Background
The prevalence of sickle cell anemia (SCA) in the Dominican Republic is approximately 4 times that in the United States.
In the pediatric sickle cell clinic at Hospital Infantil Dr. Robert Reid Cabral (HIRRC) located in the capital city of Santo Domingo, Dominican Republic, 5% to 10% of children with HbSS (homozygous for hemoglobin S) develop primary stroke, with substantial morbidity and mortality.
Transcranial Doppler (TCD) ultrasonography has been shown to effectively identify the risk for primary stroke in the United States but is not routinely accessible or available in the Dominican Republic.
Hydroxyurea is a potent disease-modifying therapy for SCA that can reduce TCD velocities and prevent conversion of conditional to abnormal velocities. Hydroxyurea is available in the Dominican Republic, but health care providers do not have experience with dosing, and most families cannot afford the cost.
Objectives
To build local capacity for primary stroke prevention in the Dominican Republic by
○Certifying local staff in TCD screening techniques, according to National Heart, Lung, and Blood Institute guidelines established in the STOP1 and STOP2 (Stroke Prevention in Sickle Cell Anemia) trials;
○Educating local health care providers about appropriate dosing and monitoring of hydroxyurea for children with SCA; and
○Training the local hematology team in principles of clinical research as part of a collaborative research project.
To conduct a prospective open-label screening and treatment research trial. The Stroke Avoidance for Children in República Dominicana (SACRED) trial includes these specific aims:
○Perform TCD screening of a large cohort of children with SCA living in the Dominican Republic to identify those with increased risk of stroke;
○Determine the prevalence of elevated (conditional or abnormal) TCD velocities in children with SCA;
○Obtain longitudinal data on the natural history of TCD velocities in this cohort; and
○Determine the ability of hydroxyurea to prevent stroke in children with conditional TCD and increased risk of stroke.
Study design
The SACRED trial (NCT02769845) is a prospective screening and treatment study of children with SCA and increased risk of stroke who live in the Dominican Republic. The three-part study design includes (1) initial TCD screening, (2) longitudinal TCD evaluations, and (3) hydroxyurea treatment for children with conditional TCD velocities.
Capacity building
The clinical performance site was the pediatric sickle cell clinic at HIRRC, Santo Domingo, Dominican Republic (Figure 1).
Recent medical school graduates were trained and certified by an experienced TCD examiner from the Cincinnati Children’s Hospital Medical Center (CCHMC) (Figures 2 and 3)
All resources (TCD machines, laboratory materials, office equipment, and hydroxyurea) were provided by the CCHMC . The SACRED trial database was built by using REDCap software for electronic online data entry, and remote monitoring was performed by the CCHMC.
The local hematology team received Collaborative Institutional Training Initiative (CITI) certification as part of their training in principles of clinical research. Training and oversight in trial operations, data collection, and managing study participants was provided to optimize efficient and accurate operations.
Clinical site coordinators were trained in special pipetting techniques for DNA and serum collection, and samples were shipped to Cincinnati for laboratory analysis (Figure 4).
Team conference calls were made twice per month via Skype, and frequent ongoing communication allowed for discussion and monitoring of study data.
Hospital Infantil Dr. Robert Reid Cabral, Santo Domingo, Dominican Republic.
Recent medical school graduates are trained and certified by an experienced TCD examiner from the Cincinnati Children’s Hospital Medical Center (Cincinnati, OH).
Recent medical school graduates are trained and certified by an experienced TCD examiner from the Cincinnati Children’s Hospital Medical Center (Cincinnati, OH).
Clinical site coordinators are trained in special pipetting techniques for DNA and serum collection.
Clinical site coordinators are trained in special pipetting techniques for DNA and serum collection.
Study management
The SACRED trial features a full academic research partnership between CCHMC and HIRRC in the Dominican Republic.
All TCD screening is performed at the clinical site by the Dominican Republic research team. TCD velocities are scored by experts at CCHMC who then provide feedback to the site team and recommendations for treatment or observation per protocol based on TCD category.
Hydroxyurea is provided at no cost to children with conditional TCD velocities. The medical team at CCHMC reviews the laboratory results and provides guidance on initiation of hydroxyurea treatment, dose calculations, and adequate patient follow-up while the patient is receiving treatment.
The SACRED protocol, consent forms, and REDCap database were prepared collaboratively and translated into Spanish. Institutional review board approval was obtained at both institutions, and training was provided onsite and remotely.
In the first year, children were screened for TCD over a 12-month period. Those with conditional TCD velocities were offered hydroxyurea at 20 mg/kg/day for 6 months, followed by escalation to maximum tolerated dose (MTD). Children with abnormal TCD velocities received transfusions once per month for primary stroke prevention.
Screening and enrollment
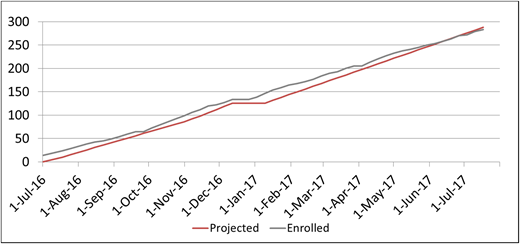
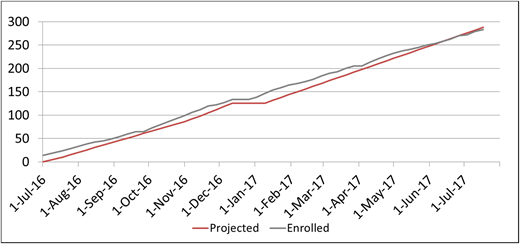
Eligible patients were identified from the local sickle cell clinic population. Enrollment was projected for an average rate of 5 to 6 children per week and for 250 to 300 children during the first year (Figure 5). Local staff from the Dominican Republic completed the consenting process and used REDCap for all data entry.
A total of 283 children with SCA were enrolled in the SACRED trial over the initial 1-year screening period.
A total of 283 children with SCA were enrolled in the SACRED trial over the initial 1-year screening period.
Results to date
Initial TCD screening of the 283 children revealed 200 normal (70.7%), 63 conditional (22.3%), 11 abnormal (3.9%), and 9 inadequate (3.1%) TCD velocities.
A total of 50 children were eligible for hydroxyurea, and 48 initiated therapy. All patients started at 20 mg/kg/day for 6 months and then escalated to MTD.
Repeat TCD screening after 6 months of hydroxyurea treatment revealed that 30 (63%) of 48 children with previous conditional velocities had reverted to normal TCD velocities. This effect has been sustained at 12 months of treatment.
Conclusions
SACRED is an important collaborative research trial that features local capacity building to help prevent stroke among children with SCA in the Dominican Republic.
The prevalence of conditional TCD velocities is >20%, which supports the observed elevated risk of stroke among children with SCA in the Dominican Republic.
Hydroxyurea treatment is associated with improved hematologic parameters, lower TCD velocities, and presumably decreased risk of stroke (Table 1).
Acknowledgments
The authors thank the patients who participate in the SACRED trial and the study teams at Hospital Infantil Dr. Robert Reid Cabral and Centro de Obstetricia y Ginecología.
This work was supported by the Cincinnati Children’s Hospital Research Foundation to help with the design and conduct of the SACRED trial.
Authorship
Conflict-of-interest disclosure: R.E.W. received research funding from Addmedica, Bristol-Myers Squibb, and Biomedomics; served as a consultant for Nova Laboratories; and was a member of the board of directors and served on advisory committees for Novartis; other, Global Blood Therapeutics, and Agios. The remaining authors declare no competing financial interests.
Correspondence: Luisanna Sánchez, Cincinnati Children’s Hospital Medical Center, Cincinnati OH; e-mail: sanchez.luisanna@gmail.com.