Key Points
HLA-DR, HLA-DQ or nonpermissive HLA-DP mm is associated with a survival and relapse benefit after haploidentical transplantation using PTCy.
HLA mm at the A locus is associated with an increased risk of chronic GVHD.
Abstract
HLA disparity is the major predictor of outcome following unrelated donor (UD) transplantation, where a single mismatch (mm) at the HLA-A, HLA-B, HLA-C, or HLA-DRB1 locus leads to increased mortality, and mismatching at multiple loci compounds this effect. In contrast, HLA disparity has not been shown to increase mortality in the context of haploidentical transplant using posttransplant cyclophosphamide (PTCy). To better define the consequences of loci-specific HLA mm, we analyzed 208 consecutive patients undergoing haploidentical transplantation for hematologic malignancy using PTCy at our institution (median age, 52 years [range, 19-75 years]; peripheral blood stem cell, 66%; reduced-intensity conditioning, 59%). Median follow-up was 65.4 months (range, 34.3-157.2 months). In univariate analysis, a single class II HLA mm at HLA-DR, HLA-DQ or a nonpermissive (np) HLA-DP mm had a protective effect on disease-free and overall survival (OS), primarily a result of reduced relapse risk. Furthermore, this survival effect was cumulative, so that patients with 3 class II mm (HLA-DR, HLA-DQ, and np HLA-DP) had the best OS. In multivariate analysis, HLA-DR mm and np HLA-DP mm were both independently associated with improved OS (hazard ratio [HR], 0.43; P =.001; and HR, 0.47; P =.011, respectively). In contrast, single or multiple mm at HLA-A, HLA-B, or HLA-C loci had no effect on acute graft-versus-host disease (GVHD), nonrelapse mortality (NRM), relapse, or survival, although the presence of an HLA-A mm was associated with increased chronic GVHD incidence. The association of class II mm with lower relapse occurred without a corresponding increase in NRM or acute or chronic GVHD. These findings will require validation in larger registry studies.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative therapy for high-risk hematologic malignancies that are not curable by chemotherapy alone. Historically, the ability to offer HCT to all patients was limited by the availability of an HLA-matched related or unrelated donor (UD). With the administration of posttransplant cyclophosphamide (PTCy) as a strategy for graft-versus-host disease (GVHD) prophylaxis, haploidentical transplantation (haplo) has become an increasingly used donor source for patients lacking a suitably matched donor, leading to comparable transplant outcomes.1,2
In the setting of UD transplantation utilizing conventional GVHD prophylaxis, HLA disparity is the major predictor of transplant outcome. In this context, a single allele– or antigen-level mismatch (mm) at the HLA-A, HLA-B, HLA-C, or HLA-DRB1 locus leads to increased mortality, and mismatching at multiple loci compounds this effect.3-5 Although mismatching at HLA-DQB1 and HLA-DPB1 has no apparent effect on survival in UD transplantation, the presence of a nonpermissive (np) mm according to the T-cell epitope (TCE) grouping6 results in excess nonrelapse and all-cause mortality.7,8 Therefore, optimal transplant outcome in UD transplantation is best achieved when donor and recipient are allele-level matched at HLA-A, HLA-B, HLA-C, and HLA-DR while avoiding an np HLA-DP mm.
In contrast to UD transplantation, there is no clear consensus regarding the consequences of HLA disparity in the context of haplo transplantation using PTCy, with most published studies showing no correlation of the number of HLA mm’s with GVHD, relapse, all-cause mortality, or nonrelapse mortality (NRM).9-11 In this study, we analyzed the contribution of mm at individual HLA loci on the incidence of acute and chronic GVHD, relapse/progression, NRM, and survival following haplo transplant with PTCy.
Methods
Patients and donors
Institutional review board approval was granted for this retrospective review of 208 consecutive patients who underwent a T-cell replete haplo-hematopoietic stem cell transplant for hematologic malignancy, transplanted between October 2005 and December 2016. Baseline characteristics were prospectively recorded in our institutional database, and events (relapse, death, cause of death, occurrence, and grading of acute and chronic GVHD) were entered into the database in real time. These data were retrospectively extracted from the database at the time of analysis.
Donors could be first- or second-degree relatives, including half-siblings, with 2 to 5 antigen or allelic mismatches at HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1 in either the graft-versus-host (GVH) or host-versus-graft direction. Donors were required to share ≥1 matched allele at each HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1 locus.
Histocompatibility testing
High-resolution HLA typing was performed on all donors and recipients at HLA-A, HLA-B, HLA-C, HLA-DRB1, HLA-DQB1, and HLA-DPB1. HLA type was determined using Sanger sequencing of at least exons 2 and 3 of class I loci and at least exon 2 of class II loci (GenDx, Utrecht, The Netherlands). HLA-DPB1 TCE permissivity was determined using the algorithm described by Crivello et al.12
Panel reactive antibody was evaluated on single-antigen class I and II beads (One Lambda, Canoga Park, CA). Mean fluorescence intensity of donor-specific antibody >1000 was considered clinically relevant. All haploidentical donors were evaluated by flow cytometric crossmatch. Positive flow cytometric crossmatch in the presence of donor-specific antibody was considered a contraindication to transplant.
Transplant
Haplo transplants were performed following nonmyeloablative (NMA)2 or myeloablative (MA)13,14 conditioning with PTCy as previously published. T-cell–replete bone marrow or peripheral blood stem cell (PBSC) grafts were administered on day 0 (CD34 dose capped at 5 × 106/kg), followed by PTCy on days +3 and +4. Tacrolimus and mycophenolate mofetil were started on day +5. Mycophenolate mofetil was stopped without taper on day +35. In the absence of GVHD, tacrolimus was discontinued on day +180. Filgrastrim was given from day +5 until neutrophil recovery.
Definitions and study end points
Primary outcomes analyzed were overall survival (OS), disease-free survival (DFS; survival without evidence of active malignancy after transplantation), relapse/progression of malignancy, and NRM. Acute GVHD was classified as clinically significant (grades 2-4) or severe (grades 3-4).15 Chronic GVHD was classified as mild, moderate, or severe by National Institutes of Health consensus criteria.16 Acute and chronic GVHD were evaluated and graded by a single practitioner within the program. NRM and relapse/progression were treated as competing risks.
Statistical methods
Cumulative incidences (CIs) of NRM, relapse/progression, acute GVHD, and chronic GVHD were computed to account for presence of competing risks.17 Probabilities of OS and DFS were estimated using the Kaplan-Meier method. We performed log-rank test and Gray’s test to compare survival probabilities and CIs, respectively. We used the Cox proportional hazards models to examine effects of HLA loci mm on hazard functions for OS and DFS, as well as on cause-specific hazard functions for GVHD, relapse/progression, and NRM, while adjusting for significant patient-, disease-, and transplant-related factors. We considered the following variables in multivariate analysis (MVA): HLA-DR mm, np HLA-DP mm, age (<55 years vs ≥55 years), recipient sex, diagnosis (acute myeloid leukemia [AML], acute lymphoblastic leukemia [ALL], or other), stem cell source (bone marrow or PBSC), intensity (MA vs NMA), Disease Risk Index (DRI) (high/very high, low/intermediate), HCT-specific comorbidity index (0-2 vs ≥3), donor age (<40 years vs ≥40 years), donor sex, cytomegalovirus (CMV) (D− R+, other), ABO compatibility (compatible, minor incompatible, or major incompatible), donor relationship (child, sibling, or parent), and year of transplantation (2005-2010, 2011-2013, and 2014-2016). The forward stepwise selection algorithm was implemented for each end point, and variables were selected by the 5% significance criterion. The final Cox models for OS, DFS, NRM, and relapse/progression were constructed by including all the variables significant for ≥1 end point. The proportional hazard assumption for a covariate Z was assessed by creating and testing the time-dependent variable Z×log(t). All covariates in the final Cox models passed the tests of proportionality. Statistical analysis was performed using the software SAS (version 9.4, the SAS Institute, Cary, NC) and the R package “cmprsk” (www.r-project.org).
Results
Patient, disease, and transplant characteristics
Baseline patient, disease, and transplant characteristics are shown in Table 1. Median (range) recipient age was 52 (19-75) years, and 54% were male. HCT-specific comorbidity index was ≥3 in 46%. Patients were most frequently transplanted for AML (34%), non-Hodgkin lymphoma/Hodgkin disease/chronic lymphocytic leukemia (25%), myelodysplastic/myeloproliferative neoplasms/chronic myeloid leukemia (20%), and ALL (17%). DRI was low, intermediate, and high/very high in 14%, 48% and 37% respectively. Seventy-seven percent were CMV seropositive; CMV-seronegative donor/CMV-seropositive recipient combination occurred in 27% of transplants. The donor was a child, sibling/half sibling, or parent in 47%, 38%, and 14%, respectively. PBSCs were used as the stem cell source in 66% of patients, and conditioning intensity was reduced in 59%.
Donor-recipient HLA disparity
A summary of donor-recipient HLA disparity, total and per loci, are shown in Table 2. The total number of HLA mm (HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1) in the GVH direction between donor and recipient was 5, 4, and ≤3 in 50%, 27%, and 23%, respectively. Individual loci mm in the GVH direction for HLA-A, HLA-B, HLA-C, HLA-DR, and HLA-DQ was seen in 79%, 89%, 84%, 85%, and 77%, respectively. HLA-DP was matched, permissive mm, and nonpermissive (np) mm in 22%, 55%, and 23%, respectively.
Transplant outcomes for the entire cohort and impact of overall HLA matching
After a median (range) follow-up for surviving patients of 65.4 (34.3-157.2) months, 5-year OS, DFS, relapse/progression, and NRM for all patients were 53%, 46%, 37%, and 17%, respectively. The CI of grade II to IV and grade III to IV acute GVHD at 6 months was 44% and 17%, respectively. The CI of all-grade and moderate-to-severe chronic GVHD at 3 years was 34% and 25%, respectively. Graft failure occurred in 5 patients (2.4%), all of whom were successfully regrafted with a second haplo transplant.
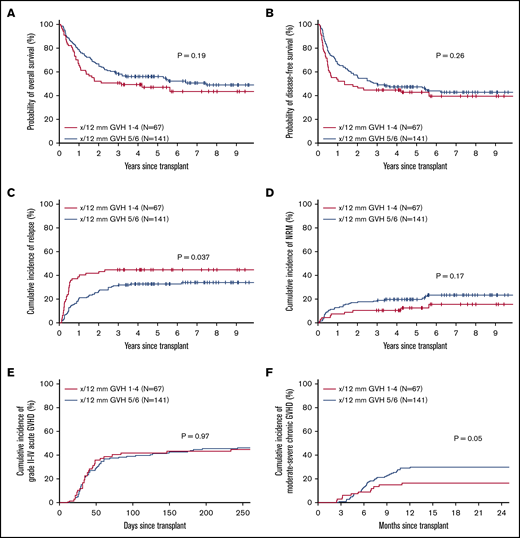
To analyze the effect of overall HLA disparity, we compared outcomes of transplants where donor and recipient had 5 or 6 (out of 12) antigen or allelic mismatches at HLA-A, HLA-B, HLA-C, HLA-DR, HLA-DQ, or HLA-DP in the GVH direction (n = 141) vs those with 1 to 4 out of 12 mismatches (n = 67). There were no significant differences in OS, DFS, NRM, or acute GVHD between the groups. However, patients with less mismatch (1-4 vs 5-6) had a statistically higher incidence of relapse and a trend toward a lower incidence of chronic GVHD (Figure 1). A similar pattern was seen when the analysis was restricted to 10 loci (HLA-A, HLA-B, HLA-C, HLA-DR, and HLA-DQ; supplemental Figure 1).
Effect of cumulative HLA allele mismatch on transplant outcomes. OS (A), DFS (B), relapse/progression (C), NRM (D), grade II-IV acute GVHD (E), and moderate-to-severe chronic GVHD (F), according to the total number of HLA mismatches (5-6 vs 1-4 out of 12).
Effect of cumulative HLA allele mismatch on transplant outcomes. OS (A), DFS (B), relapse/progression (C), NRM (D), grade II-IV acute GVHD (E), and moderate-to-severe chronic GVHD (F), according to the total number of HLA mismatches (5-6 vs 1-4 out of 12).
Survival, relapse/progression, and NRM according to individual HLA loci disparity
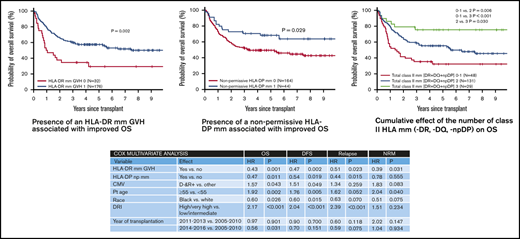
In univariate analysis, there was no effect of class I mm in the GVH direction at HLA-A, HLA-B, or HLA-C loci on OS (Figure 2A-C). Similarly, mm at HLA-A, HLA-B, or HLA-C loci had no effect on DFS or CI of relapse/progression or NRM (data not shown). In contrast, class II mm GVH had a significant impact on OS. Transplants where either an HLA-DR or HLA-DQ mm was present were associated with a significant improvement in OS (P =.002 and P =.014, respectively; Figure 2D-E). Estimated 5-year OS in patients where either HLA-DR or HLA-DQ was mismatched vs matched was 57% vs 29% and 57% vs 39%, respectively. Unlike HLA-DR and HLA-DQ, mm at HLA-DP had no effect on OS (Figure 2F), with an estimated 5-year OS of 52% vs 56%. However, if restricted only to an np HLA-DP mm, there was a significant OS benefit to having an np HLA-DP mm, with estimated 5-year OS of 68% vs 49% (P =.029; Figure 2G).
Effect of loci-specific HLA mismatch on OS. OS according to the presence/absence of a loci-specific HLA mismatch at HLA-A (A), HLA-B (B), HLA-C (C), HLA-DR (D), HLA-DQ (E), HLA-DP (F), or the presence/absence of an np HLA-DP mismatch (G).
Effect of loci-specific HLA mismatch on OS. OS according to the presence/absence of a loci-specific HLA mismatch at HLA-A (A), HLA-B (B), HLA-C (C), HLA-DR (D), HLA-DQ (E), HLA-DP (F), or the presence/absence of an np HLA-DP mismatch (G).
The impact of class II mm was further analyzed in a Cox MVA while adjusting for other significant covariates such as age (≥55 years vs <55 years), race (black vs white), DRI (high/very high vs low/intermediate), CMV (D− R+ vs other), and year of transplant (Table 3). As HLA-DR and HLA-DQ were strongly correlated, given their known genetic linkage disequilibrium, only HLA-DR was considered. In this analysis, the presence of an HLA-DR mm (hazard ratio [HR], 0.43; P =.001) or HLA-DP mm (HR, 0.47; P =.011) was independently associated with improved OS. A similar association was also noted with DFS (HR, 0.47; P =.002; and HR, 0.54; P =.019 respectively; Table 3).
The presence of an HLA-DR, HLA-DQ or np HLA-DP mm was also associated with a reduction of relapse/progression. In univariate analysis, the 5-year CI of relapse/progression was lower in transplants associated with an HLA-DR mm GVH (34% vs 50%, P =.05). Likewise, HLA-DQ and np HLA-DP mm were also associated with lower 5-year relapse risk (34% vs 44%, P =.16; and 25% vs 40%, P =.07, respectively). In Cox MVA, significant relapse reduction was noted with either an HLA-DR mm (HR, 0.51; P =.023) or an np HLA-DP mm (HR, 0.44; P =.015), while adjusting for other significant covariates (Table 3). HLA-DR mm also demonstrated a protective effect against NRM (HR, 0.39; P =.031), whereas np HLA-DP mm had no significant impact on NRM (Table 3). Class I mm at HLA-A, HLA-B, or HLA-C had no impact on either relapse/progression or NRM.
We further analyzed the cumulative effect of the number of class II mm’s (HLA-DR, HLA-DQ, and np HLA-DP). Figure 3 shows the relative impact on OS, DFS, relapse, and NRM of having 0 or 1 vs 2 vs 3 class II mm’s. Estimated 5-year OS was improved with an increasing number of class II mm’s (estimated to be 32%, 55% and 76%, respectively). Similarly, 5-year DFS was also improved with increasing class II mm’s (28%, 49%, and 62%, respectively). Risk of relapse/progression at 5 years posttransplant was highest in patients with 0 or 1 class II mm’s (50% vs 33% and 31% in patients with 2 and 3 mm’s, respectively). CI of NRM at 5 years posttransplant was lowest in patients with 3 class II mm’s (7% vs 18% and 22% in patients with 2 and 0 or 1 mm, respectively. There was no association of the cumulative number of class I mm’s (HLA-A, HLA-B, or HLA-C) with OS, DFS, NRM, or relapse/progression.
Transplant outcomes according to the total number of class II HLA mismatches. OS (A), DFS (B), relapse/progression (C), and NRM (D) according to the number of class II HLA mismatches
Transplant outcomes according to the total number of class II HLA mismatches. OS (A), DFS (B), relapse/progression (C), and NRM (D) according to the number of class II HLA mismatches
GVHD according to HLA loci disparity
In univariate analysis, there was no significant effect of mm at any class I (HLA-A, HLA-B, or HLA-C) or class II (HLA-DR, HLA-DQ, or np HLA-DP) loci on the CI of either grade II-IV or III-IV acute GVHD. Supplemental Figure 2 shows the CI of grade II to IV acute GVHD according to the presence of mm’s at each individual locus.
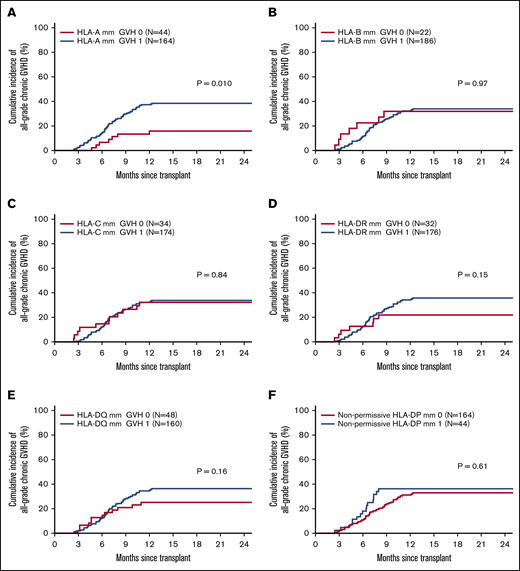
Figure 4 shows the CI of chronic GVHD according to the presence of mm’s at each individual locus. Among class I HLA loci, only the presence of an mm at the HLA-A locus was associated with increased risk of chronic GVHD (39 vs 19%, P =.01). An HLA-A mm was also associated with an increased incidence of moderate-to-severe chronic GVHD (29% vs 14%, P =.043). There was no association of class II HLA mm at any locus and the CI of chronic GVHD. The impact of HLA-A mm was further analyzed in a Cox MVA while adjusting for other significant covariates such as race (black vs white). In MVA, the association of HLA-A mm with chronic GVHD remained significant (HR, 2.17; P =.041).
HLA loci-specific chronic GVHD incidence. Cumulative incidence of chronic GVHD according to presence/absence of an HLA mismatch at HLA-A (A), HLA-B (B), HLA-C (C), HLA-DR (D), HLA-DQ (E), and nonpermissive HLA-DP (F).
HLA loci-specific chronic GVHD incidence. Cumulative incidence of chronic GVHD according to presence/absence of an HLA mismatch at HLA-A (A), HLA-B (B), HLA-C (C), HLA-DR (D), HLA-DQ (E), and nonpermissive HLA-DP (F).
Discussion
In this large, single-center, retrospective analysis with mature follow-up (median of 5 years), we demonstrate the protective effect of class II HLA mm on survival after haplo transplantation utilizing PTCy. The presence of an mm at HLA-DR, HLA-DQ or the presence of an np mm at HLA-DP are each associated with a statistically significant improvement in OS. Furthermore, this effect appears to be cumulative, so that patients with 3 class II mm’s (HLA-DR, HLA-DQ, and np HLA-DP) have the best OS. The survival advantage in patients with an HLA class II mm is primarily the result of reduced relapse risk. Remarkably, this association of class II mm with lower relapse occurs without a corresponding increase in NRM. In fact, the presence of an HLA-DR mm was associated with lower NRM in this analysis. In contrast to the significant impact of class II HLA mm’s, there is no apparent effect of class I HLA mm’s at the HLA-A, HLA-B, or HLA-C locus on survival or relapse incidence, although the presence of an HLA-A mm is associated with an increased risk of chronic GVHD.
Outside of the context of haplo transplant with PTCy, HLA disparity has generally been associated with inferior survival. For example, in UD transplantation using standard GVHD prophylaxis, the presence of a single-allele– or antigen-level mm at the HLA-A, HLA-B, HLA-C, or HLA-DRB1 locus results in excess mortality, and mismatching at multiple loci compounds this effect.3-5 Although no such association has been shown with HLA-DQ or HLA-DP mm, the presence of an HLA-DP np mm according to the TCE algorithm results in higher nonrelapse and all-cause mortality.7,8 In the context of single or double umbilical cord blood (UCB) transplantation, the consequences of HLA disparity are much less clear, with conflicting reports showing that a higher degree of HLA disparity is associated with inferior,18-21 neutral,22 or superior23-26 transplant outcomes.
Our results are in concordance with other published studies9-11 showing that survival after haplo transplant using PTCy does not appear to be significantly impacted by the total number of HLA mm’s on the unshared haplotype when specific HLA loci are not taken into account. However, the consequences of an individual HLA mm at selective loci has been less well studied and remains an open area of investigation. For example, an analysis by the Johns Hopkins group demonstrated that the presence of an antigenic HLA-DR mm was associated with decreased relapse and improved DFS following haplo transplant using PTCy. This study analyzed a uniformly treated patient population receiving NMA conditioning and bone marrow exclusively as the stem cell source.9 In contrast to those results, a European registry analysis by Lorentino et al, with more heterogenous conditioning regimens and the use of peripheral blood in addition to marrow as a stem cell source, failed to show an effect of HLA-DR mm on relapse or survival, but it did show an association with increased grade 2 to 4 acute GVHD.10
The results of our analysis strongly suggest that selective mm’s at class II loci (HLA-DR, HLA-DQ, and np HLA-DP mm) are associated with reduced relapse and improved survival in the context of haplo transplant using PTCy. On the contrary, class I mm’s (HLA-A, HLA-B, or HLA-C) had no impact on relapse or survival. Of note, both HLA-DR and HLA-DP mm’s have been associated with reduced relapse risk in the context of UD7,8,27 and UCB18,25,28 transplantation, and HLA-DR mm has been associated with improved survival in the setting of UCB transplant in one study.25 However, in the UD setting using conventional GVHD prophylaxis, HLA-DR mm and np HLA-DP mm are also associated with higher risks of GVHD and NRM, negating any beneficial effect on transplant outcome.7,29-31 We believe that the absence of compensatory increase in NRM in haplo transplant is likely the result of PTCy-based GVHD prophylaxis.
We hypothesize that the relapse/survival benefits of class II HLA mismatch, without associated increases in GVHD/NRM, are likely based on several important immunological principles: (1) the robust alloresponse that occurs when crossing HLA barriers, (2) the unique mechanisms of PTCy-mediated allodepletion, and (3) the preferential expression of class II HLA antigens on hematopoietic cells. Alloreactive T cells against foreign HLA are found naturally in high frequency in the normal T-cell repertoire.32 Such T cells are similarly present in the naive and memory T-cell compartment.33 PTCy efficiently depletes rapidly dividing alloreactive memory T cells due to their rapid activation kinetics and sensitivity of activated T cells to Cy-induced elimination, resulting in partial abrogation of the T-cell response to mismatched HLA.34 In contrast, it has been proposed that residual alloreactive T cells from the naive T-cell compartment, having slower kinetics of activation, can preferentially survive the effects of PTCy and result in preservation of at least some anti-malignancy activity with acceptable GVHD.34 Unlike HLA class I molecules, which are expressed on a wide variety of nucleated cells, HLA class II molecules are preferentially expressed on cells of hematopoietic origin. Therefore, it is plausible that CD4 T cells targeting class II antigens on hematopoietic cancer cells, partially surviving PTCy-induced allodepletion, may be the main drivers of the selective graft–versus-malignancy responses seen in this context, an idea which is supported by significant preclinical data.35-38
The finding of lower NRM associated with the presence of an HLA-DR GVH mm was an unexpected observation in this analysis. As HLA-DR disparity is a major contributor of the strength of the alloresponse in the in vitro mixed lymphocyte response,39 we hypothesize that HLA-DR disparity may accelerate the kinetics of T-cell activation in vivo and thus improve the efficiency of cyclophosphamide-induced allodepletion and/or regulatory T-cell generation,40,41 which may result in less infectious or GVHD-related mortality. In our analysis, 5-year NRM was substantially lower in HLA-DR GVH mm patients (16% vs 27%). Furthermore, GVHD-related death appeared to be higher following HLA-DR GVH matched patients, with 4 of the 8 (50%) nonrelapse deaths occurring in the context of active treatment of NIH-defined severe chronic GVHD compared with 1 of 28 (4%) nonrelapse deaths in the HLA-DR GVH mm group. In contrast to HLA-DRB1 mm, there was no NRM advantage seen with either HLA-DQB1 or HLA-npDPB1 mm, a finding that may relate to the significantly lower expression of these antigens on the surface of antigen-presenting cells compared with HLA-DRB1.42-44 This observation, although provocative, clearly needs to be validated in a larger study population.
In the context of UD transplantation, HLA disparity at HLA-A, HLA-B, HLA-C, or HLA-DR is associated with a significant increase in the incidence and severity of acute GVHD, and this effect was compounded in the setting of multiple HLA mismatches.4,45 In contrast, our data show no correlation between HLA disparity at any loci and the incidence of acute GVHD following PTCy-based haplo transplantation. With regard to chronic GVHD, however, we show that the presence of an HLA-A mm in the GVH direction is associated with an increased risk of all-grade and moderate-to-severe chronic GVHD. This association, although not previously reported in the setting of PTCy-based haplo transplant, is supported by comparable data in the context of UD transplantation.4,45,46 Furthermore, the same association of HLA-A mm with chronic GVHD has also been seen in the context of UCB transplantation, although limited to UCB transplants that were mismatched at 3 or 4 other loci.18
We believe that the association of class II mm with improved OS may have significant implications for donor selection in the context of haplo transplant using PTCy. As most patients have >1 available potential haplo donor options, choosing donors that maximize class II mm (ie, avoiding HLA-DR and HLA-DQ matching in the GVH direction and prioritizing an np HLA-DP mm) may improve overall transplant outcome. Furthermore, with the increasing use of PTCy-based GVHD prophylaxis for mismatched UD transplantation,47-49 it will be important to evaluate the impact of class II mismatching in this setting as well.
In conclusion, our results confirm historical data showing that the total number of HLA mismatches, irrespective of HLA loci, does not impact mortality risk in the context of PTCy-based haplo transplantation. Conversely, we show the importance of certain loci-specific HLA mismatches in transplant outcome. We report that class II mm’s (HLA-DR mm, HLA-DQ mm, or np HLA-DP mm) are associated with improved survival after haplo transplant utilizing PTCy, a product of lower relapse without a corresponding increase in NRM. Furthermore, we show that HLA-A mismatch is associated with a higher risk of chronic GVHD, without any corresponding effects on survival or relapse. This study does have several important limitations. First, although this represents a fairly robust single-center study, it does not have the power of a large registry study for detecting small differences in HLA-locus–specific outcomes. Second, our population is relatively heterogenous based on disease, stem cell source, and conditioning intensity. Despite these limitations, this study represents the most comprehensive analysis of HLA disparity in the context of PTCy-based haploidentical PBSC transplantation to date. These findings will require validation in larger registry studies.
Send data sharing requests via e-mail to the corresponding author, Scott R. Solomon (ssolomon@bmtga.com).
Acknowledgment
The authors are grateful to all the clinicians and support staff at the Blood and Marrow Transplant Program at Northside Hospital Cancer Institute for their excellent care of our transplant patients.
Authorship
Contribution: S.R.S. designed the research; S.R.S., M.M.S., L.E.M., H.K.H, and A.B. treated patients; M.T.A. and K.C.J. collected the data; S.R.S. and X.Z. analyzed and interpreted data; X.Z. performed the statistical analysis; S.R.S. wrote the manuscript; and all authors revised and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Scott R. Solomon, Northside Hospital Cancer Institute, 5670 Peachtree Dunwoody Rd NE, Suite 1000, 10th Floor, Atlanta, GA 30342; e-mail: ssolomon@bmtga.com.
References
Author notes
The full-text version of this article contains a data supplement.