Key Points
NT regrading of the JULIET trial by CTCAE, modified CRES, and ASTCT criteria highlighted the need for standardized NT grading practices.
CTCAE was suboptimal for grading CAR-T cell therapy-associated NT; CRES and ASTCT scales offer more accurate assessments of ICANS.
Abstract
Chimeric antigen receptor-T (CAR-T) cell therapy achieves durable responses in patients with relapsed/refractory diffuse large B-cell lymphoma (r/r DLBCL), but may be associated with neurological toxicity (NT). We retrospectively assessed differences and concordance among 3 available grading scales (the National Cancer Institute Common Terminology Criteria for Adverse Events v4.03 [CTCAE], modified CAR-T Related Encephalopathy Syndrome [mCRES], and American Society for Transplantation and Cellular Therapy [ASTCT] scales) applied to the same set of NT data from the JULIET (A Phase 2, Single Arm, Multicenter Trial to Determine the Efficacy and Safety of CTL019 in Adult Patients With Relapsed or Refractory DLBCL) trial. Individual patient-level NT data from the phase 2, single-group, global, pivotal JULIET trial (NCT02445248) were retrospectively and independently graded, using CTCAE, ASTCT, and mCRES, by 4 medical experts with experience managing patients with 3 different CD19-targeted CAR constructs. According to the US Food and Drug Administration definition of NT using CTCAE, 62 of 106 patients infused with tisagenlecleucel had NT as of September 2017. Among 111 patients infused with tisagenlecleucel (as of December 2017), the 4 experts identified 50 patients (45%) who had any-grade NT per CTCAE, 19 (17%) per mCRES, and 19 (17%) per ASTCT. Reevaluation according to the mCRES/ASTCT criteria downgraded 31 events deemed NT by CTCAE to grade 0. This is the first study to retrospectively apply CTCAE, mCRES, and ASTCT criteria to the same patient data set. We conclude that CTCAE v4.03 was not designed for, and is suboptimal for, grading CAR-T cell therapy-associated NT. The CRES and ASTCT scales, which measure immune effector cell-associated neurotoxicity syndrome, offer more accurate assessments of NT after CAR-T cell therapy.
Introduction
Chimeric antigen receptor-T (CAR-T) cell therapy uses reprogrammed T cells to target and kill cancer cells, and thus has become a promising treatment for patients with advanced hematologic malignancies.1-10 Patients with relapsed or refractory diffuse large B-cell lymphoma (r/r DLBCL) or r/r transformed follicular lymphoma may receive CD19-directed CAR-T cell therapy after 2 systemic therapy options such as R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone).11,12 Two such CD19-directed CAR-T cell therapies are currently commercially available: tisagenlecleucel and axicabtagene ciloleucel. A third, lisocabtagene maraleucel, is undergoing late-stage clinical trials (NCT02631044).13
The efficacy and safety of CAR-T cell therapies have been extensively characterized in clinical trials and demonstrate a positive benefit:risk profile. However, these therapies are associated with unique, but common, adverse events that must be identified and managed appropriately: cytokine release syndrome (CRS) and neurological toxicity (NT).3,10,14-18 NT after CAR-T cell therapy generally occurs after the onset of CRS, and higher grades of NT tend to occur concurrently with higher grades of CRS.10,19 Clinical features of CAR-T cell therapy-associated NT are numerous, and patients can experience events such as headache, dizziness, delirium, seizures, dysphasia, hallucinations, and impaired motor and language skills.1,3-5,8,10 This may be distressing to the patient and the patient’s family, but fortunately, NT and CRS generally resolve within days with standard supportive therapy such as corticosteroids. Patients with concurrent CRS also often receive anti-interleukin-6 agents.1 In severe cases, rapidly fatal cerebral edema has occurred in CAR-T cell trials (eg, the JCAR015 ROCKET [Study Evaluating the Efficacy and Safety of JCAR015 in Adult B-cell Acute Lymphoblastic Leukemia] trial in adult ALL), although none was observed in the JULIET (A Phase 2, Single Arm, Multicenter Trial to Determine the Efficacy and Safety of CTL019 in Adult Patients With Relapsed or Refractory DLBCL) lymphoma trial.10,20-22
In the JULIET trial, NT was identified and graded per protocol according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.03.10 Because it was not designed specifically for CAR-T cell therapy trials, the CTCAE scale has shortcomings in accurately capturing the severity, timing, and spectrum of NT. Specifically, the CTCAE scale leaves much room for subjectivity and does not discern the clinically relevant findings that define immune effector cell-mediated events from nonspecific ones.
Recognizing that the CAR-T-associated NT represents a unique syndrome that would benefit from a unified scale, the multiinstitution CAR-T cell-therapy-associated Toxicity (CARTOX) Working Group introduced the term CAR-T cell-Related Encephalopathy Syndrome (CRES).23 The CARTOX group created a CRES grading system that included a 10-point questionnaire (CARTOX-10), designed to capture subtle to severe cognitive and attentive dysfunction. This scale was then grouped with gradation of signs of increased intracranial pressure and presence of seizures, whereby the greatest level of toxicity in any given domain would also be captured as the overall CRES grade.
Finally, a panel of American Society for Transplantation and Cellular Therapy (ASTCT, previously known as American Society for Blood and Marrow Transplantation) members coined the term immune effector cell-associated neurotoxicity syndrome, or ICANS, effectively replacing CRES as the preferred nomenclature for the syndrome. The ASTCT grading scale for ICANS is similarly domain-based and uses a modified version of the CARTOX-10 screening tool, called the Immune Effector Cell-Associated Encephalopathy (ICE) score. In addition to the ICE score, ICANS consensus grading also takes into account consciousness, seizures, motor findings, and cerebral edema.24 The ASTCT grading tool was created to provide a means to better assess and harmonize the classification of CAR-T cell therapy-associated NT and its treatment across diseases, regions, and CAR-T cell products.
Four medical experts with experience treating patients with 3 different CD19-targeted CAR-T cell constructs retrospectively assessed and regraded NT after tisagenlecleucel treatment in patients with r/r DLBCL or r/r transformed follicular lymphoma in the JULIET trial, as reported in the US Food and Drug Administration (FDA) prescribing label. We compare the results of regrading by CTCAE to the original FDA data report, as well as regrading by CTCAE compared with a modified CRES (mCRES) score and the ASTCT ICANS score.
Methods
JULIET trial
JULIET (NCT02445248) was the first global, phase 2, single-group, pivotal trial of centrally manufactured tisagenlecleucel for adult patients with r/r DLBCL and r/r transformed follicular lymphoma. Eligible patients were at least 18 years old, with 2 or more prior lines of therapy (including rituximab and an anthracycline), and were ineligible for or had relapsed after autologous hematopoietic stem cell transplantation. Patients with primary mediastinal B-cell lymphoma were not eligible for enrollment. Other key exclusion criteria included prior anti-CD19 therapy, prior allogeneic hematopoietic stem cell transplant, and active central nervous system disease involvement. After leukapheresis, manufacturing of tisagenlecleucel was carried out at centralized facilities in Morris Plains, New Jersey, and in Leipzig, Germany. Bridging chemotherapy was permitted during the manufacturing interval.10 Lymphodepleting chemotherapy was omitted in a minority of patients with a white cell count lower than 1000 cells/mm2 1 week before tisagenlecleucel infusion.10
The primary endpoint of the JULIET trial was overall response rate (partial responses plus complete responses) by Lugano classification25 per independent review committee assessment. Secondary endpoints of the JULIET trial were duration of response, overall survival, safety, and cellular kinetics.10
In the JULIET trial, NT was graded, per protocol, using CTCAE v4.03 criteria (Table 1).
Data source
For the present retrospective analysis, NT patient-level data from case report forms were collected for the JULIET trial for the 9-month data cutoff of December 2017. Preferred term (supplemental Table 1), grade per CTCAE v4.03, and time to onset were extracted for all NT symptoms, including but not limited to headache, peripheral neuropathy, encephalopathy, dizziness, seizures, anxiety, paresthesia, insomnia, and delirium. CRS grade and use of anticytokine therapy or corticosteroids were also obtained. Only NT with at least temporal association with CAR-T cell therapy was considered for regrading. Symptoms that occurred up to 1 year after infusion were considered.
Adjudication of NT regrading
Four medical experts with experience treating patients with different CAR-T cell therapy products independently reviewed patient-level data from the JULIET trial, using the broadly defined NT criteria (ie, any nervous system or psychiatric disorders) in the FDA label, and they regraded NT for each patient based on the case report forms. This analysis had 2 objectives. First, NT was regraded by CTCAE criteria retrospectively, giving one overarching CTCAE grade to each patient (eg, overarching CTCAE grade 3 was given for a patient who had the following individual neurological events: grade 3 encephalopathy, grade 2 paresthesia, and grade 1 dyskinesia), and compared with the FDA label. Second, NT was regraded by assessment tools more focused to elaborate degrees of encephalopathy/delirium, as developed by CARTOX (CRES scale) and ASTCT (ICANS scale), and was compared with the expert regrade by CTCAE.
The CARTOX-10 questionnaire is a new tool proposed to prospectively assess overall cognitive function that could not be used in this retrospective study. Therefore, an mCRES scale was used for this analysis, wherein grades 1 and 2 (distinguished by the CARTOX-10 score) were combined. Key definitions of each NT grade for the 3 assessment tools are outlined in Table 1. CRS was also regraded according to the Lee and ASTCT scales (S.J.S., R.T.M., E.S.R., J.L., J.E.S., V.V.R., F.L.L., D.G.M., manuscript in preparation). Gradings by independent experts were compiled along with the investigator’s initial grading. As expected, especially when introducing new grading methods, some variance was observed among the 4 experts’ independent and blinded grading assessments. Thus, as done in real-world practice, complex patient cases went through an adjudication discussion by the 4 experts, similar to a clinical tumor board, referring back to the source documents when necessary. The clinically most appropriate grade was selected as the final grade. As per the investigational charter, the most conservative final assessment (ie, the highest grade) of any expert reviewer determined the final grading for any individual case. For example, if an event could not be reconciled by the 4 experts and was graded as 2, 3, 3, and 4, then grade 4 was the final grading. Last, NT grading using all 3 systems was summarized for all patients, and all patients were stratified according to presence of CRS by the Penn scale.
Results
As of December 2017, 111 patients were infused with tisagenlecleucel in the JULIET trial. Median follow-up from time of infusion was 14 months; 93 patients had at least 3 months of follow-up and made up the efficacy analysis set. Detailed patient characteristics were previously described.10 Ninety-two percent of patients received bridging therapy before tisagenlecleucel infusion.10 Sixty-four of 111 patients (57.7%) had CRS events, and 24 patients (21.6%) had grade 3/4 CRS events as defined by the Penn scale. No grade 5 CRS or NT events occurred.
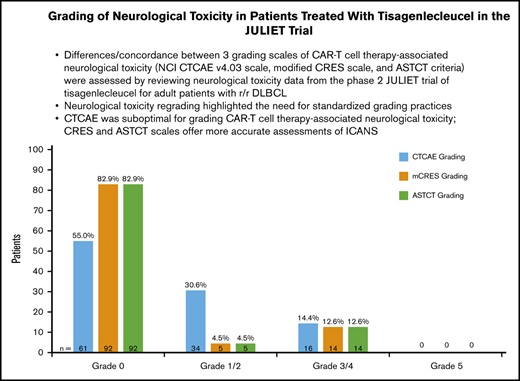
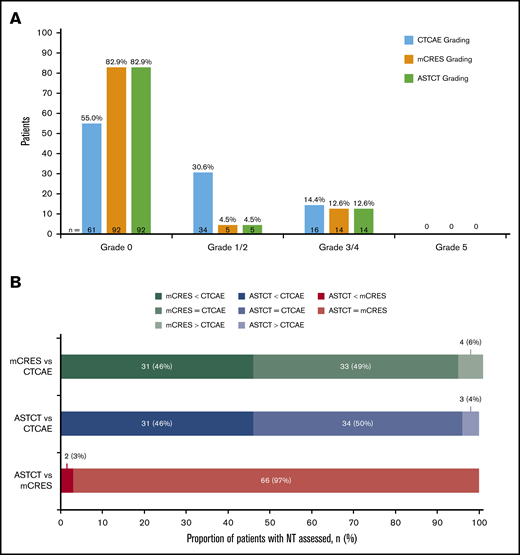
Sixty-eight patients (61.3%) identified as having NT were retrospectively evaluated by CTCAE, mCRES, and ASTCT criteria. Fifty patients (45.0%) were considered to have any-grade NT when regraded by CTCAE, 19 patients (17.1%) were identified as having NT by mCRES, and 19 patients (17.1%) were identified as having NT by ASTCT criteria (Figure 1A). Thus, the CTCAE scale identified 31 more patients as having NT than did either the mCRES system or the ASTCT system. These 31 patients generally presented with either nervous system disorders such as syncope, dizziness, peripheral neuropathy, and hypotonia that seemed distinct from and did not raise clinical suspicion of encephalopathy, or psychiatric disorders such as anxiety and insomnia (Table 2). Finally, some patients had headache, which was considered a nonspecific symptom and is not part of the ASTCT ICANS grading scale.24 Corticosteroid treatment by CTCAE, mCRES, and ASTCT grade is shown in Table 3. Only 2 of the 31 patients who had NT per CTCAE, but grade 0 NT by mCRES and ASTCT, had received corticosteroids (Table 4). NT by ASTCT criteria provided concordance for 66 patients, a lower grade for 2 patients, and a higher grade for no patients compared with the mCRES scale (Figure 1B). NT by ASTCT criteria provided concordance for 34 patients, a lower grade for 31 patients, and a higher grade for 3 patients compared with the CTCAE scale (Figure 1B). NT by mCRES provided concordance for 33 patients, a lower grade for 31 patients, and a higher grade for 4 patients compared with the CTCAE scale (Figure 1B). Among 68 regraded patients, 33 (48.5%) patients were graded as the same score across the 3 grading scales.
Regrade of JULIET trial patient-level data showed 50 patients as having any-grade NT by CTCAE, 19 patients by mCRES, and 19 patients by ASTCT criteria. (A) Classification of NT by CTCAE, mCRES, and ASTCT grading systems (N = 111). (B) Cross-classification of NT by 3 grading scales: CTCAE, ASTCT, and mCRES.
Regrade of JULIET trial patient-level data showed 50 patients as having any-grade NT by CTCAE, 19 patients by mCRES, and 19 patients by ASTCT criteria. (A) Classification of NT by CTCAE, mCRES, and ASTCT grading systems (N = 111). (B) Cross-classification of NT by 3 grading scales: CTCAE, ASTCT, and mCRES.
The CTCAE grades by medical experts also varied from those reported by the FDA, using a broader definition based on the CTCAE system (Table 5). Among 106 patients receiving tisagenlecleucel included in the FDA label, 62 (58.5%) patients were reported as having NT, including 43 (40.6%) with grade 1/2 and 19 (17.9%) with grade 3 or higher NT. By comparison, the expert regrading of the 62 patients identified as having NT in the FDA label yielded 50 patients (45.0%) with NT, including 34 patients (30.6%) with grade 1/2, 11 patients (9.9%) with grade 3, and 5 patients (4.5%) with grade 4 NT. Two patients received corticosteroids for persistent neurotoxicity after resolution of CRS.26
In the subgroup analysis of patients with or without CRS, all 3 grading systems identified more patients with CRS as having NT compared with patients without CRS (Table 6). Among the subgroup of 64 patients with CRS by the Penn scale, the CTCAE, mCRES, and ASTCT systems identified a rate of grade 3 or higher NT of 17.2%, 15.7%, and 15.7%, respectively (Table 6). For 47 patients without CRS, the CTCAE, mCRES, and ASTCT systems identified a rate of grade 3 or higher NT of 10.6%, 8.5%, and 8.5%, respectively (Table 6). More patients with CRS (per the Penn scale) had NT during the study than those without CRS (NT by ASTCT criteria: 15/64 [23.4%] vs 4/47 [8.5%], χ2 test: P = .039). For 39 regraded patients with CRS, 22 (56.4%) were graded the same across all 3 scales. For 29 regraded patients without CRS, 11 (37.9%) were graded the same across all 3 scales. The medical experts reached independent agreement for 19/68 patients (27.9%) for the mCRES grading scale and 47/68 patients (69.1%) by ASTCT criteria. All discrepancies were resolved during the adjudication conference that followed group meetings and discussions. In the majority of patients who had higher-grade NT per the CTCAE scale than the mCRES and ASTCT scales, the less specialized CTCAE scale identified NT not considered relevant for CRES or ICANS, resulting in grades of 0 by mCRES and ASTCT.
Discussion
Per protocol, NT events in the JULIET trial were identified and graded using the CTCAE v4.03 criteria. However, the CTCAE scale was not specifically developed to capture the scope and severity of the NT syndrome that can occur after CAR-T cell therapy, and new grading systems have since emerged that are more appropriate for this purpose. To gain a better understanding of tisagenlecleucel’s NT safety profile, NT-related data collected in the JULIET trial were assessed retrospectively by a panel of medical experts and regraded using the CTCAE criteria in parallel with the mCRES system and the ASTCT criteria. This study is the first to retrospectively apply a modified version of the CARTOX Working Group’s CRES grading system and the ASTCT consensus ICANS criteria to the same CAR-T cell-related NT data set from a registrational trial.
Medical experts were able to achieve agreement regarding NT grading using all 3 grading systems applied to data extracted from the JULIET trial after discussions. Overall, fewer cases of CAR-T cell therapy-related NT were identified by both the mCRES system and the ASTCT criteria compared with the CTCAE scale. For example, mCRES and ASTCT criteria categorized 31 patients as having grade 0 NT compared with NT ranging from grade 1 to 3, using the CTCAE scale. Furthermore, the medical experts in this study identified fewer cases of clinically relevant CAR-T cell therapy-related NT by CTCAE criteria compared with those listed in the FDA label. This analysis highlights the unsuitability of CTCAE v4.03 for effectively capturing CAR-T cell therapy-related NT. For example, encephalopathy and delirium are the principal points of focus, or cognitive domains, of the more clinically sensitive mCRES and ASTCT systems. In contrast, as originally graded in the trial and included in the FDA label, NT by CTCAE includes numerous nervous system or psychiatric events not indicative of neurotoxic effects of CAR-T cell therapies (eg, anxiety, late-onset dizziness, headache with onset up to 2 months after CAR-T cell infusion, peripheral neuropathy, and sleep disorder). Finally, based on the individual examples given here, evaluating NT using the CTCAE system is highly subjective when used by practitioners to capture CAR-T-associated encephalopathy. Using a diffuse and overlapping variety of CTCAE NT terms can create confusion, misreporting, and suboptimal clinical management of NT associated with CAR-T cell therapy. In addition, the proportion of likely nonattributable events picked up by the CTCAE system, and included in the FDA label, in the JULIET trial is very high compared with the NT identified by mCRES and ASTCT criteria. This suggests that the CTCAE scale would pose difficulties in reliable clinician training outcomes as well as consistent global institutional implementation. With this study, we showed that the first step in investigating the complex clinical syndrome of NT associated with CAR-T cell therapies is the accurate grading, which can then be used to investigate further associations of NT and clinically relevant markers (eg, age, tumor burden).27,28
Limitations of this analysis include its retrospective nature and the consequent insufficient detail for full implementation of the CARTOX grading system (eg, the prospective part of the CARTOX-10 score questionnaire), thus requiring the grouping of grade 1/2 NT events together. In addition, the mCRES scale used here may have underestimated the actual CRES grade 1/2 because the CARTOX-10 score might pick up subtle mental status changes not recognized or reported by the investigators using CTCAE. The same limitation applies to the ICE score, which is a modified version of the CARTOX-10 score and is used in the ASTCT ICANS consensus criteria. Nevertheless, as management for NT is usually initiated at grade 3/4 events, differentiating between grades 1 and 2 in this analysis may not be clinically important, and this limitation does not preclude the distinction between mild and severe NT.
Care must be taken to compare the data generated here with NT results from other clinical trials using other CD19 CAR-T cell therapies for DLBCL. Indeed, the ZUMA-1 (Long-Term Safety and Activity of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma) trial and TRANSCEND (Study Evaluating the Safety and Pharmacokinetics of JCAR017 in B-cell Non-Hodgkin Lymphoma) trials have not been regraded by an expert panel paying special attention to attribution and causation of NT. It is possible that a similar process would also lead to decreased numbers of NT events attributable to CAR-T cell therapy for ZUMA-1 and TRANSCEND. In addition, inpatient care, as mandated in the ZUMA-1 trial, may have allowed more opportunity to detect sensitive changes in low-grade ICANS, which may not be as clearly identifiable in the outpatient setting in which approximately 25% of CAR-T cell therapy infusions were performed in JULIET. It is anticipated that future studies will have prospective data collected using more specific ICANS grading and allowing more precise comparisons of clinical trial adverse events.
In conclusion, this is the first study to retrospectively apply the CTCAE, mCRES, and ASTCT systems to the same patient data set. We conclude that the CTCAE system is suboptimal for the grading of CAR-T cell therapy-associated NT, as it captures a high number of nonattributable and nonspecific nervous system and psychiatric events. In addition, this is evidenced by the discrepancy between the FDA report and the retrospective regrade, both using CTCAE applied to the same JULIET patient data set, as the CTCAE system is highly subjective in capturing CAR-T cell therapy-associated NT. Our data indicate that the CRES/mCRES and ASTCT criteria both offer more accurate assessments of the occurrence and severity of CAR-T cell-related NT events. Both the CRES/mCRES and ASTCT scales appear to suit clinicians’ needs, with small nuances separating them; however, ICANS scoring per ASTCT is now being adopted by most physicians and regulatory bodies, and we expect it to become the universal grading scale for CAR-T cell therapy-associated NT.
Novartis is committed to sharing with qualified external researchers access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. The data availability of these trials is according to the criteria and process described on www.clinicalstudydatarequest.com.
Acknowledgments
The investigators thank the patients, their families, and the clinical study teams who participated in the JULIET trial.
Medical writing support was provided by Ina Nikolaeva (Healthcare Consultancy Group) and was funded by Novartis Pharmaceuticals Corporation. Editorial assistance was provided by Marie Louise Edwards, Lei Yin, and Yichen Lu from Analysis Group, Inc., and was supported by Novartis Pharmaceuticals Corporation. The study was sponsored by Novartis Pharmaceuticals Corporation.
Authorship
Contribution: R.T.M., S.J.S., D.G.M., and F.L.L. contributed to the study design; S.J.S. and R.T.M. provided study materials or patients; V.V.R. introduced the concept for this study for review; and all authors provided data analysis and interpretation, manuscript writing, and final approval of manuscript and are accountable for all aspects of the work.
Conflict-of-interest disclosure: R.T.M. received honoraria, membership on the board of directors or advisory committees, and research funding from Novartis; consultancy and honoraria from CRSPR Therapeutics, Incyte, and Juno Therapeutics; honoraria from Kite Therapeutics; patents and royalties from Athersys, Inc.; and is employed by Oregon Health & Science University. R.T.M. also provided consultant services to and received payment from Novartis. This potential conflict of interest has been reviewed and managed by Oregon Health & Science University. S.J.S. received honoraria, membership on the board of directors or advisory committees, and research funding from Celgene; consultancy and honoraria from Dava Oncology; honoraria and research funding from Genentech; membership on the board of directors or advisory committees for Gilead; consultancy, honoraria, and research funding from Merck; honoraria, membership on the board of directors or advisory committees, and research funding from Novartis; and consultancy, honoraria, and membership on the board of directors or advisory committees for Nordic Nanovector. V.V.R. is employed by Novartis. E.S.R. is employed by Novartis. J.L. is employed by the Analysis Group, which received funding from Novartis. J.E.S. is employed by the Analysis Group, which received research funding from Novartis. D.G.M. receives research funding from Kite Pharma, a Gilead Company, and Celgene; he also receives research funding from and has patents licensed or pending with Juno Therapeutics, a Celgene/Bristol-Myers Squibb company; has participated in advisory board and/or data monitoring committee meetings for which he receives honoraria for BioLine RX, Kite Pharma, Gilead, Pharmacyclics, Novartis, Juno Therapeutics, and Celgene; and is a scientific advisory board member for which he receives honoraria from and has stock options in A2 Biotherapeutics. F.L.L. is a scientific advisor to Kite/Gilead, Novartis, Celgene/BMS, GammaDelta Therapeutics, and Wugen; and an allogene consultant with grant options for Cellular Biomedicine Group, Inc.
Correspondence: Richard T. Maziarz, Adult Blood and Marrow Stem Cell Transplant & Cellular Therapy Program, Knight Cancer Institute, Oregon Health and Science University, Mail code: OC14HO, 3181 SW Sam Jackson Park Rd, Portland, OR 97239; e-mail: maziarzr@ohsu.edu.