Key Points
The incidence rate of venous thromboembolism in patients with chronic lymphocytic leukemia was 8.2 per 1000 person-years.
Second primary cancer was a stronger risk factor for venous thromboembolism than prognostic markers of chronic lymphocytic leukemia.
Abstract
Venous thromboembolism (VTE) is associated with inferior survival in cancer patients. The risk of VTE and its effect on survival in chronic lymphocytic leukemia (CLL) patients remains unclear. The present study investigated the impact of patient-related factors, CLL prognostic markers, and CLL treatment on the risk of VTE and assessed overall survival relative to VTE. All patients in the Danish National CLL Registry (2008-2015) were followed from the date of CLL diagnosis to death, VTE, emigration, or administrative censoring. Hazard ratios (HRs) were estimated using Cox models, and second primary cancers and anticoagulation treatment were included as time-varying exposures. During a median follow-up of 2.6 years, 92 VTEs occurred among 3609 CLL patients, corresponding to a total incidence rate of 8.2 VTEs per 1000 person-years (95% confidence interval [CI], 6.7-10.1). A history of VTE or second primary cancer was associated with HRs of VTE of 5.09 (95% CI, 2.82-9.17) and 3.72 (95% CI, 2.15-6.34), respectively, while β2-microglobulin >4 mg/L, unmutated immunoglobulin HV and unfavorable cytogenetics had lower HRs. CLL patients with VTE had marginally higher mortality, which was most pronounced among patients <60 years of age (HR, 7.74; 95% CI, 2.12-28.29). Our findings suggest that markers of unfavorable CLL prognosis contribute to an increased risk of VTE; however, previous VTE or a second primary cancer is more strongly associated with the risk of VTE than any CLL-specific marker. Focusing attention on this preventable complication may improve survival in young CLL patients.
Introduction
Chronic lymphocytic leukemia (CLL) is a hematological cancer type with a heterogeneous disease course. Patient-specific factors, clinical disease stage, and CLL-specific markers of prognosis influence the 5-year overall survival rate, which ranges from 95% in the most indolent phenotypes to 19% for the group with poorest overall survival.1-3 Venous thromboembolism (VTE) is associated with decreased survival and impaired quality of life in cancer patients.4,5 Recent studies have shown a considerable incidence of VTE among CLL patients.6-9 The incidence appears high throughout the clinical course of CLL, which contrasts solid tumors, wherein the incidence of VTE is highest during the initial 6 to 12 months after diagnosis.6,7,10,11 A considerable proportion of CLL patients are diagnosed with a second primary cancer following CLL diagnosis.12-15 It is, however, not known if second primary cancers, CLL-specific markers, or CLL treatments are associated with the incidence of VTE. A greater understanding of the epidemiology of VTE in the context of CLL is crucial because of the implications on prophylaxis, treatment, and prognosis.
The aim of the present study is to investigate the etiology of VTE in CLL patients. Our underlying hypotheses are that patients with previous VTE, second primary cancer, negative prognostic CLL-specific markers, and CLL treatment have a higher risk of VTE than CLL patients free of these exposures. Furthermore, we hypothesize that the mortality of CLL patients with VTE after the CLL diagnosis is higher compared with CLL patients without VTE after the CLL diagnosis. Finally, we hypothesized that the cumulative incidence of VTE is higher in analysis where solely death was treated as a competing risk than analyses where death plus second primary cancer were treated as competing risks.
Methods
Study population and data sources
Denmark has a publicly funded health care system where the expense of prescribed medicine is partially reimbursed. Since 1977, the Danish National Patient Registry (DNPR) has prospectively collected information related to the hospital funding and relevant to our study, discharge dates, and diagnoses classified by International Classification of Diseases (ICD) codes from all Danish hospitals.16 Prescribed medicine received from Danish community pharmacies is registered in the Danish National Prescription Registry by Anatomical Therapeutic Chemical codes.17 Vital status and full address and dates of moving of all Danish residents is registered in the Danish Civil Registration System. For emigrants, the date and country of emigration is registered.18
In addition to being registered in the DNPR, all CLL patients (ICD-10: DC911) in Denmark are registered systematically in the Danish National CLL Registry. This registry was established in 2008 in order to monitor the quality of care and diagnostics for CLL patients. The 9 hematology centers in Denmark report to the Danish National CLL Registry prospectively from the date of CLL diagnosis until death or emigration of the patient, certain predefined variables not provided by the DNPR, including World Health Organization performance status (WHO-PF),19 clinicopathological findings, and first-line treatment. The CLL diagnosis date and ICD-10 codes are confirmed by cross-referencing with the DNPR. The Danish National CLL Registry’s overall coverage is 99%, and the completeness of its data is 98%; however, delay in data submission for the most recent years has resulted in lower completeness percentages for these years.20 We included all patients diagnosed with CLL who were recorded in the Danish National CLL Registry. The unique civil personal registration number that is assigned to every Danish resident at birth or immigration enables linking, at the patient level, of information from different registers and databases.16
Exposures
Information about age, sex, WHO-PF, CLL-specific markers, and first-line CLL treatment were retrieved from the Danish National CLL Registry. Patients with Binet Stage C, unmutated immunoglobulin HV (IgHV), IgHV 3-21 variable, or deletions 11q (del11q) or 17p were classified as high-risk CLL according to the Danish National Guidelines for CLL (supplemental Table 1).21 Information about other primary cancers, excluding nonmelanoma skin cancers (ICD-8: 140-209, except 173.x; ICD-10: CC00-96, except CC44), both before and after the CLL diagnosis were retrieved by linkage to the DNPR. VTEs preceding the CLL diagnosis were identified by linkage to the DNPR (ICD-8: 450 and 451; ICD-10: I26, I80.1-I80.9). Information about anticoagulation treatment (Anatomical Therapeutic Chemical codes B01AA03, B01AE07, B01AF02, B01AF01, B01AB10,B01AB04, and B01AB05) was obtained by linkage to the Danish National Prescription Registry, which includes the types and amounts of medications dispensed. Information regarding hospital admissions were obtained from the DNPR and periods of hospitalization were coded as “exposed to anticoagulation treatment” if patients were anticoagulated before hospital admission based on information from the Danish National Prescription Registry.
Outcomes
Due to the low positive predictive value of VTE diagnosis codes from emergency departments in the DNPR, we solely included VTEs coded in hospital wards and VTE diagnoses coded in emergency departments only when they were subsequently coded in a ward or an outpatient clinic (ICD-10: I26, I80.1-I80.9) or if concurrent prescribing of anticoagulation therapy was registered.22 Last available information regarding VTE diagnosis was 31 December 2015. Information regarding vital status and emigration was obtained from the Danish Civil Registration System.
Validity of the VTE diagnosis
The validity and origin of VTE diagnoses from the DNPR before and after diagnosis of CLL was assessed by a medical doctor’s systematic review of the medical records as well as vascular diagnostic images from all newly diagnosed CLL patients at Aalborg University Hospital between 2008 and 2016. The ICD-10 codes I26 and I80.1 to 80.9 of the CLL patients were identified in the DNPR. The validation process is described in details in supplemental Methods. Validation study data were collected and managed using Research Electronic Data Capture hosted at Aalborg University Hospital.23
Statistics
Study entry was the date of CLL diagnosis, and the CLL patients contributed with person-time at risk of VTE until VTE, death, emigration, or administrative censoring, which was 31 December 2015 (last follow up for VTE). Continuous variables are presented as medians with 25th and 75th percentiles, and categorical variables are reported as frequencies and percentages.
Second primary cancers, anticoagulation treatment, and CLL treatment were treated as time-varying exposures in time-to-event analyses. This means that during the study period, subjects switched from unexposed to exposed on the date of secondary primary cancer/anticoagulation/CLL treatment exposure. For anticoagulation, study subjects switched back to unexposed on the date of last anticoagulation treatment. In the analysis concerning CLL treatment, administrative censoring was defined as 1 year after the CLL diagnosis in order to minimize the potential proportion of CLL patients either no longer being exposed to CLL treatment following a switch to the “CLL treated” group or being exposed to second-line CLL treatment.
To describe rates of VTE according to patient-related factors, CLL-specific markers, and CLL treatment, incidence rates (IRs) of VTE expressed as events per 1000 person-years (×10−3 p-y) and associated 95% confidence intervals (CIs) were calculated for the entirety of CLL patients and for patient-specific factors, CLL prognostic factors, periods of watch and wait, and CLL treatment. Cox proportional hazards regression models were constructed to assess the influence of a priori–identified possible confounders on the hazard of VTE after the CLL diagnosis; anticoagulation and second primary cancer were included as time-varying variables. Assumptions of proportional hazards were checked by log-log plots.
The cumulative overall mortality of the CLL patients with respect to VTE is described with VTE as a time-varying exposure. The CLL patients began in the “no VTE” group and were switched to the “VTE after CLL” group on the date of their VTE diagnosis. The cumulative mortality in the VTE after CLL group was calculated by the Kaplan-Meier estimator, while in the no VTE group it was calculated treating VTE as a competing risk by use of the Aalen-Johansen estimator. In Cox proportional hazards regression models stratified by patient-related factors and CLL-specific markers, we compared the mortality of CLL patients who experienced a VTE during follow-up with the mortality of CLL patients who did not experience a VTE during follow-up.
To graphically summarize the occurrence of VTE according to patient-related factors and CLL-specific markers, we depicted cumulative incidence of VTE allowing for the competing risk of death and second primary cancer, when relevant.
To illustrate the magnitude of events competing with VTE, stacked cumulative incidences of possible outcomes (VTE, second primary cancer, and death) were depicted treating second primary cancers, VTE, and death as competing risks, when relevant. To illustrate the possible overestimation of the VTE risk in CLL patients, in the situation where a second primary cancer was not considered a competing event, we depicted the cumulative incidences of VTE in CLL patients, both when solely death was treated as a competing risk and when death plus second primary cancer were treated as competing risks.
SAS version 9.4 (SAS Institute, Cary, NC) was used for merging data from the different data sources. Statistical analyses were conducted by use of STATA MP version 14 (Stata Corporation, College Station, TX).
Ethics
The study was approved by the Danish Data Protection Agency (2008-58-0028, internal reference 2017-93). Register-based studies do not require ethical approval in Denmark.
Data-sharing statement
The syntaxes will be available upon reasonable request by contacting inlg@rn.dk. Individual study participant data will not be shared due to the restrictions by the Danish Data Protection Agency.
Results
Patient characteristics
A total of 4040 CLL patients were diagnosed with CLL and registered in the Danish CLL Registry from 2008 to 2017. Due to administrative censoring, 429 CLL patients who were diagnosed after 31 December 2015, were excluded from the study. Two patients were excluded due to errors in data merging, leaving 3609 CLL patients for follow-up. Their median age at CLL diagnosis was 70.4 years (25th-75th percentile, 63.4-78.1 years), and 60% were male. Median follow-up was 2.6 years (25th-75th percentile, 1.2-4.8 years).
In total, 225 VTEs were registered among the CLL patients, 123 of which preceded the CLL diagnosis (47 pulmonary embolisms and 76 deep vein thromboses). The median time from the first VTE diagnosis to the CLL diagnosis was 2585 days (25th-75th percentile, 583-5091). Baseline characteristics are described in Table 1.
Validity of the VTE diagnoses
A total of 469 CLL patients, among whom 32 VTEs were coded in the DNPR and an additional six VTEs were identified during the validation process, were followed at Aalborg University Hospital from 2008 through 2016. Five (15.6%) of the VTEs registered in the DNPR were not confirmed by reviewing diagnostic images, patient records and biochemistry. The positive predictive value of a VTE diagnosis from DNPR in CLL patients was 84.4% (95% CI, 67.2-97.4) and the sensitivity was 81.8% (95% CI, 64.5-93.0) (supplemental Table 2).
Outcomes
In the study period, 102 VTEs occurring subsequent to CLL diagnosis were registered in the DNPR. Ten of the 102 registered VTEs were coded solely in an emergency department without a concurrent anticoagulation prescription or subsequent VTE coding from hospital wards or outpatient clinics, leaving 92 VTEs to study (47 pulmonary embolisms and 45 deep vein thromboses). The overall IR of VTE in the CLL patients was 8.2 × 10−3 p-y (95% CI, 6.7-10.1). Twenty-two (24%) of the VTEs occurred in CLL patients who were diagnosed with a second primary cancer.
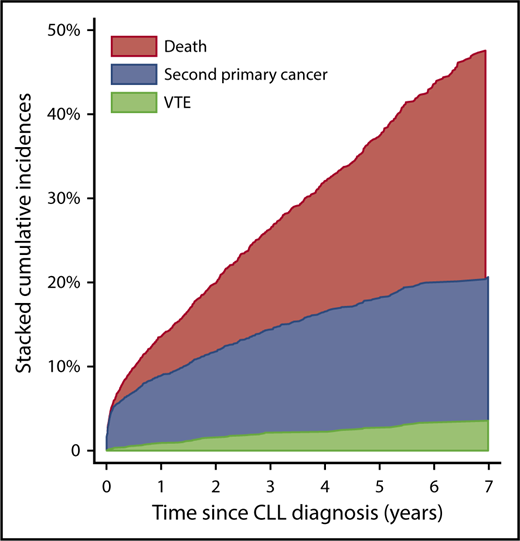
In total, 32.1% of the CLL patients had either a VTE, a second primary cancer, or died within the initial 4 years following CLL diagnosis. The 4-year cumulative incidence of VTE prior to possible second primary cancer or death was 2.3%. The 4-year cumulative incidence of second primary cancer before possible death or VTE was 14.3%, while 15.5% died within the first 4 years as the first event (Figure 1). For further details, see supplemental Results. In total, 721 of the CLL patients died during the study period (IR, 63.4 × 10−3 p-y); 13 patients free of VTE after the CLL diagnosis emigrated before administrative censoring.
Stacked cumulative incidences of VTE, second primary cancer, and death. Exclusively contingent first events are depicted.
Stacked cumulative incidences of VTE, second primary cancer, and death. Exclusively contingent first events are depicted.
Patient-related factors and VTE
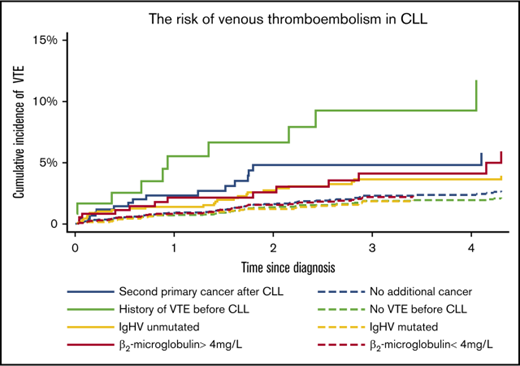
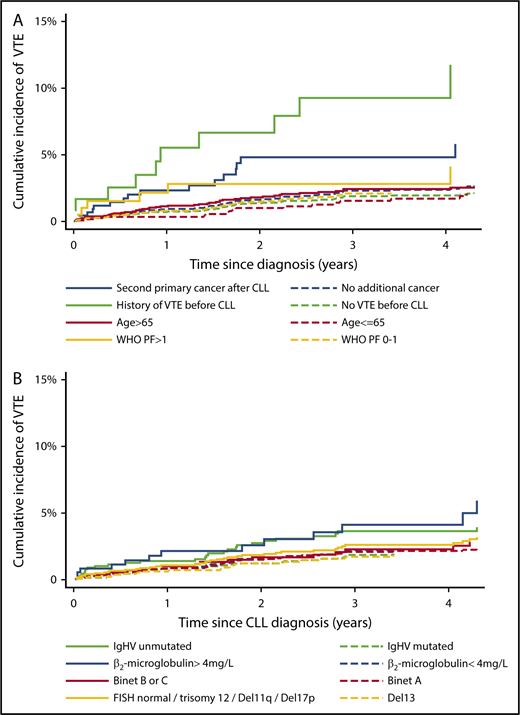
The 2-year cumulative incidence of VTE for patients with previous VTE was 7.9% (95% CI, 3.7%-14.3%), while for those with no previous VTE, it was 1.4% (95% CI, 1.0%-1.8%). For patients with a second primary cancer after the CLL diagnosis, the 2-year cumulative incidence of VTE was 4.8% (95% CI, 2.5%-7.4%), while for those not exposed to second primary cancer, it was 1.7% (95% CI, 1.3%-2.2%) (Figure 2A).
Cumulative incidence of VTE according to patient-related factors and CLL-specific markers. Shown are cumulative incidences of VTE in 4 different exposure categories; all patients are thus at risk in 1 of the 2 groups in each exposure category. CLL patients without a second primary cancer (yet) contributed to number at risk of VTE from the CLL diagnosis date (t0); death and second primary cancer were treated as competing risks. CLL patients exposed to a second primary cancer contributed to number at risk of VTE from the second primary cancer diagnosis date (t0); death was treated as a competing risk (A). Too few events among CLL patients with Binet C, when a second primary cancer was treated as a competing risk, precluded a separate graph for this group (B).
Cumulative incidence of VTE according to patient-related factors and CLL-specific markers. Shown are cumulative incidences of VTE in 4 different exposure categories; all patients are thus at risk in 1 of the 2 groups in each exposure category. CLL patients without a second primary cancer (yet) contributed to number at risk of VTE from the CLL diagnosis date (t0); death and second primary cancer were treated as competing risks. CLL patients exposed to a second primary cancer contributed to number at risk of VTE from the second primary cancer diagnosis date (t0); death was treated as a competing risk (A). Too few events among CLL patients with Binet C, when a second primary cancer was treated as a competing risk, precluded a separate graph for this group (B).
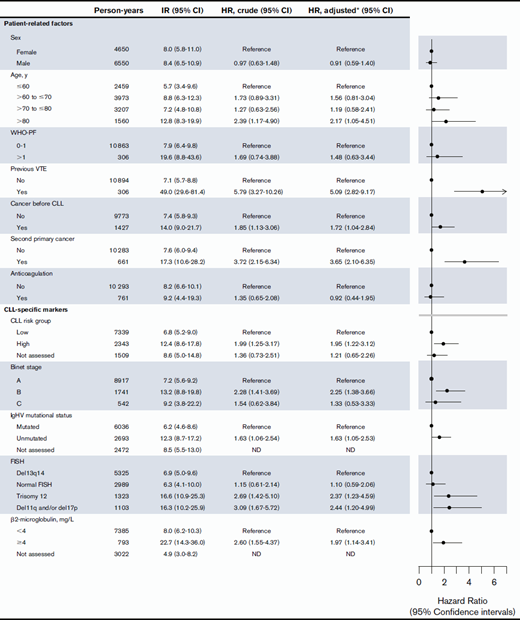
The high cumulative incidence of VTE among CLL patients with previous VTE was to some extent a result of a higher proportion of second primary cancers in CLL patients with previous VTE (23.9%) compared with the total study population (12.8%). However, the hazard ratio (HR) of VTE in cases with previous VTE compared with no previous VTE was 5.09 (95% CI, 2.82-9.17) after adjustment for second primary cancer, anticoagulation treatment, WHO-PF, previous VTE, sex, age, and CLL risk group (Table 2). The relative risk of VTE differed less according to age, sex, cancer before the CLL diagnosis, and WHO-PF (Table 2).
Treating death as the lone competing risk for VTE, the 4-year cumulative incidence of VTE in CLL patients was 3.1% (95% CI, 2.5-3.9), whereas in analysis treating both death and second primary cancer occurrence as competing risks, the 4-year cumulative incidence of VTE was 2.6% (95% CI, 2.0-3.4) (Figure 3).
Cumulative incidence of VTE in CLL depicted, treating solely death and death plus second primary cancer as competing risks.
Cumulative incidence of VTE in CLL depicted, treating solely death and death plus second primary cancer as competing risks.
CLL-specific markers and VTE
The 2-year cumulative incidence of VTE for patients with β2-microglobulin levels >4 mg/L was 3.0% (95% CI, 1.5% to 5.5%), while for those with β2-microglobulin levels below 4 mg/L, it was 1.5% (95% CI, 1.1% to 2.2%). For patients with unmutated IgHV, the 2-year cumulative incidence of VTE was 2.9% (95% CI, 1.9% to 4.3%), while for those with mutated IgHV, it was 1.2% (95% CI, 0.7% to 1.9%) (Figure 2B). Crude and adjusted HRs of VTE did not differ markedly for β2-microglobulin levels >4 mg/L, unmutated IgHV status, and unfavorable FISH, indicating that the higher cumulative incidences of VTE in these groups were not mainly due to confounding by second primary cancers, higher age, or previous VTE (Table 2).
CLL treatment and VTE
The IR of VTE was higher during exposure to CLL treatment (IR, 22.0 ×10−3 p-y [95% CI, 9.9-48.9]) compared with watch-and-wait periods (IR, 9.6 ×10−3 p-y [95% CI, 6.6-12.8]). None of the CLL patients who experienced a VTE during CLL treatment received fludarabine-containing regimens, and 50% received a combination of chemotherapy and immunotherapy. Several types of chemotherapy and immunotherapy were represented in the CLL treatment regimens under which VTE occurred. The higher IR of VTE in CLL treatment was not explained by CLL risk group, second primary cancer, or previous VTE, as the HR remained unchanged after adjustment for these factors; crude HR of VTE was 2.38 (95% CI, 0.96-5.93) while adjusted for the aforementioned factors, HR of VTE during CLL treatment was 2.40 (95% CI, 0.91-6.30).
Survival of CLL patients according to VTE
The cumulative mortality risk among patients with VTE following CLL diagnosis was 35.7% (95% CI, 23.6-51.7) at 4 years from CLL diagnosis, while among CLL patients with no VTE, the cumulative mortality risk was 21.4% (95% CI, 19.9-23.2) (Figure 4). In multivariable Cox regression models including sex, age, WHO-PF, second primary cancer, IgHV mutational status, and β2-microglobulin levels, VTE after the CLL diagnosis was associated with a HR for death of 2.13 (95% CI, 1.39-3.27) in the study period. The effect of VTE on the mortality was lower than the effect of a second primary cancer and age but on par with unmutated IgHV and β2-microglobulin >4 mg/L. (Table 3). The mortality associated with VTE following CLL diagnosis was sevenfold higher in CLL patients <60 years compared with CLL patients <60 years who were not diagnosed with VTE and threefold higher in CLL patients >80 years who experienced VTE, while the association was weaker in the older age groups of 60 to 70 years and 70 to 80 years (Table 4). We observed no interaction between previous VTE and VTE after the CLL diagnosis and thus similar effects of VTE after CLL diagnosis on the mortality in the 2 groups (hazard rate ratio for interaction, 0.44 [95% CI 0.12-1.60]). The effect of VTE after the CLL diagnosis on mortality was lower in age groups >60 to 70 years, >70 to 80 years, and >80 years compared with those ≤60 years (hazard rate ratios for interaction, 0.06 [95% CI 0.01-0.32], 0.09 [95% CI, 0.02-0.39], and 0.18 [95% CI, 0.05-0.62], respectively). In summary, this means that the observed increased relative mortality in patients with VTE after the CLL diagnosis is mainly due to a higher risk of death in the youngest CLL patients in case of VTE after the CLL diagnosis.
Cumulative mortality according to diagnosis of VTE. VTE is treated as competing risk among those not (yet) exposed to VTE.
Cumulative mortality according to diagnosis of VTE. VTE is treated as competing risk among those not (yet) exposed to VTE.
Discussion
By use of the systematical registration of all Danish CLL patients in the Danish National CLL Registry, this study of VTE in 3609 CLL patients demonstrated that the risk of VTE was markedly higher in patients who were diagnosed with a second primary cancer following CLL diagnosis and patients with a history of VTE. The effects of unmutated IgHV, β2-microglobulin levels ≥4 mg/L, and unfavorable FISH on the VTE risk were weaker. A higher proportion of second primary cancers in the group exposed to VTE before the CLL diagnosis explained some of the effect of previous VTE on the risk of VTE after the CLL diagnosis, even though the adjusted effect estimate showed a fivefold increased risk of VTE in CLL patients with a history of VTE. Exposure to first-line CLL treatment within the first year after the CLL diagnosis was associated with a higher IR of VTE compared with watch and wait, and this was not confined to a specific regimen of chemotherapy or immunotherapy. CLL patients with VTE following CLL diagnosis had marginally higher mortality than CLL patients who did not experience a VTE, particularly the youngest CLL patients.
A considerable proportion of the CLL patients were exposed to a second primary cancer following CLL diagnosis, which has also been observed in previous studies.12-15 Simple censoring in the case of a second primary cancer would introduce informative right censoring (ie, those who left the study due to a second primary cancer would have a markedly different [higher] risk of VTE compared with those who stayed in the study). A second primary cancer following CLL diagnosis should therefore be treated as a competing risk when estimating the cumulative incidence of VTE, as described for death.24,25 To the best of our knowledge, we are the first to illustrate and incite deliberation of second primary cancer as competing risk in addition to death. This observation is relevant for a substantial amount of studies in cancer populations with long follow-up periods, especially in studies of more common long-term outcomes.
Our study indicates that unfavorable CLL-specific markers increase the risk of VTE, as also indicated in a recent study by Šimkovič et al.7 We found that unmutated IgHV, del11q/del17p, and trisomy 12 were associated with VTE. Šimkovič et al7 found that 79% of CLL patients with VTE had unmutated IgHV genes and 58% had del11q/del17p. It has been hypothesized that β2-microglobulin is related to the activity of the CLL cells.26-28 Cancer cells, particularly those within advanced tumor types with a more rapid course of disease, shed procoagulant microvesicles.29 We speculate that more active CLL cells release more β2-microglobulin and also shed more procoagulant microvesicles, thus increasing the risk of VTE. This might also be the case for other CLL-specific markers and second primary cancers.
The etiology of VTE in CLL patients is sparsely described in the existing scientific literature. As in earlier studies concerning VTE in CLL patients, the overall IR of VTE for CLL patients in this study was higher than the IR of VTE found in the general population.7,8,30 A history of VTE was associated with a fivefold increased risk of VTE in CLL patients, which is similar to observations from other cancer studies and equivalent to the risk in patients without cancer but with a previous VTE.31-34 In the present study, the effect of age on the risk of VTE was surpassed by both second primary cancers following CLL diagnosis and previous VTE, which is in line with several studies in cancer populations where increasing age was not associated with increased risk of VTE.11,35-37 A higher risk of VTE was observed in periods when patients were exposed to CLL treatment than in periods of watch and wait, which has been shown in large studies and common cancers and indicated by the study on VTE in CLL by Šimkovič et al.7,9,38-41 Interestingly, Šimkovič et al7 also mentioned a second cancer as a risk factor for VTE in CLL patients, but it was not further investigated, perhaps due to the study design and its aim.
The validity of the VTE diagnosis codes among CLL patients has not been investigated previously, and the local validation study was thus highly relevant for the assessment of the data quality for the main outcome in our study. The positive predictive value of VTE diagnosis observed in the validation population in our study (84.4%) is consistent with recent reports of positive predictive values of VTE diagnoses in cancer-specific subsets of the population (85% and 86%) and the in general population (88%).42-44 In addition, we found that the sensitivity of the DNPR codes was actually “only” 76.2%. A limitation of our study, however, remains that only ∼10% of the VTE diagnoses were validated, and the validation study was confined to 1 hospital. However, the positive predictive value is mainly dependent on disease prevalence, and we thus consider the results from our single-center validation study valid to extrapolate to the remaining study population, as it is unlikely that the prevalence of VTE would be markedly different or higher in CLL patients followed up in one of the other 8 CLL centers in Denmark.
Another limitation is that due to the potential long period of undiagnosed CLL, some portion of the VTEs that occurred before the diagnosis of CLL actually occurred in a patient with undiagnosed CLL. This would be especially true for the events that occurred in the few months prior to CLL diagnosis, but as the diagnostic workup is longer for CLL than VTE, this misclassification is unavoidable. In the present study, we did not have access to information regarding fractures and surgery, which is also a limitation.
Our study showed that previous VTE and second primary cancer were associated with the risk of VTE. We thus recommend patient information regarding VTE risk and symptoms in CLL patients with previous VTE and in those exposed to second primary cancer. Since VTE increased the mortality markedly in the youngest CLL patients, we would recommend careful consideration of thromboprophylaxis in CLL patients <60 years if exposed to thrombogenic types of second primary cancer, particularly in case of a history of VTE and/or unfavorable FISH changes/unmutated IgHV/high β2-microglobulin levels. The observations from our study prompt further investigation of the impact of second primary cancers on outcomes in cancer populations. However, an additional cancer is probably not an important competing risk in all cancer types; likewise, in a recent publication, we observed a surprisingly high IR of VTE in CLL patients, despite a similar amount of person-time at risk, no events were observed among patients with indolent lymphomas.6
In conclusion, our study showed that CLL patients with a second primary cancer following CLL diagnosis and those with a history of previous VTE have a considerable risk of VTE. CLL-specific markers were also correlated to VTE, but to a lesser extent. Furthermore, we observed that a second primary cancer after study entry should be considered as a competing risk in future cancer studies.
The full-text version of this article contains a data supplement.
Acknowledgment
The authors thank all physicians at the Danish Hematology Centers who collected data for the Danish National CLL Registry.
Authorship
Contribution: I.L.G. designed the study, validated the VTE diagnoses codes, performed data merging, analysis, and interpretation, and wrote the manuscript; S.J.R. performed data merging, analysis, and interpretation and reviewed the manuscript; I.C., L.E., C.B.P., O.J.B., D.B.G., R.S.P., L.N., A.R., and M.F. were responsible for collection and submission of clinical data in their respective CLL centers, and they reviewed the manuscript; C.T.-P. and H.H.E. collected data from the Danish National Registries at Statistics Denmark, provided derived data sets for merging with data from the Danish National CLL Registry, and reviewed the manuscript; and S.R.K. and M.T.S. designed the study, interpreted data, and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Inger Lise Gade, Department of Clinical Medicine, Aalborg University, Sdr Skovvej 15, 9000 Aalborg, Denmark; e-mail: inlg@rn.dk.