimated the number of children with a confirmed thrombophilia defect. However, this did not affect our primary study objective. We were also unable to determine if thrombophilia testing affected management in asymptomatic family members. Furthermore, our median follow-up time may not have been long enough to determine a statistically significant difference in the risk for recurrent TEEs in patients with catheter-related TEEs who were or were not tested.
In conclusion, our retrospective study demonstrates that thrombophilia testing during the acute setting does not impact clinical management and was not cost effective at our institution. Even in patients with a confirmed thrombophilia defect, long-term management was only rarely altered. Based on these results, we recommend against routine thrombophilia testing during the acute TEE setting except for rare conditions such as patients with purpura fulminans, vitamin K antagonist-induced skin necrosis, heparin resistance, or in the setting of a clinical trial.
Acknowledgment
A.Z. is supported by a grant from the National Institutes of Health, National Heart Lung, and Blood Institute (1K23HL132054-01).
Authorship
Contribution: C.G. gathered the data, analyzed the results, and drafted the manuscript; and R.S. and A.Z. critically edited the manuscript.
Conflict-of-interest disclosure: R.S. serves as a consultant for CSL Behring and Octapharma. The remaining authors declare no competing financial interests.
Correspondence: Ayesha Zia, Division of Pediatric Hematology/Oncology, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75390; e-mail: ayesha.zia@utsouthwestern.edu.