Key Points
One dose of MCV4 was safe and immunogenic after HCT.
Serogroup-specific responses varied between 52% and 77% after 1 vaccine dose, suggesting that a second dose may be beneficial after HCT.
Abstract
Immunization with the conjugated quadrivalent (serogroups A, C, Y, and W-135) meningococcal vaccine (MCV4) after hematopoietic cell transplantation (HCT) is recommended. However, immune responses to MCV4 have not been prospectively studied after HCT. We conducted a vaccine response study among 67 adults who received 1 MCV4 dose a year after autologous or allogeneic HCT from January to September 2014. Pre- and postvaccination serogroup serum bactericidal antibody (SBA) titers were measured a median of 57 days after vaccination. Serogroup-specific responses were defined as a fourfold increase in SBA titer with postvaccination titers ≥1:8. Prior to vaccination, 44 (65.7%) patients had no protective titers (<1:8) to any meningococcal serogroup, and 3 (4.5%) patients had protective titers to all 4 serogroups. The median serogroup-specific postvaccination SBA titers were 1:2048 for A, 1:64 for C, 1:128 for W-135, and 1:128 for Y (P < .001 for all pre- and postvaccination pairwise comparisons; similar among serogroups, Spearman ρ 0.5-0.6, P < .0001). Among serogroup-specific nonimmune patients prior to vaccination, serogroup-specific response rates were 76.9%, 65.5%, 51.7%, and 65% to serogroups A, C, W-135, and Y, respectively. One dose of MCV4 elicited protective titers in the majority of patients. These data suggest that a second vaccine dose may be beneficial.
Introduction
Neisseria meningitidis remains a leading cause of bacterial meningitis among children and adults.1 Current guidelines recommend that individuals at increased risk for invasive meningococcal disease (IMD) because of exposure or medical conditions receive the conjugated quadrivalent meningococcal vaccine (MCV4).2 Risk factors for IMD include functional or anatomic asplenia, infection with human immunodeficiency virus, antibody deficiencies, and complement deficiencies, including the use of anti-C5 therapies such as eculizumab.2,3
The immunogenicity of the MCV4 vaccine among adult hematopoietic cell transplant (HCT) recipients has not been prospectively studied. Although these patients usually recover immunoglobulin G1 levels within a few months after transplant,4 which is the predominant immunoglobulin G subclass involved in the immune response to N meningitidis,5 these patients remain at increased risk of infection with encapsulated organisms. Retrospective data suggest that the immune responses to the MCV4 vaccine are poor after HCT and that a 2-dose regimen may be required to achieve protective antibody titers6 ; however, the immunogenicity of a single- or multiple-dose vaccine regimen has not been prospectively evaluated. Given the knowledge gaps regarding the safety and immunogenicity of MCV4 after HCT,7 we conducted a vaccine response study among adult HCT recipients.
Methods
Patients 18 years of age or older who underwent HCT at Dana-Farber Cancer Institute and provided written informed consent to have pre- and postvaccination samples obtained were eligible. The study was approved by the Office for Human Research Studies at Dana-Farber Cancer Institute. Autologous and allogeneic HCT recipients were included in this study if they received the meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria-toxoid conjugate vaccine (Menactra; Sanofi Pasteur Inc, Swiftwater, PA) after transplantation. Per institutional practice, a single dose of meningococcal vaccine is administered by intramuscular injection at the 1-year HCT visit and is documented in the patient’s immunization record.
To assess the immunogenicity of MCV4, pre- and postvaccination serogroup serum bactericidal antibody (SBA) titers were measured. Prevaccination titers were measured on the day of vaccination, whereas postvaccination titers were measured at their next follow-up visit 1 to 2 months later. Patient serum was kept frozen at −80°C until testing was performed. SBA titers were measured using serial dilutions of patient serum in the presence of human complement to determine the minimum antibody titer capable of killing N meningitidis in vitro. A single batch of human complement was used for the entire study. This assay was performed at the Vaccine Evaluation Unit of Public Health England, Manchester, United Kingdom,8 by personnel blinded to patient characteristics.
A vaccine response was defined as a fourfold increase in SBA titers between pre- and postvaccination samples as well as a postvaccination titer that would be deemed protective (conventionally defined as SBA titers ≥1:8).9 A seroreversion was defined as a fourfold decrease in SBA titers. The primary study end point was N meningitidis serogroup A, C, W-135, and Y vaccine response rates after MCV4 administration.
Wilcoxon signed-rank test was used to assess vaccine responses on paired samples, and Spearman’s rank correlation coefficient was used to compare vaccine responses between serogroups. Logistic regression analysis was used to identify patient characteristics that were associated with a protective postvaccination titer, including age, sex, type of HCT, HLA matching (for allogeneic HCT), ablative conditioning, date of vaccination relative to HCT, presence of graft-versus-host disease (GVHD) at time of vaccination, absolute lymphocyte count, and immunosuppressant medication usage. A multivariable model was not pursued because of the lack of statistically significant covariates on univariate analysis. Statistical analyses were conducted using JMP Pro 13.0 (SAS Institute, Gary, NC).
Results
Eighty-two HCT patients consented to participate in the study, of which 67 underwent MCV4 vaccination between January and September 2014 and had paired samples available for analysis. Patients were followed until 1 December 2017. Their demographic and clinical data are shown in Table 1.
Median age at vaccination was 58 years (range, 21-77); 58.2% were male. Thirteen (19.4%) patients underwent autologous HCT, and 54 (80.6%) underwent allogeneic HCT. Among allogeneic-HCT recipients, 2 patients received antithymocyte globulin at transplantation, and 1 patient was on a clinical trial with blinded treatment allocation that may have included antithymocyte globulin as well. No patient received anti–B-cell antibodies within 1 year from vaccination. Thirty-two patients (59.3%) were being treated for GVHD at the time of vaccination, and 43 patients (79.6%) were on immunosuppressive therapy. Five patients (7.5%) received intravenous immunoglobulins within 3 weeks of vaccination.
The MCV4 conjugated vaccine was administered at a median of 369 (range, 327-405) days after HCT. Adverse events were assessed at the subsequent visit, and none were documented within 60 days of vaccine administration. Prior to vaccination, 44 patients (65.7%) did not have protective titers to any of the different meningococcal serogroups, and only 3 patients (4.5%) had protective titers to all 4 serotypes.
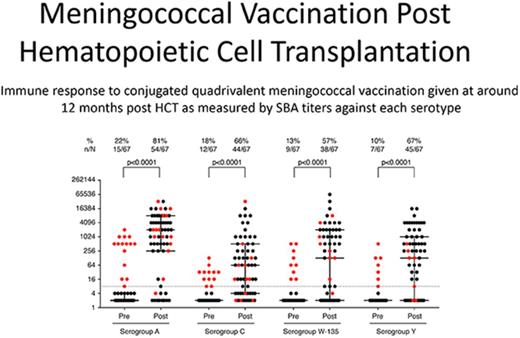
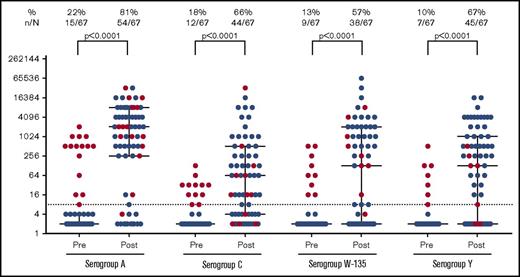
The vaccine response rate was measured a median of 57 days (IQR, 41-91) after vaccination (Figure 1). The median postvaccination SBA titers were 1:2048 for serogroup A, 1:64 for serogroup C, 1:128 for serogroup W-135, and 1:128 for serogroup Y. Response rates were statistically significant when compared with baseline (P < .001 for all pre- and postvaccination pairwise comparisons) and similar among serogroups (Spearman ρ = 0.5-0.6, P < .0001). Among nonimmune patients at baseline, serogroup specific response rates were 76.9%, 65.5%, 51.7%, and 65.0% to serogroups A, C, W-135, and Y, respectively. Fifty-one patients (76.1%) had protective titers to 2 or more meningococcal serogroups, and 29 patients (43.3%) had a protective titer to all 4 serogroups. Five patients exhibited a seroreversion to a single serogroup, and 1 patient exhibited a seroreversion to 3 serogroups.
Pre- and postvaccine SBA titers by meningococcal serogroup. Pre- and postvaccination serogroup SBA titers were measured. SBA titers were measured using serial dilutions of patient serum in the presence of human complement to determine the minimum antibody titer capable of killing N meningitidis in vitro. Vaccine response rates were measured a median of 54 days after vaccination. The dashed line indicates a titer of 1:8. Subjects with a prevaccination titer ≥1:8 for a given serogroup are indicated with red dots. Median and IQR bars are shown.
Pre- and postvaccine SBA titers by meningococcal serogroup. Pre- and postvaccination serogroup SBA titers were measured. SBA titers were measured using serial dilutions of patient serum in the presence of human complement to determine the minimum antibody titer capable of killing N meningitidis in vitro. Vaccine response rates were measured a median of 54 days after vaccination. The dashed line indicates a titer of 1:8. Subjects with a prevaccination titer ≥1:8 for a given serogroup are indicated with red dots. Median and IQR bars are shown.
Interestingly, no patient who received a mismatched allogeneic HCT developed a protective antibody titer to serogroup W-135, but responses to serogroups other than W-135 were achieved. Patients with prevaccine titers ≥1:8 to serogroup W-135 were more likely to have protective postvaccination titers to that serogroup, but this observation was not seen in other serogroups (Table 2). Given the relative lower immunogenicity of serogroup W-135, as well as the small proportion of patients who underwent mismatched HCT in our study, these potential risk factors require further evaluation. No other patient covariate was associated with a decreased risk of developing a protective titer to any meningococcal serotype, including the type of HCT (Table 3). However, our study is limited in ability to determine the risk factors that affect vaccine responses after HCT given our sample size. After 172 person-years of follow-up, no patient developed an episode of IMD.
Discussion
As a vaccine-preventable disease with an estimated mortality rate of 14.9%,10 IMD is an important public health concern. As a majority of patients in our cohort mounted a protective immune response to at least 1 meningococcal serogroup after vaccination, these data suggest that the MCV4 vaccine is immunogenic and likely beneficial among adult HCT recipients. However, as 57% of patients did not have a protective titer to at least 1 serogroup post vaccination, these results suggest that a second dose should be considered for HCT recipients, as is currently recommended by the Centers for Disease Control and Advisory Committee on Immunization Practices for persons who are at increased risk for IMD.2
In conclusion, this study demonstrates that MCV4 vaccination after transplantation is both safe and immunogenic. However, the optimal timing and number of vaccine doses after HCT requires further study. The evaluation of longer-term immunogenicity and clinical efficacy of conjugated meningococcal vaccination among adult HCT recipients is warranted.
Acknowledgment
M.P.C. receives salary support from the Detweiler Travelling Fellowship, provided by the Royal College of Physicians and Surgeons of Canada.
Authorship
Contribution: N.C.I. conceptualized the study; M.P.C., S.R.W., L.R.B., F.M.M., and N.C.I. performed data analysis; M.P.C. wrote the manuscript with critical input from all coauthors; and all authors reviewed the manuscript and agreed to its submission in its current form.
Conflict-of-interest disclosure: This manuscript was prepared as part of routine work. N.C.I. received research grants from GlaxoSmithKline (unrelated to this study) and Astellas. S.R.W. has received clinical trial support from Janssen. The remaining authors declare no competing financial interests.
Correspondence: Matthew P. Cheng, Division of Infectious Diseases, Brigham and Women’s Hospital, Dana-Farber Cancer Institute, 75 Francis St, PBB-A4, Boston, MA 02115; e-mail: mcheng@bwh.harvard.edu.