Background
Despite the improved results achieved with dose-intense treatments, the prognosis of acute myeloid leukemia (AML) patients remains poor, with cure rates ranging from 60% to 70% in patients <60 years old, and overall cure rates of only 35% to 40%. Both disease-related genetic heterogeneity and treatment-related toxicity contribute to such low rates of success.1,2 In developing countries, the treatment outcome of AML is still significantly inferior to that reported in Europe and the United States. Delay in diagnosis and the lack of availability of laboratory assays to inform risk stratification contribute to the poor outcome.3-8 The identification of leukemic residual cells through minimal residual disease (MRD) analyses has been postulated as a significant prognostic factor for relapse and survival.9-11 Multiparametric flow cytometry (MPFC) for MRD detection can identify leukemia-associated aberrant immunophenotypes (LAIPs) in ∼85% of patients at diagnosis or detect progenitor cells with antigenic expression that differs from the normal counterparts in virtually all the cases.12,13 However, MRD studies are rarely performed in developing countries mainly due to their high costs and lack of infrastructure. In addition, there are few multicenter clinical trials conducted in developing countries that incorporate MRD studies adapted to local resources.
Rationale and aims
Considering that MRD positivity has a clear prognostic value for relapses and provides additional risk information to pretreatment cytogenetics/molecular characteristics, we are particularly interested in implementing a standardized multicenter protocol for MRD evaluation to further assess treatment outcome of AML in a large and developing country like Brazil. As part of a current International Consortium of Acute Leukemia Brazilian study, the Feasibility Study of the Use of Intermediate Doses of Cytarabine Associated with Autologous Hematopoietic Stem Cells as Consolidation Treatment of Adults with Low- or Intermediate-risk de Novo Acute Myeloid Leukemia, we are conducting a multicenter study, the main goal of which is to assess the feasibility of a MRD protocol for low- and intermediate-risk AML patients treated with a chemotherapy-based protocol that includes or does not include autologous stem cell transplantation as consolidation therapy.
Study design
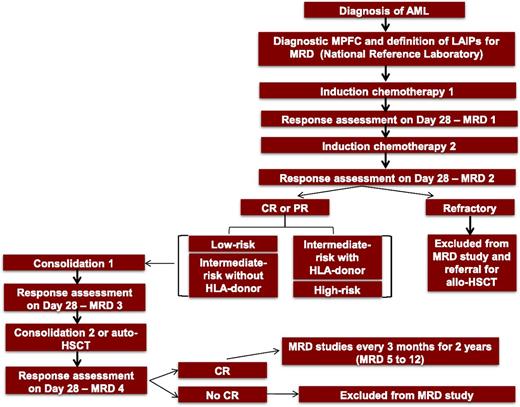
Patients with AML (excluding acute promyelocytic leukemia) according to the World Health Organization Classification of Hematopoietic Tumors, between 18 and 65 years old, including de novo or secondary to myelodysplastic syndrome cases, are included. Patients are stratified into low-, intermediate- or high-risk categories according to the European LeukemiaNet classification.2 All diagnostic and MRD tests are performed by a single central laboratory at the Hematology Laboratory of the Medical School of Ribeirão Preto, University of São Paulo. MRD assessment time points are detailed in Figure 1. The following variables were analyzed: (1) period of time from collection to acquisition in the flow cytometer; (2) quality of the sample based on MPFC quality control standard criteria; and (3) number of cases with LAIPs suitable for MRD detection with a sensitivity of ≥0.1%. This study has been approved by the institutional ethics committee since July 2015.
Study workflow. Before immunophenotypic analysis, BM slides are reviewed to confirm diagnosis or posttreatment morphological remission. In cases where morphology cannot define the acute leukemia phenotype as myeloid or lymphoid, a screening study is performed by using an orientation antibody panel including CD45, CD34, myeloperoxidase, CD19, CD79a, CD3, CD7. LAIPs are defined in BM samples at diagnosis, after each course of therapy, pre–autologous stem cell transplantation, post–autologous stem cell transplantation, and every 3 months thereafter for 2 years. In addition, a sample of stem cell–enriched peripheral blood harvested for autologous transplant is analyzed.
Study workflow. Before immunophenotypic analysis, BM slides are reviewed to confirm diagnosis or posttreatment morphological remission. In cases where morphology cannot define the acute leukemia phenotype as myeloid or lymphoid, a screening study is performed by using an orientation antibody panel including CD45, CD34, myeloperoxidase, CD19, CD79a, CD3, CD7. LAIPs are defined in BM samples at diagnosis, after each course of therapy, pre–autologous stem cell transplantation, post–autologous stem cell transplantation, and every 3 months thereafter for 2 years. In addition, a sample of stem cell–enriched peripheral blood harvested for autologous transplant is analyzed.
Establishment of a minimal panel of antibodies
Table 1 shows the panel of antibodies selected for the study. A negative control tube (cells only) is included in all studies. The strategy was adapted from the experience of Dario Campana's laboratory in the Department of Pediatrics, Yong Loo Lin School of Medicine, Singapore and adapted for a 6- to 8-color MPFC (Table 1).
Training of personnel and standardization of analysis
A member of the Brazilian team was trained in Singapore and was responsible for training a 4-member team in the Brazilian Central Laboratory on his return. After the first cases were analyzed, a Web-based case discussion between the 2 labs was set up to confirm if the analyses were properly applied.
Logistics
Bone marrow (BM) samples were collected in preservative-free heparin and shipped at room temperature to the Brazilian Central Laboratory. The samples from 7 centers travelled by road (distance range, 160-400 km) and from the remaining center by plane and road (∼1400 km).
Flow cytometry method
Mononuclear cells are isolated by Ficoll-Hypaque density gradient centrifugation. After washings, ≥5 × 105 or 1 × 106 cells in 100 μL, at diagnosis or MRD time points, respectively, are distributed per staining tube. Before staining as detailed in Figure 2, cells are incubated with rabbit serum to block Fc receptors. Acquisition from all cells in each test tube (≥105 for diagnosis and 5 × 105 events for MRD) is performed in a FACSCanto II flow cytometer and analyzed by the FACSDiva software (BD Biosciences). The results of the MRD analysis are expressed as the percentage of viable nucleated cells harboring the abnormal phenotype. For the future statistical correlations, both MRD positivity, defined as >0.1% of cells harboring a certain LAIP, and the quantitation of MRD as a continuous variable will be used.
Representative MPFC analysis of AML at diagnosis (upper panels) and MRD1 (lower panels). After sample preparation, cells are labeled with the antibody mixture to identify the backbone leukemic blasts (CD45, CD34, CD117 and CD33, panel A) followed by combinations of fluorochrome-conjugated monoclonal antibodies directed to the leukemic phenotypes (CD38, HLA-DR, CD11b, CD7, CD4, CD56, CD13, CD19, CD11c, CD64, CD133, CD15, NG2, and CD41 (examples in B) at room temperature in the dark. Blast cells are identified by using the gating templates defined at diagnosis. The staining tubes that define the LAIPs at diagnosis are then repeated at the MRD points of study.
Representative MPFC analysis of AML at diagnosis (upper panels) and MRD1 (lower panels). After sample preparation, cells are labeled with the antibody mixture to identify the backbone leukemic blasts (CD45, CD34, CD117 and CD33, panel A) followed by combinations of fluorochrome-conjugated monoclonal antibodies directed to the leukemic phenotypes (CD38, HLA-DR, CD11b, CD7, CD4, CD56, CD13, CD19, CD11c, CD64, CD133, CD15, NG2, and CD41 (examples in B) at room temperature in the dark. Blast cells are identified by using the gating templates defined at diagnosis. The staining tubes that define the LAIPs at diagnosis are then repeated at the MRD points of study.
Results
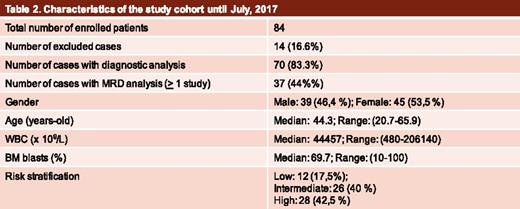
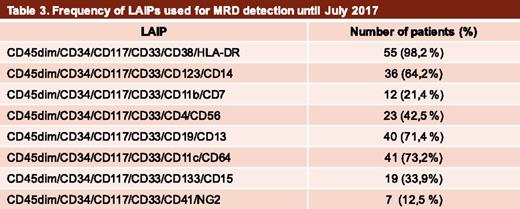
Until the present date, 84 patients have been enrolled in the MRD study (Table 2). Of these, 2 (2.5%) diagnostic samples were discarded because the material was either inadequate or they were collected at >72 hours from the arrival at the central laboratory. Additional diagnostic samples that did not fill in the inclusion criteria, such as equivocal diagnosis (4 acute lymphoblastic leukemia, 4 acute promyelocytic leukemia, and 2 myelodysplastic syndrome cases) or high-risk AML (2 cases), were further excluded (12, 14.2%). Therefore, of the initially enrolled AML cases, diagnostic material to define LAIPs was available in 70. LAIPs suitable for MRD detection with a sensitivity of ≥0.1% have been identified for all patients. The frequency of LAIPs used for MRD detection is shown in Table 3. Of note, 46.4%, 28.5%, 10.7%, and 6% of the included samples have already been analyzed at MRD1, MRD2, MRD3, and MRD4 time points, respectively.
Conclusions
Our results demonstrate the feasibility of the development of MRD studies in a developing country with a large territory. The training of personnel, establishment of a network, and minimal infrastructure resulted in 97.5% of samples suitable for MPFC analyses and the use of a minimal panel of antibodies allowed the identification of LAIPs suitable for MRD detection in all patients. The validation of the proposed MRD detection method in the context of a common clinical protocol will probably contribute to the future implementation of a uniform protocol that may improve interlaboratory standardization in the country.
Acknowledgments:
This work was supported by the São Paulo Research Foundation (grant 2013/08135-2) as part of the Research, Innovation and Dissemination Center Program of the Center for Cell-Based Therapy hosted by the Ribeirão Preto Hemotherapy Center at the Medical School of Ribeirão Preto, University of São Paulo.
Authorship
Conflict-of-interest disclosure: No competing financial interests declared.
Correspondence: Lorena Lobo de Figueiredo-Pontes, Division of Hematology, Department of Medicine, Medical School of Ribeirão Preto, Ribeirão Preto, São Paulo, Brazil; e-mail: lorenafigdo@yahoo.com.br.