Key Points
rVWF contains no factor VIII but has higher VWF multimers and a longer half-life than pdVWF.
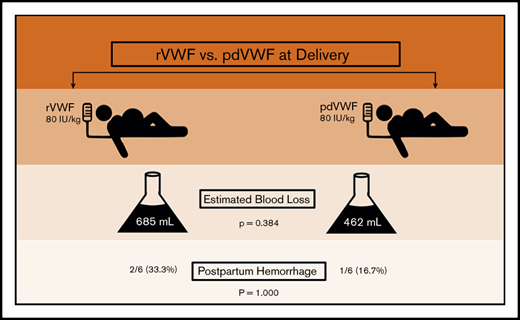
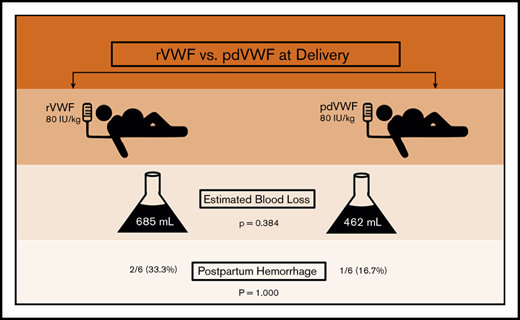
PPH occurred in 25% of women with VWD, despite a higher VWF dose (80 IU/kg).
Abstract
von Willebrand disease (VWD) is a congenital bleeding disorder characterized by deficient or defective von Willebrand factor (VWF). Among women with VWD, postpartum hemorrhage (PPH) is common. Treatment options at delivery include plasma-derived VWF (pdVWF) and recombinant VWF (rVWF). However, limited data are available regarding their efficacy. We conducted a retrospective observational study comparing PPH in women with VWD treated at the Hemophilia Center of Western Pennsylvania between 1 February 2017 and 31 January 2018 with either rVWF or pdVWF. We compared postpartum outcomes, including PPH frequency and estimated blood loss (EBL) at delivery. There were a total of 12 deliveries, 7 vaginal and 5 cesarean. At delivery and for 3 days postpartum, 6 women received 80 IU/kg of rVWF and 6 received 80 IU/kg of pdVWF, based on prepregnancy weight, insurance, and/or patient choice. Treatment groups had similar demographics, including median age (32.0 vs 27.0 years; P = .075), bleeding scores (3.0 vs 3.5; P = .734), and prepregnancy body mass index (29.0 vs 29.2 kg/m2; P = .691). PPH occurred in 3 (25.0%) of 12 deliveries, with no difference by treatment group (2 of 6 rVWF vs 1 of 6 pdVWF; P = 1.000) and no difference in EBL by treatment group (685 vs 462 mL; P = .384) or delivery type (vaginal, P = .722 vs cesarean, P = .531). In summary, PPH occurred in one-fourth of the deliveries in women with VWD, despite a higher dose (80 IU/kg) of rVWF or pdVWF. Future trials are needed to develop and assess novel strategies to prevent PPH in VWD.
Introduction
von Willebrand disease (VWD) is the most common congenital bleeding disorder, affecting 1% of the population, and is characterized by deficient or defective von Willebrand factor (VWF).1 Among women with VWD, reproductive tract bleeding is common, including menorrhagia and postpartum hemorrhage (PPH).2 Women with VWD have a 1.5-fold greater risk of PPH, and the incidence of PPH in this group ranges from 40% to 60%.3,4 PPH is associated with an increased risk for significant morbidity, including increased risk of postpartum depression, iron deficiency, length of hospital stay, and difficulties with lactation, as well as increased mortality.5
Pregnancy is associated with dramatic increases in VWF and factor VIII (FVIII). These physiologic increases are also seen in women with VWD, primarily in those with type 1 VWD, resulting in normalization of VWF and FVIII in many patients by the time of delivery.6 However, most women with VWD do not achieve levels that occur during normal pregnancy.7 Current guidelines suggest evaluating VWF activity, or ristocetin cofactor (VWF/RCo), during the eighth month of gestation, and, if it is found to be <0.50 IU/mL, treating at delivery with 50 IU/kg of plasma-derived VWF (pdVWF).8 Despite factor replacement, PPH continues to occur in women, particularly those with the lowest VWF/RCo before delivery, even when VWF levels have normalized.9,10 Women with VWD do not achieve VWF levels comparable to those of controls without VWD, even when they receive factor replacement,9 and experience greater blood loss at delivery.3,9,10 One possible explanation for the high incidence of PPH despite factor replacement is that the current dosing strategy does not account for the 1.5-fold increase in blood volume during pregnancy.11 This approach, however, is the basis for current dosing strategies in children and obese adults with congenital bleeding disorders.12,13 To account for this physiologic change in blood volume during pregnancy, a VWF dose of 80 IU/kg, which is a dose ∼1.5-fold higher than that currently recommended, has been proposed14 and was initiated at our institution and assessed as an objective of this study.
In the United States, currently approved factor replacement options for VWD include human pdVWF (Humate) and recombinant human VWF (rVWF; Vonvendi). These products differ in multimeric composition, FVIII, and half-life. Specifically, compared with pdVWF, rVWF contains a higher amount of high–molecular weight multimers, no FVIII, and a 1.4-fold greater half-life.15-17 Whether these differences improve clinical hemostasis with rVWF compared with pdVWF remains unknown, because no direct comparisons between the products have been performed. A recent case series of 2 patients with severe VWD, 1 with type 3 VWD requiring prophylaxis for severe mucosal bleeding and the other with type 2A undergoing knee replacement surgery, reported higher FVIII and VWF levels with longer half-life compared with historical treatment of patients with pdVWF and, in the latter patient, higher and longer-lasting high–molecular weight multimers vs those with pdVWF for similar surgery 3 years previously.18 Whether rVWF reduces postpartum bleeding to a greater degree than pdVWF has not been studied. We, therefore, conducted a retrospective observational study of delivery outcomes in women with VWD at a single institution, comparing rVWF and pdVWF.
Methods
We conducted a retrospective observational study to compare postpartum outcomes in women with VWD treated at the Hemophilia Center of Western Pennsylvania (Pittsburgh, PA) who were admitted for childbirth between 1 February 2017 and 31 January 2018 and received rVWF or pdVWF. Primary PPH was defined per the American College of Obstetrics and Gynecology at the time of this study (specifically, 24-hour blood loss >500 mL after vaginal delivery or >1000 mL after cesarean delivery),19 although this definition was updated in 2017 to >1000 mL in the first 24 hours after either vaginal or cesarean delivery.20 Blood loss at delivery was calculated as total blood loss, combining blood loss subtracted from an under-buttock drape (amniotic fluid, urine, stool) with blood loss from weighed laps, sponges, and pads, and recorded in the medical record. Type of factor administered at delivery was based on insurance coverage and/or patient choice, per standard of care; in no case was the decision based on clinical assessment. We reviewed deidentified medical records of all women with VWD admitted for childbirth receiving rVWF or pdVWF during the 12-month period. The study was approved by the University of Pittsburgh Institutional Review Board as an exempt study (PRO18010198).
Data were evaluated by descriptive statistics, including mean, median, and standard deviation or frequency (percentage). Clinical variables, including age, race, VWD type, bleeding score, bleeding history, body surface area, estimated blood loss, and comorbidity, were compared, if continuous, by Student t test and, if discrete, by χ2 or Fisher’s exact test. P < .05 was considered statistically significant.
Results
A total of 12 women with VWD, 11 with type 1 and 1 with type 2B, underwent delivery during the 12-month time period, including 7 by vaginal and 5 by cesarean section. All 12 received VWF clotting factor concentrate at delivery, including 80 IU/kg of rVWF in 6 and 80 IU/kg of pdVWF in 6, with the first dose administered just before delivery and 1 additional daily dose each on days 1 and 2 postpartum. The dose was based on prepartum weight, and the type of factor treatment was based on insurance and/or patient choice.
Demographic data were comparable between treatment groups, by age, race, and blood type (Table 1). The baseline prepregnancy bleeding history was similar between groups, as was median prepregnancy bleeding score (3.0 vs 3.5; P = .734). There were no differences in frequency of comorbidities between groups, including anemia (P = .455) diabetes (P = 1.0000), hypertension (P = 1.000) obesity (P = 1.000), and smoking (P = 1.000). Only 1 patient, with 2B VWD, had experienced PPH before the study; she received rVWF in the study.
By the eighth month of pregnancy, VWF levels increased from baseline to the same degree in both groups. The VWF/RCo activity increased nearly 2.0-fold from prepregnancy baseline; VWF antigen increased ∼2.5-fold from baseline; FVIII clotting activity increased ∼2.2-fold from baseline (Table 1). Similarly, body mass index increased from baseline by ∼1.2-fold at the eighth month and by 1.2-fold at delivery in the 2 groups, respectively.
At delivery, PPH occurred in 3 (25.0%) of the 12 women, despite a higher dose of VWF (ie, 80 IU/kg), with no difference by VWF treatment type (P = 1.000), and in 2 of 6 in the rVWF-treated group, compared with 1 of 6 in the pdVWF-treated group (Table 2). The proportion of patients with PPH also did not differ within treatment groups by the type delivery (vaginal, P = 1.000 vs cesarean section, P = 1.000), nor was the median EBL different between groups (500 vs 375 mL; P = .722 in vaginal and 1000 vs 950 mL; P = .531 in cesarean deliveries, respectively). The treatment groups had comparable median hemoglobin (11.9 vs 11.6 g/dL; P = .845) and platelet counts (177 vs 185 × 103/μL; P = .618) at delivery, and none required transfusion. The median length of hospital stay seemed longer in the rVWF group (4.0 vs 2.5 days; P = .078). One patient had uterine atony, requiring uterotonics, and 1 had a perineal tear, but none required hysterectomy as a result of PPH. One patient developed HELLP syndrome (hemolysis, elevated liver enzymes, low platelet count), which resolved. None developed coagulopathy or secondary PPH. None of the patients received oral tranexamic acid (Lysteda) or IV tranexamic acid (Cyclokapron). There were no significant differences in delivery complications by treatment group (P = 1.000), nor were there any thromboses or deaths.
Discussion
We found that one-fourth of women with VWD developed PPH, despite a higher VWF dose of 80 IU/kg, with no difference in estimated blood loss by type of VWF factor replacement (rVWF vs pdVWF). Although the number of treated patients was small, there were no significant differences in baseline characteristics, bleeding score, VWF level, or medical comorbidities between the groups that might have contributed to differences in their delivery outcomes. One fourth of these women experienced blood loss meeting criteria for PPH per 2017 US American College of Obstetrics and Gynecology guidelines, a rate observed in only 3% to 5% of non-VWD obstetric patients.18 These data confirm that despite pregnancy-associated increases in VWF levels, and despite higher VWF dosing of 80 IU/kg, with rVWF or pdVWF at delivery, postpartum bleeding occurs. Furthermore, there is no apparent relationship between VWF level and PPH risk. Although VWF levels were not monitored in this study, this lack of correlation between bleeding and VWF risk at delivery has been shown by others.3,21 We conclude that VWF replacement, whether rVWF or pdVWF or at high dose, is not sufficient to prevent PPH in women with VWD. Clearly, novel approaches are needed to reduce PPH and reduce PPH in women with VWD.
The question is why VWF at a higher dose (ie, 80 IU/kg), whether rVWF or pdVWF, failed to prevent PPH. Some potential explanations include the concomitant bleeding risk in patients with an underlying bleeding disorder together with well-established acquired coagulopathies associated with pregnancy, including the dilutional coagulopathy associated with the increased blood volume of pregnancy,11,14 rapid hormonal-associated decrease in VWF and FVIII at delivery,3,21 platelet functional defects associated with pregnancy,22 and/or activation of the fibrinolytic system within the first 3 hours of delivery.23 If PPH in women with VWD is a combined coagulation disorder (ie, a result of defective hemostasis associated with VWD and defective hemostasis associated with pregnancy), perhaps a combined approach is needed to prevent PPH. Specifically, in addition to VWF replacement, use of an antifibrinolytic agent within the first 3 hours of delivery might be helpful. In that regard, IV tranexamic acid, which inhibits plasmin-mediated fibrinogen and fibrin breakdown, was shown to reduce blood loss and mortality after trauma in the CRASH-2 trial24 and PPH and PPH-related mortality in pregnant women with PPH in the WOMAN trial, when administered IV within 3 hours of delivery.25 Although the latter trial showed tranexamic acid was effective in women without bleeding disorders, it suggests the potential for IV tranexamic acid administered within 3 hours of delivery to prevent PPH in women with VWD, for which a trial is planned (registered at www.clinicaltrials.gov as #NCT04344850).
This study has several limitations. This was a small retrospective observational study, which limits power and applicability, was subject to confounding, and has insufficient capacity to establish causality. Furthermore, the measurement of estimated blood loss by which PPH was quantitated by weighing blood-soaked drapes, pads, and towels may have been subject to differences between the delivering obstetrician, nurse, or anesthesiologist and may be associated with variability and error. Finally, no prospective coagulation monitoring of VWF levels was performed in this observational study, limiting conclusions drawn from the dosing schema.
Despite these limitations, this study demonstrates the first comparison of rVWF and pdVWF in the postpartum setting and confirms that although rVWF and pdVWF dosed at 80 IU/kg, based on the increase in blood volume, were safe, neither were effective in preventing PPH at delivery in women with VWD. Future studies are needed to evaluate novel strategies. One such strategy, the IV administration of tranexamic acid within 3 hours of delivery, shown to prevent PPH in women without coagulation disorders, is a next step in the quest to prevent PPH in women with VWD.
The authors are available for data and protocol sharing via e-mail to the corresponding author, Margaret V. Ragni (ragni@pitt.edu).
Acknowledgments
The authors thank Jonathan Yabes for his statistical expertise and advice on data analysis.
This study was supported by the Pennsylvania Department of Health (Harrisburg PA), State Support of Hemophilia Center of Western Pennsylvania (41000058531), and Health Resources and Services Administration Federal Hemophilia Treatment.
Authorship
Contribution: N.M. and M.V.R. contributed to the study design, data acquisition, data interpretation, analysis, and preparation of the manuscript.
Conflict-of-interest disclosure: M.V.R. reports research support from Alnylam/Sanofi, Biomarin, Bioverativ, Sangamo, SPARK, and the American Thrombosis Hemostasis Network; advisory board consulting for Alnylam/Sanofi, Biomarin, Bioverativ, and SPARK; and nonfinancial support (study drug) from Shire/Takeda. N.M. declares no competing financial interests.
Correspondence: Margaret V. Ragni, Division Hematology/Oncology, University of Pittsburgh, Hemophilia Center of Western PA, 3636 Blvd of the Allies, Pittsburgh, PA 15213-4306; e-mail: ragni@pitt.edu.