Key Points
ITK inhibitors perturb functional changes due to polarizing culture conditions in normal human tonsil CD4+ T cells.
Primary human PTCL cells alter their functional properties in culture and ITK inhibitors modify these changes.
Introduction
Normal immunity requires several effector CD4+ T-cell subsets (T helper 1 [Th1], Th2, Th9, Th17, follicular helper [Tfh] T cells, and regulatory T cells [Treg]), each of which expresses characteristic surface markers, cytokines, and transcription factors.1,2 The T-cell–specific kinase, interleukin-2–inducible T-cell kinase (ITK),3 which is activated through the T-cell receptor (TCR), and modulates calcium signaling,4 is involved in T-cell development, proliferation, and migration5-7 and is also an essential regulator of CD4+ T-cell differentiation to effector subsets.8 For example, ITK is required for Th2 and Th9 development and modifies the reciprocal balance between activating Th17 and suppressive Tregs.9
Peripheral T-cell lymphomas (PTCLs) are a diverse group of diseases10 with the major subtypes being angioimmunoblastic T-cell lymphoma (AITL) and PTCL not otherwise specified (PTCL-NOS). Clinical outcomes are generally poor with a 5-year overall survival of 25% to 35%.11 Gene expression profiling has demonstrated that AITL and ∼20% of PTCL-NOS are derived from Tfh cells,12,13 whereas some of the remaining cases of PTCL-NOS may arise from Th1 or Th2 cells. ITK is highly expressed in PTCL-NOS and AITL but not other subtypes,14 and the enzyme is activated by TCR signaling in T-cell lymphomas.15 There is interest in developing molecules that inhibit the kinase function of ITK, and it has recently been found that ibrutinib, which is clinically well tolerated and effective as an inhibitor of the B-cell homolog of ITK, Bruton tyrosine kinase, in some mature B-cell malignancies,16 also inhibits ITK.17
Small molecule ITK inhibitors (ITKi) have not previously been investigated for their effects on differentiation of either primary human tonsillar or PTCL CD4+ T cells. We demonstrated that in specific culture conditions primary human lymphoma cells had the potential to differentiate toward various functionally polarized states and that ITKi modify differentiation of both tonsillar T cells and lymphoma cells. These results have implications for the design of clinical trials using these small molecules in the treatment of PTCLs.
Methods
A detailed description of patients, cell culture, flow cytometric analyses, western blotting, and immunofluorescence microscopy is given in the supplemental Materials and methods.
Results and discussion
ITKi repress in vitro differentiation of normal tonsillar T cells
In order to assess the effects of small molecule ITKi on normal human T cells, we stimulated tonsillar CD4+ T cells with anti-CD3/anti-CD28/interleukin-12 (IL-12) to induce CD4+CXCR5hiPD-1hi cells, the phenotype of germinal center Tfh cells functionally associated with production of IL-21 early after immunization.18 The fraction of CD4+CXCR5hiPD-1hi T cells (supplemental Figure 1) increased from ∼10% (Figure 1A) to ∼30% (Figure 1B) with stimulation and was repressed, almost to baseline levels, by ITKi: ibrutinib (paired Student t test, P = .0048), PF-6465469 (P = .0068), BMS509744 (P = .026), and ONO7790500 (P = .037) (Figure 1C).
ITKi perturb in vitro functional polarization of tonsil CD4+T cells. (A) Flow cytometry dot plot showing PD-1 and CXCR5 expression of untreated tonsillar CD4+ T cells. (B) Flow cytometry dot plots showing PD-1 and CXCR5 expression of tonsillar CD4+ T cells treated with anti-CD3/anti-CD28 and IL-12. There was either no inhibitor included in the culture (dimethyl sulfoxide [DMSO]) or ibrutinib or ONO7790500. The numbers indicate the percentage of CD4+ T cells within the gate. (C) Percentage of CD4+CXCR5hiPD-1hi cells relative to cells stimulated with anti-CD3/anti-CD28 and IL-12 (red column). The percentage of cells without stimulation (Unstim; pink) or in stimulated cells treated with ITKi (shades of blue as shown in the legend) is indicated. Mean ± standard error of the mean (SEM). ITKi repress the fraction of CXCR5hiPD-1hi cells: ibrutinib (paired Student t test, P = .0048), PF-6465469 (P = .0068), BMS509744 (P = .026), and ONO7790500 (P = .037). (D) Flow cytometry dot plots showing IL-17A and FoxP3 expression following polarizing culture in the absence (DMSO) or presence of either ibrutinib or ONO7790500. The numbers indicate the percentage of CD4+ T cells within the quadrant. (E) Column charts show the percentage of CD4+FoxP3+ or CD4+IL-17A+ (mean ± SEM). No stimulation (pink), stimulation (red), and stimulation with either ibrutinib (light blue) or ONO7790500 (dark blue) are indicated. Th17-like cells were significantly reduced by ITKi (paired Student t test, ibrutinib, P = .02; ONO7790500, P = .03), whereas Treg-like cells were increased (ibrutinib, P = .04; ONO7790500, P = .03). (F) Flow cytometry dot plots showing IL-4 expression following polarizing culture in the absence (DMSO) or presence of either ibrutinib or ONO7790500. The numbers indicate the percentage of total CD4+ T cells within the quadrant. (G) Column chart shows the percentage of CD4+IL-4+ (mean ± SEM). No stimulation (pink), stimulation (red), and stimulation with either ibrutinib (light blue) or ONO7790500 (dark blue) are indicated. CD4+IL-4+ cells were significantly reduced by ITKi (ibrutinib, P = .01; ONO7790500, P = .01). For the flow cytometry experiments (A-G), the results shown are representative of 3 separate experiments. (H-I) Western blots showing total ITK and phosphorylated ITK in cells stimulated by anti-CD3/anti-CD28 in the absence or presence of 4 ITKi (PF-6465469, ibrutinib, ONO7790500, BMS509744) as indicated. GAPDH, glyceraldehyde-3-phosphate dehydrogenase, is a loading control. Patient 1 (H) and patient 2 (I). (J-K) Immunofluorescence microscopy of lymph node sections taken from patients 1 and 2. Magnification is indicated at the top right. Images at ×80 are taken from the area and are indicated by the white rectangle in the ×20 image. (J) Sections were stained with anti-CD4 and anti-BCL6. (K) Sections were stained with anti-CD4, anti-FoxP3, and anti-RORγt. *P < .05, **P < .01.
ITKi perturb in vitro functional polarization of tonsil CD4+T cells. (A) Flow cytometry dot plot showing PD-1 and CXCR5 expression of untreated tonsillar CD4+ T cells. (B) Flow cytometry dot plots showing PD-1 and CXCR5 expression of tonsillar CD4+ T cells treated with anti-CD3/anti-CD28 and IL-12. There was either no inhibitor included in the culture (dimethyl sulfoxide [DMSO]) or ibrutinib or ONO7790500. The numbers indicate the percentage of CD4+ T cells within the gate. (C) Percentage of CD4+CXCR5hiPD-1hi cells relative to cells stimulated with anti-CD3/anti-CD28 and IL-12 (red column). The percentage of cells without stimulation (Unstim; pink) or in stimulated cells treated with ITKi (shades of blue as shown in the legend) is indicated. Mean ± standard error of the mean (SEM). ITKi repress the fraction of CXCR5hiPD-1hi cells: ibrutinib (paired Student t test, P = .0048), PF-6465469 (P = .0068), BMS509744 (P = .026), and ONO7790500 (P = .037). (D) Flow cytometry dot plots showing IL-17A and FoxP3 expression following polarizing culture in the absence (DMSO) or presence of either ibrutinib or ONO7790500. The numbers indicate the percentage of CD4+ T cells within the quadrant. (E) Column charts show the percentage of CD4+FoxP3+ or CD4+IL-17A+ (mean ± SEM). No stimulation (pink), stimulation (red), and stimulation with either ibrutinib (light blue) or ONO7790500 (dark blue) are indicated. Th17-like cells were significantly reduced by ITKi (paired Student t test, ibrutinib, P = .02; ONO7790500, P = .03), whereas Treg-like cells were increased (ibrutinib, P = .04; ONO7790500, P = .03). (F) Flow cytometry dot plots showing IL-4 expression following polarizing culture in the absence (DMSO) or presence of either ibrutinib or ONO7790500. The numbers indicate the percentage of total CD4+ T cells within the quadrant. (G) Column chart shows the percentage of CD4+IL-4+ (mean ± SEM). No stimulation (pink), stimulation (red), and stimulation with either ibrutinib (light blue) or ONO7790500 (dark blue) are indicated. CD4+IL-4+ cells were significantly reduced by ITKi (ibrutinib, P = .01; ONO7790500, P = .01). For the flow cytometry experiments (A-G), the results shown are representative of 3 separate experiments. (H-I) Western blots showing total ITK and phosphorylated ITK in cells stimulated by anti-CD3/anti-CD28 in the absence or presence of 4 ITKi (PF-6465469, ibrutinib, ONO7790500, BMS509744) as indicated. GAPDH, glyceraldehyde-3-phosphate dehydrogenase, is a loading control. Patient 1 (H) and patient 2 (I). (J-K) Immunofluorescence microscopy of lymph node sections taken from patients 1 and 2. Magnification is indicated at the top right. Images at ×80 are taken from the area and are indicated by the white rectangle in the ×20 image. (J) Sections were stained with anti-CD4 and anti-BCL6. (K) Sections were stained with anti-CD4, anti-FoxP3, and anti-RORγt. *P < .05, **P < .01.
We focused further work on ibrutinib (ITK IC50 [50% inhibitory concentration] 2.2 nM) and ONO779050019,20 (ITK IC50 <4 nM). ONO7790500 is more selective than ibrutinib. ONO7790500 produces >80% inhibition of only 3/311 kinases at 0.3 μM,19,20 whereas ibrutinib targets 10 kinases with structurally similar ATP binding sites, including the B-cell homolog of ITK Bruton tyrosine kinase.21 At a concentration of 1 µM, neither inhibitor produced apoptosis above baseline after 1 day in culture (supplemental Figure 2A). TCR signaling through ITK also regulates cell proliferation.5 After 6 days of culture with ibrutinib or ONO7790500 (both agents at 0.2 µM), tonsillar CD4+ T-cell proliferation was severely repressed (supplemental Figure 2B).
Next, we investigated in vitro polarization of tonsillar CD4+ T-cell populations by determining changes in functionally important cytokines (ie, IL-17 and IL-4) and the transcription factor, FoxP3. Th17 and Treg cells are coordinately regulated,22 and ITK has an essential role in this process.9 We observed that ITKi-treated cultures showed significantly reduced Th17-like (CD4+IL-17A+) cells (paired Student t test, ibrutinib, P = .02; ONO7790500, P = .03), whereas Treg-like (CD4+FoxP3+) populations increased (ibrutinib, P = .04; ONO7790500, P = .03) (Figure 1D-E) in line with results from Itk-deficient mice. ITKi also repressed IL-4 expressing populations (ibrutinib, P = .01; ONO7790500, P = .01) (Figure 1F-G).
Perturbations of Tfh differentiation in Itk-deficient animals have not been reported, but here we show that ITKi suppressed CD4+CXCR5hiPD-1hi cells in culture at concentrations that do not have significant effects on apoptosis.
ITKi modify functional polarization of PTCL cells
To determine responses of human PTCL cells to ITKi, we investigated 2 cases (patient 1 with PTCL-NOS and patient 2 with AITL) from which it was possible to isolate large numbers of monoclonal CD4+ T cells (supplemental Figure 3) from pleural fluid (patient 1) or peripheral blood (patient 2). Both lymphomas demonstrated PD-1 and BCL6 expression consistent with the recently established category of Tfh lymphoma.10 Western blots showed expression of ITK (Figure 1H-I). Phosphorylated ITK was induced by anti-CD3/anti-CD28/IL-12 stimulation and repressed by ITKi (Figure 1H-I). Apoptosis was not increased above baseline by either ibrutinib or ONO7790500 at concentrations up to 1 µM after 3 days in culture, and both inhibitors repressed proliferation at 6 days in culture (supplemental Figure 2C).
By immunofluorescence, we observed CD4+BCL6+, CD4+RORγt+, and CD4+FoxP3+ populations in patients' lymph node (Figure 1J-K; supplemental Figure 4) consistent with functional heterogeneity being present in vivo. We investigated the ability of patient cells to respond to anti-CD3/anti-CD28/IL-12 (Figure 2A-B) and found that after 3 days of culture the fraction of CD4+PD-1hiCXCR5hi cells increased, and this was significantly repressed by ibrutinib (paired Student t test, patient 1, P = .017; patient 2, P = .016) and ONO7790500 (patient 1, P = .010; patient 2, P = .027).
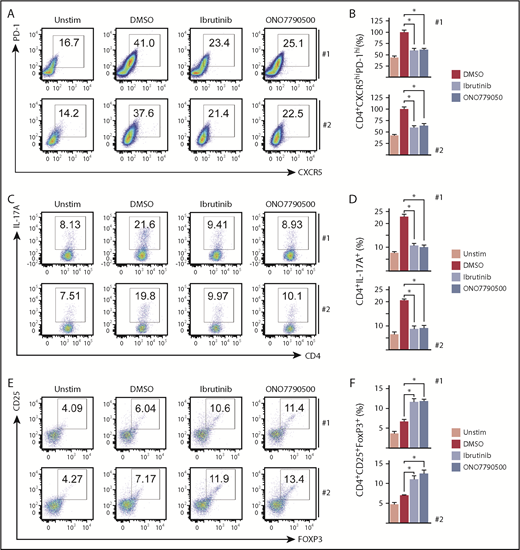
ITKi alter functional polarization of PTCL cells. (A) Flow cytometry dot plots showing PD-1 and CXCR5 expression of unstimulated cells and cells following 4 days’ stimulation with anti-CD3/anti-CD28/IL-12 in the absence (DMSO) or presence of ITKi (ibrutinib or ONO7790500) for patients 1 and 2. Numbers indicate the percentage of CD4+ T cells within the gate. (B) Column plots for patients 1 and 2 showing Tfh-like (CD4+CXCR5hiPD-1hi) cells as a percentage of total CD4+ cells relative to the percentage obtained with anti-CD3/anti-CD28 and IL-12 in the absence of drug (DMSO) (red columns). Blue columns represent drug treatment with ibrutinib or ONO7790500 as indicated. Drug treatment caused significant reduction in percentage of CD4+CXCR5hiPD-1hi cells. Ibrutinib (paired Student t test, patient 1 P = .017; patient 2, P = .016) and ONO7790500 (patient 1, P = .010; patient 2, P = .027). (C) Flow cytometry dot plots showing CD4 and IL-17A expression of unstimulated cells and cells following 2 days’ stimulation under Th17 polarizing conditions in the absence (DMSO) or presence of ITKi (ibrutinib or ONO7790500) for patients 1 and 2. Numbers indicate the percentage of CD4+ T cells within the gate. (D) Column plots for patients 1 and 2 showing CD4+IL-17A+ cells as a percentage of total CD4+ cells. Blue columns represent drug treatment with ibrutinib or ONO7790500 as indicated. CD4+IL-17A+ cells as a percentage of total CD4+ cells, without stimulation (Unstim), is represented by the pink column. Drug treatment caused significant reduction in CD4+IL-17A+ cells. Ibrutinib (paired Student t test, patient 1, P = .017; patient 2, P = .016) and ONO7790500 (patient 1, P = .013; patient 2, P = .013). (E) Flow cytometry dot plots showing FoxP3 and CD25 expression of unstimulated cells and cells following 2 days’ stimulation under Treg polarizing culture conditions in the absence (DMSO) or presence of ITKi (ibrutinib or ONO7790500) for patients 1 and 2. Numbers indicate the percentage of CD4+ T cells within the gate. (F) Column plots for patients 1 and 2 showing CD4+CD25+FoxP3+ Treg-like cells as a percentage of total CD4+ cells. Blue columns represent drug treatment with ibrutinib or ONO7790500 as indicated. Unstimulated cells are represented by the pink column. Drug treatment caused a significant increase in percentage of Treg-like cells. Ibrutinib (paired Student t test, patient 1, P = .045; patient 2, P = .043) and ONO7790500 (patient 1, P = .021; patient 2, P = .034). *P < .05.
ITKi alter functional polarization of PTCL cells. (A) Flow cytometry dot plots showing PD-1 and CXCR5 expression of unstimulated cells and cells following 4 days’ stimulation with anti-CD3/anti-CD28/IL-12 in the absence (DMSO) or presence of ITKi (ibrutinib or ONO7790500) for patients 1 and 2. Numbers indicate the percentage of CD4+ T cells within the gate. (B) Column plots for patients 1 and 2 showing Tfh-like (CD4+CXCR5hiPD-1hi) cells as a percentage of total CD4+ cells relative to the percentage obtained with anti-CD3/anti-CD28 and IL-12 in the absence of drug (DMSO) (red columns). Blue columns represent drug treatment with ibrutinib or ONO7790500 as indicated. Drug treatment caused significant reduction in percentage of CD4+CXCR5hiPD-1hi cells. Ibrutinib (paired Student t test, patient 1 P = .017; patient 2, P = .016) and ONO7790500 (patient 1, P = .010; patient 2, P = .027). (C) Flow cytometry dot plots showing CD4 and IL-17A expression of unstimulated cells and cells following 2 days’ stimulation under Th17 polarizing conditions in the absence (DMSO) or presence of ITKi (ibrutinib or ONO7790500) for patients 1 and 2. Numbers indicate the percentage of CD4+ T cells within the gate. (D) Column plots for patients 1 and 2 showing CD4+IL-17A+ cells as a percentage of total CD4+ cells. Blue columns represent drug treatment with ibrutinib or ONO7790500 as indicated. CD4+IL-17A+ cells as a percentage of total CD4+ cells, without stimulation (Unstim), is represented by the pink column. Drug treatment caused significant reduction in CD4+IL-17A+ cells. Ibrutinib (paired Student t test, patient 1, P = .017; patient 2, P = .016) and ONO7790500 (patient 1, P = .013; patient 2, P = .013). (E) Flow cytometry dot plots showing FoxP3 and CD25 expression of unstimulated cells and cells following 2 days’ stimulation under Treg polarizing culture conditions in the absence (DMSO) or presence of ITKi (ibrutinib or ONO7790500) for patients 1 and 2. Numbers indicate the percentage of CD4+ T cells within the gate. (F) Column plots for patients 1 and 2 showing CD4+CD25+FoxP3+ Treg-like cells as a percentage of total CD4+ cells. Blue columns represent drug treatment with ibrutinib or ONO7790500 as indicated. Unstimulated cells are represented by the pink column. Drug treatment caused a significant increase in percentage of Treg-like cells. Ibrutinib (paired Student t test, patient 1, P = .045; patient 2, P = .043) and ONO7790500 (patient 1, P = .021; patient 2, P = .034). *P < .05.
We next determined the effects of ITKi on functional polarization of malignant T cells. The proportion of CD4+IL-17A+ increased under polarizing culture conditions and was significantly reduced by ibrutinib (paired Student t test, patient 1, P = .017; patient 2, P = .016) and ONO7790500 (patient 1, P = .013; patient 2, P = .013) (Figure 2C-D). Conversely, the addition of ibrutinib induced CD4+CD25+FoxP3+ cells (paired Student t test, patient 1, P = .045; patient 2, P = .043) and ONO7790500 (patient 1, P = .021; patient 2, P = .034) (Figure 2E-F).
CD4+ T-cell phenotypes are not fixed, but the various subsets can interconvert, suggesting the concept of plasticity.2 We propose that PTCL cells are capable of functional polarization under specific culture conditions, features reminiscent of the plasticity observed between normal CD4+ T-cell subsets, and that ITK has a role in regulating this process. However, it is also possible that culture conditions allowed outgrowth of polarized cells within the lymphoma, but reduction in proliferation combined with the observed changes in cell phenotype supports the view that T-cell lymphoma cells have the potential to change their functional properties.
A pilot clinical trial has recently been reported in which the effects of ibrutinib on, mainly, cutaneous T-cell lymphoma were investigated.23 Patients were not selected for ITK expression, and the study only showed limited clinical activity, and the efficacy of this drug in patients with Tfh lymphoma remains an open question. Future trial designs might combine standard-of-care chemotherapy, to provide cytotoxic activity, with ITKi to repolarize the malignant T cells and disrupt tumor/microenvironment crosstalk. Epigenetic regulation is crucial to T-cell differentiation,24 and the hypomethylating agent, azacytidine, produced remissions in AITL in a recent clinical trial.25 The combined effects of ITKi and azacytidine might profoundly alter the behavior of malignant T cells.
We report a study of 2 patients, and although our results need confirmation with more cases, we suggest that ibrutinib or other ITKi might be a route to perturb differentiation of ITK-expressing Tfh lymphoma to promote suppressive Treg cells at the expense of activating Tfh or Th17 cells for therapeutic benefit. This novel concept requires testing in stratified clinical trials enrolling patients with PTCLs defined on molecular criteria.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors are grateful to the patients whose generosity allowed the studies reported here. ONO7790500 was a gift from Toshio Yoshizawa, ONO Pharmaceuticals.
This work was supported by a studentship from the Higher Committee for Education Development in Iraq and Erbil Polytechnic University, Erbil, Iraq (S.M.) and a clinical research fellowship from the Ernest and Helen Scott Haematological Research Institute (R.L.A.).
Authorship
Contribution: S.M. designed and carried out experiments and analyzed data; M.C. analyzed data; S.D.W. designed experiments and wrote the paper; and R.L.A. and M.J.A. analyzed data and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Simon D. Wagner, Leicester Cancer Research Centre, Room 104, Hodgkin Building, University of Leicester, Lancaster Rd, Leicester LE1 7HB, United Kingdom; e-mail: sw227@le.ac.uk.

![Figure 1. ITKi perturb in vitro functional polarization of tonsil CD4+ T cells. (A) Flow cytometry dot plot showing PD-1 and CXCR5 expression of untreated tonsillar CD4+ T cells. (B) Flow cytometry dot plots showing PD-1 and CXCR5 expression of tonsillar CD4+ T cells treated with anti-CD3/anti-CD28 and IL-12. There was either no inhibitor included in the culture (dimethyl sulfoxide [DMSO]) or ibrutinib or ONO7790500. The numbers indicate the percentage of CD4+ T cells within the gate. (C) Percentage of CD4+CXCR5hiPD-1hi cells relative to cells stimulated with anti-CD3/anti-CD28 and IL-12 (red column). The percentage of cells without stimulation (Unstim; pink) or in stimulated cells treated with ITKi (shades of blue as shown in the legend) is indicated. Mean ± standard error of the mean (SEM). ITKi repress the fraction of CXCR5hiPD-1hi cells: ibrutinib (paired Student t test, P = .0048), PF-6465469 (P = .0068), BMS509744 (P = .026), and ONO7790500 (P = .037). (D) Flow cytometry dot plots showing IL-17A and FoxP3 expression following polarizing culture in the absence (DMSO) or presence of either ibrutinib or ONO7790500. The numbers indicate the percentage of CD4+ T cells within the quadrant. (E) Column charts show the percentage of CD4+FoxP3+ or CD4+IL-17A+ (mean ± SEM). No stimulation (pink), stimulation (red), and stimulation with either ibrutinib (light blue) or ONO7790500 (dark blue) are indicated. Th17-like cells were significantly reduced by ITKi (paired Student t test, ibrutinib, P = .02; ONO7790500, P = .03), whereas Treg-like cells were increased (ibrutinib, P = .04; ONO7790500, P = .03). (F) Flow cytometry dot plots showing IL-4 expression following polarizing culture in the absence (DMSO) or presence of either ibrutinib or ONO7790500. The numbers indicate the percentage of total CD4+ T cells within the quadrant. (G) Column chart shows the percentage of CD4+IL-4+ (mean ± SEM). No stimulation (pink), stimulation (red), and stimulation with either ibrutinib (light blue) or ONO7790500 (dark blue) are indicated. CD4+IL-4+ cells were significantly reduced by ITKi (ibrutinib, P = .01; ONO7790500, P = .01). For the flow cytometry experiments (A-G), the results shown are representative of 3 separate experiments. (H-I) Western blots showing total ITK and phosphorylated ITK in cells stimulated by anti-CD3/anti-CD28 in the absence or presence of 4 ITKi (PF-6465469, ibrutinib, ONO7790500, BMS509744) as indicated. GAPDH, glyceraldehyde-3-phosphate dehydrogenase, is a loading control. Patient 1 (H) and patient 2 (I). (J-K) Immunofluorescence microscopy of lymph node sections taken from patients 1 and 2. Magnification is indicated at the top right. Images at ×80 are taken from the area and are indicated by the white rectangle in the ×20 image. (J) Sections were stained with anti-CD4 and anti-BCL6. (K) Sections were stained with anti-CD4, anti-FoxP3, and anti-RORγt. *P < .05, **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/3/5/10.1182_bloodadvances.2018027821/2/m_advances027821f1.png?Expires=1769240730&Signature=jaCOaIeFlsTrhC8-a1cm-y34Tj-waR9D4peB7G61Mdeq5HzXkzf0hneu2IPExh5UPkqgtKeyv3RkqucsJAkZk-1k4qtYhM~rO0Qlf-q1HqB1VDl267NVHzepo4crX-zJKnx7BHYisJu6U0K06B2mtKKtPxh8C9gP8XuFX3q42B4dLtEbi29zohpdtJZA9B2qDjF0TFSZELgFF7n2YzmuTHQym8kqDpoj2Kkz~MIXGStVMmsowucRyqrWPfdrl1N24GiplijRfkkck7OWTnNId0yntyzG6KgRB3RMl6-dgkbAEiP9Qad13ErA8ojrYQe5flTHV84tCyKjjkWTvu8jPA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)