Key Points
Children treated with blinatumomab for B-ALL with MRD had few side effects and proceeded to hematopoietic cell transplant without delay.
Blinatumomab given prior to transplant reduces MRD and results in favorable leukemia-free survival, toxicity, and overall survival.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is indicated for patients with relapsed or refractory B acute lymphoblastic leukemia (B-ALL), where outcomes remain poor with chemotherapy alone. Numerous studies have shown that patients undergoing HCT with minimal residual disease (MRD) are at significant risk of disease relapse.1-6 The best method to eliminate MRD prior to HCT is unclear and whether further attempts to convert a patient to MRD negativity will improve post-HCT outcomes remains unproven. Attempts to do so with standard chemotherapy has had mixed responses and added toxicity, including infection and organ injury, which may delay or preclude HCT. Cellular therapies (ie, chimeric antigen–expressing T cells) have the burden of cell collection and manufacture and require ongoing chemotherapy to control the disease. Antibody therapies such as inotuzumab ozogamicin7 have also shown efficacy; however, this agent specifically comes with a high risk of veno-occlusive disease post-HCT. Blinatumomab is a US Food and Drug Administration–approved bispecific T-cell engager for patients with relapsed or refractory B-ALL or those with persistent MRD. It was designed to recognize the lymphoid marker CD19 expressed by most B-ALL. Engagement of both the target antigen (CD19) and the CD3 present on the patient’s cytotoxic T cells leads to activation of the T cell and subsequent lysis of the CD19-expressing leukemia.8,9 Here, we review our experience using blinatumomab as bridging therapy in children with relapsed or refractory B-ALL with residual MRD prior to planned transplantation.
Methods
Study population
This retrospective analysis included patients with B-ALL aged 0 to 21 years who were transplanted at 5 Foundation for the Accreditation of Cellular Therapy–accredited pediatric HCT centers. Patients were referred in complete morphological remission (CR; <5% blasts in the bone marrow) but were found to have persistent MRD. All MRD testing was performed by flow cytometry per the standards of the Children’s Oncology Group reference laboratory. All patients received blinatumomab between 2016 and 2017 with the goal of reducing or eliminating MRD prior to HCT. The analysis of deidentified patient data was performed in compliance with all applicable federal regulations pertaining to the protection of human subject research and ethical standards, as set forth in the Declaration of Helsinki.
Statistical analysis
Overall survival (OS) and leukemia-free survival (LFS) were reported using the Kaplan-Meier function. To report time to relapse, the cumulative incidence function was used, and death from transplantation was treated as a competing risk. All statistical analyses were performed using R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria; http://www.Rproject.org/).
Results and discussion
A total of 15 pediatric patients with B-ALL were identified and met criteria for analysis. The median age of the study population at the time of blinatumomab treatment was 9 years (range, 0.5-19 years), and a large variety of cytogenetic abnormalities were represented in the cohort (Table 1). Ten of the 15 patients were in their first remission (CR1) at the time of blinatumomab/HCT. The indication for transplant was persistent MRD at the end of consolidation. The median MRD level prior to blinatumomab treatment was 0.57% (range, 0.01% to 2.2%) of the mononuclear cell compartment. The median time of follow-up was 371 days post-HCT (range, 134-749 days).
Most patients (n = 12) received a single 28-day course of blinatumomab at 15 μg/m2 per day prior to reassessment and proceeding to the intended HCT. Two patients had their initial cycle of blinatumomab shortened to start HCT preparative therapy (at days 18 and 20), and 1 patient received 2 courses for a total of 66 days. Pretransplant use of blinatumomab had minimal impact on the patient’s peripheral blood counts. The absolute neutrophil count (ANC) was only mildly affected by blinatumomab therapy, with a median ANC at start of therapy of 3.39 × 109/L (range, 0.04-4.14) and end of therapy 2.21 × 109/L (range 0.06-4.1). Similarly, the absolute lymphocyte count (ALC) underwent a clinically insignificant increase, with a starting median ALC of 0.866 × 109/L (range 0.176-2.01) and end of therapy median ALC at 1.115 × 109/L (range, 0.14-2.84). A single patient experienced a grade 3 seizure (graded retrospectively) during blinatumomab therapy. Of note, this patient also had CNS3 leukemia (>5 blasts in the CSF at diagnosis/relapse) and had received CNS directed medication associated with lowering the seizure threshold at the time of the event. There were no other grade 3 or 4 toxicities or CRS events.
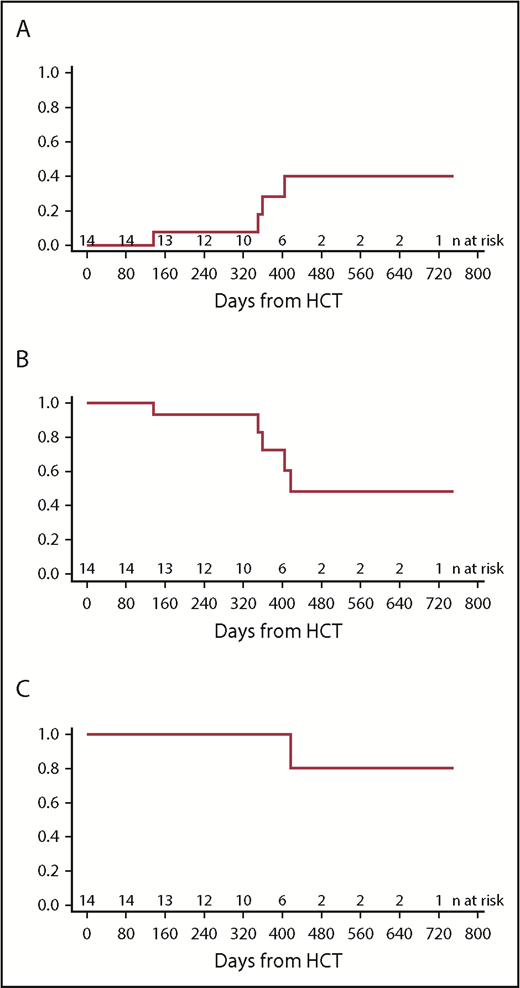
Fourteen of 15 patients were MRD negative following blinatumomab bridging therapy and proceeded to HCT. The median time from end of blinatumomab therapy to start of preparative regimen was 14 days, with a range of 1 to 35. All patients had successful neutrophil engraftment in the expected timeframe, with a median time of 19 days (range, 11-35). In the first 30 days after HCT, only 1 patient experienced any significant HCT related complication (respiratory distress from mucositis). Two of the 14 patients (14.3%) experienced grade II or III acute graft-versus-host disease (GVHD), while 3 of the 14 patients (21.4%) experienced extensive chronic GVHD; all of whom received alternative donor HCT. Four patients experienced a relapse of CD19+ ALL at a median time of 355 days post-HCT, for a cumulative incidence at 1-year post-HCT of 27.8% (Figure 1A-B); all 4 patients successfully achieved a subsequent remission with CD19+-directed therapy and remain in a CR at the time of this report. The 1-year OS was 93.3% (Figure 1C). There was no transplant-related mortality in the first 100 days. The causes of death included disease progression in 1 patient who did not proceed to HCT and complications related to chronic GVHD in a second patient.
Post-HCT outcomes. (A) Cumulative incidence of relapse. (B) LFS. (C) OS.
In recent years, there have been several reports on the safety and efficacy of blinatumomab for adult and pediatric patients with B-ALL,9-13 as well as data reflecting that blinatumomab provides better quality of life than standard chemotherapy.14 However, very few have addressed the treatment of MRD with blinatumomab,15,16 and there have been no reports of the treatment of MRD-positive pediatric leukemia. The impact of this therapy on post-HCT outcomes is still unclear. It is well established that outcomes for ALL patients with persistent MRD1,5,6 are poor and that MRD present prior to HCT is correlated with an LFS of 0% to 33%.3,4,17 In this multi-institutional retrospective study, we report the outcomes using blinatumomab as “bridging” therapy for pediatric patients who are MRD positive prior to HCT. We demonstrate that blinatumomab was safe and effective in pediatric leukemia, and more specifically, MRD was reduced to undetectable levels in most patients (93.3%) without serious infection or organ toxicity. Likewise, patients proceeded to definitive HCT therapy without delay, in some cases starting the myeloablative preparative regimen within a few hours of completing the blinatumomab infusion. In patients where the unrelated donor was not readily available, this approach provided the advantage of a low-toxicity therapeutic bridge while waiting for an alternative donor. Because blinatumomab activates the immune system and can result in cytokine release syndrome, there is some concern that any lymphocyte activation prior to HCT could negatively influence donor engraftment or perhaps cause greater rates of GVHD. However, all patients successfully engrafted and overall rates of grades II to IV acute GVHD and chronic GVHD were low, despite alternative donors being the prominent stem cell source. While this is a relatively small and retrospective study, these data represent the first report of blinatumomab bridging therapy as a safe and effective method to eliminate MRD prior to HCT for pediatric patients with B-ALL.
Authorship
Contribution: All authors contributed to the acquisition, analysis, or interpretation of the data for this article, revised the manuscript critically, approved the final version for publication, and agreed to be accountable for the results presented; and A.K.K. generated first and subsequent drafts of manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Amy K. Keating, University of Colorado School of Medicine, Department of Pediatrics, Children’s Hospital of Colorado, 13123 East 16th Ave, Aurora, CO 80045; e-mail: amy.keating@ucdenver.edu.