Key Points
EBV+ DLCL-NOS is a heterogenous entity with regards to its pathological and clinical presentation, as well as its outcome.
Elderly EBV+ DLBCL-NOS patients have poorer OS than elderly EBV− DLBCL-NOS patients.
Abstract
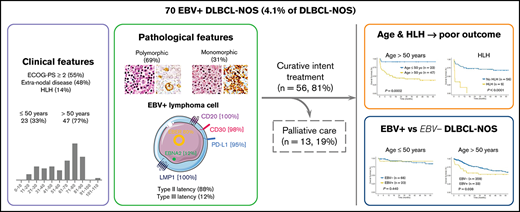
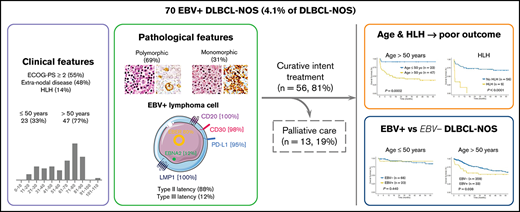
In this retrospective study, we report 70 cases of Epstein-Barr virus (EBV)+ diffuse large B-cell lymphoma not otherwise specified (DLBCL-NOS) among 1696 DLBCL-NOS cases diagnosed between 2006 and 2019 (prevalence of 4.1%). At diagnosis, median age was 68.5 years; 79% of the cases presented with an advanced-stage disease (III-IV), 48% with extranodal lesions, and 14% with an hemophagocytic lymphohistiocytosis (HLH) (8 at diagnosis and 1 on therapy). A total of 46 cases presented a polymorphic pattern, and 21 were monomorphic. All had a non-germinal center B phenotype, with the majority of tumor cells expressing CD30 and programmed death ligand 1 (98% and 95%, respectively). Type II and III EBV latency was seen in 88% and 12% of the cases, respectively. Patients were treated with immunochemotherapy (59%) or chemotherapy (22%), and 19% received palliative care due to advanced age and altered performance status. After a median follow-up of 48 months, progression-free survival (PFS) and overall survival (OS) at 5 years were 52.7% and 54.8%, respectively. Older age (>50 years) and HLH were associated with shorter PFS and OS in multivariate analysis (PFS: hazard ratio [HR], 14.01; 95% confidence interval [CI], 2.34-83.97; and HR, 5.78; 95% CI, 2.35-14.23; OS: HR, 12.41; 95% CI, 1.65-93.53; and HR, 6.09; 95% CI, 2.42-15.30, respectively). Finally, using a control cohort of 425 EBV− DLBCL-NOS, EBV positivity was associated with a shorter OS outcome within patients >50 years (5-year OS, 53% [95% CI, 38.2-74] vs 60.8% [95% CI, 55.4-69.3], P = .038), but not in younger patients.
Introduction
Epstein-Barr virus (EBV)+ diffuse large B-cell lymphoma not otherwise specified (DLBCL-NOS) is a rare entity that was initially described in 2003 by Oyama et al in 22 elderly Japanese patients1 and introduced in the 2008 World Health Organization (WHO) classification as “EBV+ DLBCL of the elderly,”2 eventually favored by underlying immunosenescence. In 2016, after cases were described in young patients,3-5 the definition was updated for “EBV+ DLBCL-NOS”6 without age restriction. At that date, the WHO established clear criteria for the diagnosis of EBV+ DLBCL-NOS that include EBV+clonal B-cell lymphoid proliferation with >80% of the malignant cells expressing EBV-encoded small RNA (EBER) in patient with no documented predisposing immunodeficiency. The “NOS” designation has been added to emphasize the exclusion of the more specific types of EBV-associated lymphomas (lymphomatoid granulomatosis, plasmablastic lymphoma, DLBCL associated with chronic inflammation, and EBV+ mucocutaneous ulcer).6 Despite this recent clarification, inconsistency remains in the literature since 2016, with different cutoff values used for EBER positivity (range, 10% to 90%).7-13
Among DLBCLs, the prevalence of EBV+ DLBCL-NOS is low in Western countries8,9 (4.4% to 5.8%) and slightly higher in Asian and Latin America countries11-14 (4.7% to 28%), and only 1 study reported 7% of cases in South Africa15 (supplemental Table 1). Pathological features have mainly been described as 2 distinct patterns, namely monomorphic, resembling DLBCL, and polymorphic, more closely related to T-cell/histiocytes rich large B-cell lymphoma,16-24 but clinical and outcome correlates with these patterns have not been fully established. Lack of uniform criteria and heterogenous geographic distribution may account for differences reported in disease prevalence and prognostic features. Outcome data of EBV+ DLBCL-NOS compared with EBV− DLBLCL-NOS remain a matter of debate; some series argue that EBV positivity is associated with adverse prognosis,5,12,13,23,25-27 while others suggest it does not affect survival.7,8,10,25,28,29 Additional work showed that the prognosis impact of EBV positivity might be related to age, with a poor prognosis in elderly patients,5,11,18,30,31 but not young patients.3,27
In this retrospective series, we aimed to describe the prevalence, clinical presentation, histopathological features, and prognosis factors of EBV+ DLBCL-NOS diagnosed according to the current WHO criteria.
Methods
Case selection
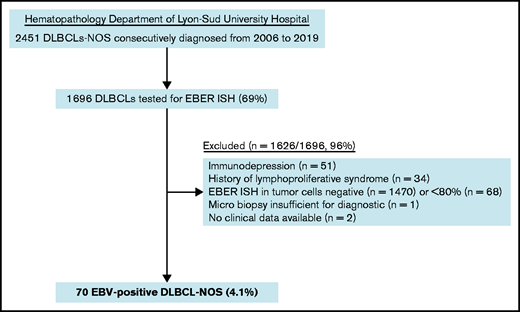
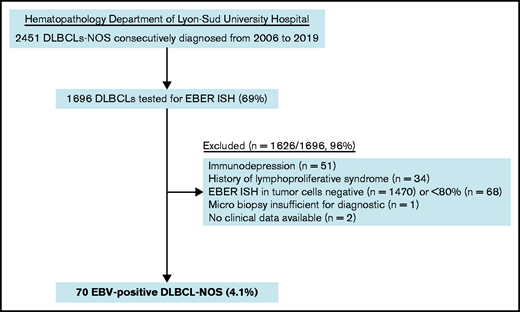
Pathological records of all DLBCL-NOS cases consecutively diagnosed from routine and consultation in the Hematopathology Department of Lyon-Sud University Hospital from 2006 to 2019 were reviewed to select cases with an EBER in situ hybridization (ISH) performed. As a second step, a histological review and scoring of EBER ISH were performed on original slides for each case with an EBER positivity notified in the chart (A.T.-G. and E.B.).32 Cases in which ≥80% of malignant CD20+ cells were positive for EBER ISH were included as EBV+ DLBCL-NOS6 (Figure 1). Clinical and laboratory data were collected from each patient’s medical chart, and staging was checked at the time of inclusion according to the latest recommendations.6,33 Patients with impairment of the immune system secondary to primary immunodeficiency, HIV infection, history of lymphoid malignancies, solid organ transplantation, allogenic stem cell transplantation, or immunosuppressive drugs were excluded. Among the 70 included cases, 52 (74%) came from hospitals in the Rhône-Alpes region and 18 (26%) came from hospitals in other French regions (supplemental Table 2). The study was conducted with the approval of the Lyon-Sud Est III ethics committee (17-090), according to French law and the Declaration of Helsinki.
Tissues samples and immunohistochemistry
Twenty patients (29%) had a core needle biopsy at diagnosis and 50 (71%) an excisional biopsy. Tissue microarrays were constructed, when possible, on excisional biopsy (n = 48/70) using standard techniques, containing 3 1-mm cores per case. IHC stainings used to establish the initial diagnosis were reviewed, and these included CD20, CD30, CD15, MUM1, CD10, BCL-2, BCL-6, c-MYC, Ki67, and CD3. Additional IHC studies were performed on available formalin-fixed paraffin-embedded or tissue microarray tissue sections with an automated immunostainer (Benchmark Ultra, Ventana Medical Systems; Roche Diagnostics, Meylan, France), with antigen retrieval and antibody dilutions performed per the manufacturer’s recommendations. These additional stainings included PAX5, OCT-2, BOB1, CD138, latent membrane protein 1 (LMP1), EBV nuclear antigen 2 (EBNA-2), and programmed death ligand 1 (PD-L1) and PD-L2 for the tumor cells and programmed cell death 1 (PD1) for tumor microenvironment (TME) cells. The panel of antibodies, clones, and positivity cutoff are listed in supplemental Table 3. Scoring for percentage of positive tumor cells was carried out for CD20 and CD30 (0-1 [<10%], 2 [10% to 90%], or 3 [100%]). PD-L1 expression was quantified using PD-L1 histoscore (H-score), calculated by multiplying the percentage of malignant cells with positive staining (0% to 100%) and average staining intensity (1 [weak], 2 [moderate], or 3 [strong]).34
Statistical analysis
The date of the diagnosis refers to the date of the diagnostic biopsy. The cutoff date of the study was set on 7 February 2020. Patients ≥50 years were considered elderly, in line with previous reports.2,18,20,35-39 Overall survival (OS) was defined as time from diagnosis to death from any cause or last follow-up. Progression-free survival (PFS) was defined from date of diagnosis until date of progression, relapse, death from any cause, or last follow-up. Patients were censored at date of last follow-up. Survival distributions were estimated with the Kaplan-Meier method and compared with the log-rank test. For prognostic modeling, univariate and subsequent multivariate analyses were performed using the proportional hazard model including a smooth function for the basal rate. Statistical investigations were conducted using GraphPad PRISM 8 and R Core Team 2019.40 Models were built with the SurvPen package41 and the figures with the ggplot package.42
For original data, please contact the corresponding author.
Results
Prevalence estimates and clinical characteristics of EBV+ DLBCL-NOS patients
Among the 1696 DLBCL-NOS cases diagnosed between 2006 and 2019 in the Pathology Department of Lyon-Sud University Hospital and tested for EBER ISH, 70 fulfilled the diagnosis criteria of EBV+ DLBCL-NOS, corresponding to a prevalence of 4.1% (Figure 1). Of note, 16 cases came from outside the institute and referred for expert review.
Clinical and biological characteristics of the patients at diagnosis are summarized in Table 1. Median age at diagnosis was 68.5 years (range, 16 to 103 years), 33% being <50 years (23/70). The sex ratio showed a male predominance (44/70 [63%]). The majority of the patients presented with an advanced-stage disease (III-IV) (53/67 [79%]), B symptoms (50/65 [77%]), and elevated lactate dehydrogenase (43/58 [74%]), and half of them (36/66 [55%]) had an altered ECOG-PS ≥ 2. Thirty-two patients (32/67 [48%]) had an extranodal disease, including mainly liver (n = 15), bones (n = 10), digestive tract (n = 10), lungs (n = 4), and adrenals (n = 4). Bone marrow involvement was found in 10 of the 35 patients (29%) who underwent a bone marrow biopsy, and 2 additional patients had a positron emission tomography with diffuse bone marrow fluorodeoxyglucose fixation but negative bone marrow biopsy findings. Lymphopenia was frequent (35/52 [67%]), and hypogammaglobulinemia was observed in only 4 patients (4/29 [14%]). EBV serology was available for 21 patients, and all of them indicated past infection with positivity for immunoglobulin G (IgG) EBV viral capsid antigen, negativity for IgM EBV viral capsid antigen, and positivity for IgG EBNA-1. EBV viral load in peripheral blood was positive in 11 of the 16 tested patients (69%), with a median of 3.7 log10 IU/mL (2.3 to 6.1 log10 IU/mL). HLH, defined according to the HLH-2004 trial criteria,43 was present at diagnosis (n = 8) or during treatment (n = 1, after 3 cycles while progressing) in 9 cases (9/65 [14%]). No differences were observed at diagnosis between patients with HLH and those without (supplemental Table 5).
Compared with young patients, elderly patients (>50 years) had a poorer ECOG-PS (≥2 in 74% vs 17%, P < .001) and higher aaIPI (≥2 in 83% vs 55%, P = .032) and tended to present with more extranodal involvement (57% vs 30%, P = .070) (Table 1).
Histological classification of EBV+ DLBCL-NOS
The majority of the cases (n = 67/70) were classified within 1 of the 2 histological patterns described in the 2016 WHO classification, based on the characteristics of their tumor infiltrate (cytology, architecture, and density) and microenvironment. For 3 cases whose diagnosis was made on a core needle biopsy, histological subclassification was not possible.
Of note, 50 patients (71%) underwent an excisional biopsy and 20 patients (29%) a core needle biopsy at diagnosis, and these latter patients were significantly older and had more frequently extranodal and less nodal disease as compared with patients with excisional biopsy (supplemental Table 6).
Polymorphic EBV+ DLBCL-NOS (46/67 [69%]).
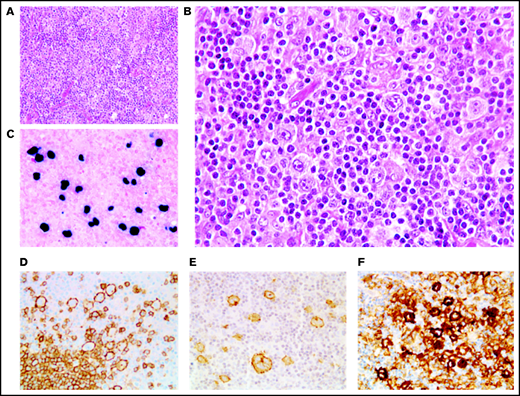
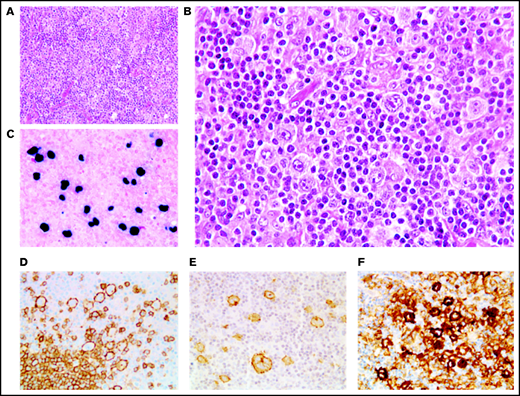
In 46 cases (27 of whom had been included in previous studies44,45), the lymph node architecture was erased by a prominent and rich inflammatory background, in which a variable number (from 2% to 40% of the cellular infiltrate) of large neoplastic cells were embedded (Figure 2). The large neoplastic B-cells resembled Hodgkin/Reed-Sternberg cells or lymphocyte predominant cells and were scattered either singly or in loose clusters (n = 41), rarely forming sheets (n = 5). The extensive reactive background included mainly small T lymphocytes and histiocytes and, in lower proportion, scattered plasma cells and neutrophils or eosinophils. Residual B follicles were present in half of the cases (23/46 [50%]), and 4 patients presented with central giant-cell granuloma. Reticular (n = 11) and/or nodular (n = 13) fibrosis was found 21 cases (21/46 [46%]), with 6 (29%) harboring a grade 3 nodular sclerosis. Foci of necrosis were observed in 6/46 cases (13%). The large neoplastic cells had a conserved B-cell phenotype (defined with the expression of ≥3 of the following B-cell markers) with co expression of CD20 (46/46 [100%]), PAX5 (40/42 [95%]), OCT-2 (37/40 [93%]) and BOB1 (32/39 [82%]). Large B-cells were positive for CD30 in all the tested cases but one (44/45 [98%]). CD15 was coexpressed in 10 out of 44 cases (23%), along with a conserved B-cell program (PAX5: 9/9 [100%], OCT-2: 7/7 [100%], and BOB1: 6/8 [75%]). Using Hans algorithm,46 all 36 analyzed cases presented a non-germinal center B immunophenotype (supplemental Figure 1). Twenty-six cases expressed c-MYC (26/35 [74%]), 12 out of 36 expressed BCL-2 (33%), and 9 out of 35 were double expressors (26%). PD-L1 was expressed in >5% of tumor cells in 37 out of 40 cases (93%), with a median PD-L1 H-score of 240 (95% confidence interval [CI], 210-280), and PD-L2 in 7 out of 40 cases (18%). In the TME, PD1 expression was found in 35 out of 41 cases (85%). LMP1 was detected in all the tested cases (40/40), and 4 also expressed EBNA-2, suggesting that EBV harbored a type II latency in 90% and a type III in 10% of the polymorphic cases (supplemental Table 4).
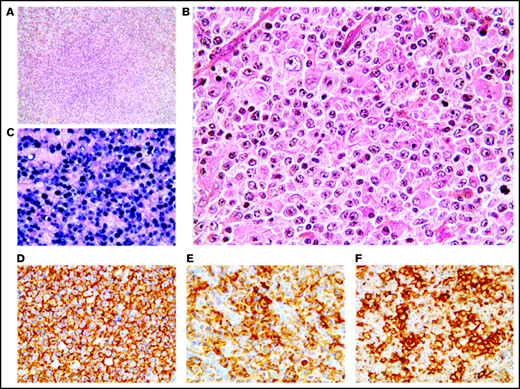
Polymorphic pattern of EBV+ DLBCL-NOS. (A-B) The lymph node architecture is effaced by a proliferation of malignant Hodgkin/Reed-Sternberg-like cells embedded in an inflammatory background. Neoplastic cells express EBER (C) and are positive for CD20 (D), CD30 (E), and PD-L1 (F). Original magnification ×200 (A) and ×400 (B-F).
Polymorphic pattern of EBV+ DLBCL-NOS. (A-B) The lymph node architecture is effaced by a proliferation of malignant Hodgkin/Reed-Sternberg-like cells embedded in an inflammatory background. Neoplastic cells express EBER (C) and are positive for CD20 (D), CD30 (E), and PD-L1 (F). Original magnification ×200 (A) and ×400 (B-F).
Monomorphic EBV+ DLBCL-NOS (21/67 [31%]).
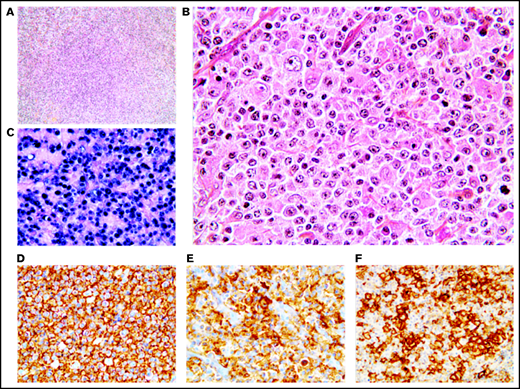
Twenty-one cases presented histological features more closely related to DLBCL with effacement of the lymph node architecture by a diffuse of focally diffuse proliferation of monomorphic medium to large lymphoid cells (Figure 3). The neoplastic B cells were monomorphic and resembled centroblasts (n = 21) and/or immunoblasts (n = 3). Partial fibrous septa (n = 9) and/or larger fibrosis bands (n = 7) were observed in 13 out of 21 cases (62%), being moderate to important in one-third of the cases (7/21 [33%]), and necrosis was identified in 9 out of 21 patients (43%), with 1 case exhibiting 80% of necrosis. The tumor cells coexpressed CD20 (21/21 [100%]), PAX5 (17/17 [100%]), OCT-2 (15/16 [94%]), and BOB1 (17/17 [100%]). CD30 was positive in all 21 cases, and none expressed CD15. The 21 monomorphic cases exhibited a non-germinal center B phenotype (supplemental Figure 1). BCL-2 and c-MYC were expressed in 5 out of 20 (25%) and 8 out of 19 (42%) cases, respectively, with 4 out of 18 being double expressors (22%). All 17 evaluable cases were positive for PD-L1, with a median PD-L1 H-score of 210 (95% CI, 130-260), and only 1 case expressed PD-L2 (1/17 [6%]). Within the TME cells, PD1 expression was observed in 13 out of 17 (76%) cases. LMP1 was positive in all 17 cases and EBNA-2 was positive in 3 of them, corresponding to a type II latency in 82% and type III in 18% of the cases (supplemental Table 4).
Monomorphic pattern of EBV+ DLBCL-NOS. (A-B) Diffuse proliferation of medium to large centroblastic lymphoid cells. (C-F) All tumor cells express EBER (C), CD20 (D), CD30 (E), and PD-L1 (F). Original magnification ×100 (A) and ×400 (B-F).
Monomorphic pattern of EBV+ DLBCL-NOS. (A-B) Diffuse proliferation of medium to large centroblastic lymphoid cells. (C-F) All tumor cells express EBER (C), CD20 (D), CD30 (E), and PD-L1 (F). Original magnification ×100 (A) and ×400 (B-F).
Among the 57 cases tested, there was no difference in age at diagnosis between patients with type II and III latency (median age of 58 years vs 64.5 years, respectively; P = .653).
When comparing the biological and clinical characteristics associated with these 2 patterns, polymorphic cases expressed CD15 (23% vs 0%, P = .049) and c-MYC (74% vs 42%, P = .037) more frequently than monomorphic cases. The distribution of type II and III latency was similar between monomorphic and polymorphic cases ([82% and 18%] vs [90% and 10%], respectively; P = .413) (supplemental Table 4). There was no significant difference in clinical characteristics at diagnosis, especially in terms of age distinction (median age of 73 years vs 64.5 years in monomorphic vs polymorphic cases, respectively; P = .327) (supplemental Table 7). A polymorphic pattern was present in 74% of the patients <50 years and 66% in patients ≥50 years vs 26% and 34%, respectively, for monomorphic cases (P = .587). Supplemental Figure 2 shows the age distribution according to histological pattern, with a bimodal distribution in both groups.
Treatments and outcome of EBV+ DLBCL-NOS patients
Treatment data were available for 69 out of 70 patients; 56 patients received an intent-to-cure therapy (56/69 [81%]) in the rituximab era, and 13 patients received palliative care only (13/69 [19%]) (Table 2). Palliative care was applied mainly due to advanced age and altered ECOG-PS (supplemental Table 8) and included steroids (n = 3), etoposide and steroids (n = 1), radiation (n = 1), or no treatment (n = 8). Among the 56 patients treated with curative intent, 41 out of 56 (73%) received an anthracycline-based chemotherapy combined with anti-CD20 antibody. Three of them received a consolidation with high-dose chemotherapy, including carmustine, etoposide, cytarabine, and melphalan, followed by autologous stem cell transplantation in first line. Within the 15 other patients (15/56 [27%]), 11 received an anthracycline-based chemotherapy alone (n = 8) or in association with RT (n = 3), and 4 progressed and died rapidly after an initial COP (cyclophosphamide, vincristine, and prednisone) (supplemental Table 9).
Overall complete response (CR) rate to first-line therapy for the 56 patients who received an intent-to-cure regimen was 73% (41/56). Ten patients (10/56 [18%]) had a progressive disease (PD), and only 1 of them could be efficiently salvaged. Finally, 5 patients (5/56 [9%]) died before response assessment from infection (n = 4, all after cycle 1) or severe toxic epidermal necrolysis (n = 1). Elderly patients more frequently experienced primary refractory disease with a higher PD rate (30% vs 0%, P = .003) following front-line therapy compared with younger patients (Table 2). Among all patients with relapsed/refractory (R/R) EBV+ DLBCL-NOS (n = 4/n = 10, respectively) , 8 received salvage therapies (8/14 [57%]) with CR in 2 cases [25%], and 4 patients did not receive any treatment due to rapid unfavorable evolution; for 2 patients, data were unknown (supplemental Table 10).
After a median follow-up of 48 months for the entire cohort, 39 patients (56%) were alive in CR, 1 (1%) with disease evolution, and 30 (43%) died. The main cause of death was lymphoma (n = 23 [77%]), followed by infection (n = 4 while on treatment [13%]), severe toxic epidermal necrolysis (n = 1 [3%]), or unknown (n = 2 [7%]).
Survival analysis in the EBV+ DLBCL-NOS cohort
For the 70 EBV+ DLBCL-NOS patients, 5-year PFS and OS were 52.7% (95% CI, 46.5-58.9) and 54.8% (95% CI, 48.5-61.1), respectively (supplemental Figure 3).
Upon univariate analysis, ECOG-PS ≥ 2 (PFS: hazard ratio [HR], 16.26; 95% CI, 4.32-61.28; P < .001; OS: HR, 15.91; 95% CI, 3.75-67.61; P < .001]), age >50 years (PFS: HR, 10.74; 95% CI, 2.72-42.43; P < .001; OS: HR, 9.93; 95% CI, 2.34-41.09; P = .002), and HLH (PFS: HR, 7.35; 95% CI, 3.11-17.37; P < .001; OS: HR, 8.31; 95% CI, 3.44-20.06; P < .001) were the strongest factors associated with PFS and OS, along with aaIPI score ≥ 2, B symptoms, clinical stage III-IV, and extranodal disease (Table 3). Given the small size of the cohort and limited number of events, only the most relevant factors in DLBCL, with a significant HR (P < .05) and the highest HR upon univariate analysis, were included in the multivariate analysis (except for the ECOG-PS, which was included in the aaIPI score). In this model, age >50 years (PFS: HR, 14.01; 95% CI, 2.34-83.97; P = .004; OS: HR, 12.41; 95% CI, 1.65-93.54; P = .014) and HLH (PFS: HR, 5.78; 95% CI, 2.35-14.23; P < .001; OS: HR, 6.09; 95% CI, 2.42-15.30; P < .001) were independently associated with a worse outcome, but not aaIPI (Table 3; supplemental Figure 4). Importantly, the univariate prognostic analysis performed in the population of 56 patients treated with curative intent highlighted the same results (supplemental Table 11; supplemental Figure 5).
Impact of EBV status on survival in DLBCL-NOS
We compared the 56 EBV+ DLBCL-NOS cases treated with curative intent to an extensive cohort of 425 EBV− DLBCL-NOS patients. This control cohort included all DLBCL-NOS patients treated with intent-to-cure immunochemotherapy (413/425 [97.2%]) or chemotherapy (12/425 [2.8%]) in the Hematology Department of Lyon-Sud University Hospital during the same period of time from 2006 to 2019 and with a negative EBER ISH result (supplemental Figure 6).
Baseline characteristics of the 56 EBV+ DLBCL-NOS cases were compared with the 425 EBV− DLBCL-NOS cases (supplemental Table 12). EBV+ patients were significantly younger than EBV− patients, with a median age of 62.7 vs 67 years (P = .008), and presented more B symptoms (P < .001) and altered ECOG-PS ≥ 2 (P = .013). The age distribution in both groups is shown in supplemental Figure 7. In the elderly population, both B symptoms and altered ECOG-PS ≥ 2 were more frequent in EBV+ cases than in EBV− cases (70% vs 33%, P < .001 and 81% vs 35%, P < .001, respectively), while it was only B symptoms in young patients (74% vs 19%, P < .001). There was no significant difference in R/R rate between EBV+ DLBCL-NOS (14/56 [25%]) and EBV− DLBCL-NOS (139/425 [32.7%]) (P = .287), with similar results in elderly patients (36% vs 35% respectively; P = .572).
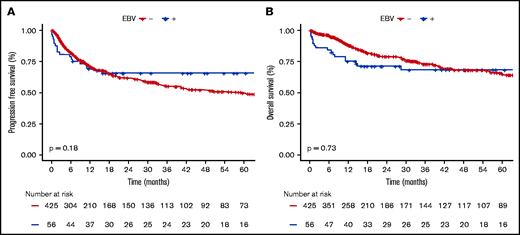
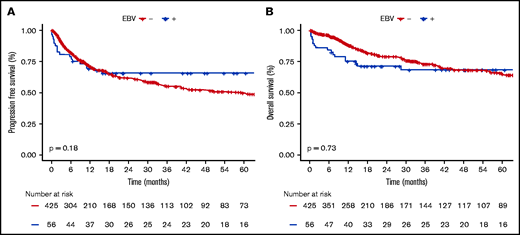
EBV+ and EBV− DLBCO-NOS cases had comparable PFS and OS (5-year PFS: 65.6%; 95% CI, 59.2-72; and 49.6%; 95% CI, 46.5-52.7, respectively; P = .180; 5-year OS: 68.3%; 95% CI, 61.9-74.7; and 64.8%; 95% CI, 61.6-68, respectively; P = .730) (Figure 4). When restricting the analysis to young patients, EBV status had no significant impact on PFS or OS (5-year PFS: 91.3%; 95% CI, 85.4-97.2; vs 69%; 95% CI, 62-76; P = .066; and 5-year OS: 91.3%; 95% CI, 85.4-97.2; vs 83.8%; 95% CI, 78-89.6; P = .438 for EBV+ vs EBV− cases). Whereas in the elderly population, EBV positivity was associated with similar PFS (5-year PFS: 47.9%; 95% CI, 33.7-68.7; vs 45.8%; 95% CI, 40-53.4; P = .405) but shorter OS (5-year OS: 53%; 95% CI, 38.2-74; vs 60.8%; 95% CI, 55.4-69.3; P = .038) (supplemental Figure 8).
Survival probabilities of EBV+ DLBCL-NOS vs EBV− DLBCL-NOS patients treated with a curative-intent regimen. (A-B) PFS (A) and OS (B) for the 56 EBV+ DLBCL-NOS compared with the 425 EBV− DLBCL-NOS cases. Both PFS and OS are not significantly between EBV+ DLBCL-NOS and EBV− DLBCL-NOS. At 5 years, PFS of EBV+ DLBCL-NOS vs EBV− DLBCL-NOS is 65.6% (95% CI, 59.2-72) and 49.6% (95% CI, 46.5-52.7), respectively (P = .180); and OS of EBV+ DLBCL-NOS vs EBV− DLBCL-NOS is 68.3% (95% CI, 61.9-74.7) and 64.8% (95% CI, 61.6-68), respectively (P = .730).
Survival probabilities of EBV+ DLBCL-NOS vs EBV− DLBCL-NOS patients treated with a curative-intent regimen. (A-B) PFS (A) and OS (B) for the 56 EBV+ DLBCL-NOS compared with the 425 EBV− DLBCL-NOS cases. Both PFS and OS are not significantly between EBV+ DLBCL-NOS and EBV− DLBCL-NOS. At 5 years, PFS of EBV+ DLBCL-NOS vs EBV− DLBCL-NOS is 65.6% (95% CI, 59.2-72) and 49.6% (95% CI, 46.5-52.7), respectively (P = .180); and OS of EBV+ DLBCL-NOS vs EBV− DLBCL-NOS is 68.3% (95% CI, 61.9-74.7) and 64.8% (95% CI, 61.6-68), respectively (P = .730).
Discussion
We report here clinical, pathological, and outcome findings in the largest cohort of EBV+ DLBCL-NOS, selected according to the latest 2016 WHO criteria with EBER cutoff > 80%. The prevalence was low (4.1%), and median age was 68.5 years, with 33% of the patients being <50 years. Histologically, 69% of the cases had a polymorphic pattern and 31% monomorphic, with a trend toward worse PFS and OS. EBV latency type II was seen in 88% and type III in 12%. Age at diagnosis and presence of an HLH were key prognostic factors in this cohort. Indeed, patients >50 years had a worse clinical presentation at diagnosis with a higher ECOG-PS score and worse aaIPI score than young cases. Elderly EBV+ DLBCL-NOS patients, but not younger patients, had worse OS compared with EBV− cases treated in the same period of time. All in all, our data emphasize the clinical, pathological, and outcome heterogenicity of EBV+ DLBCL-NOS.
The prevalence of EBV+ DLBCL-NOS in this cohort was 4.1% among all DLBCL-NOS, in accordance with other studies conducted in Western countries reporting prevalence from 2.4% to 5.8%.8,9,18,29,36,38,39 In all of these series, including ours, lack of systematic research of EBER ISH before the introduction of “EBV+ DLBCL of the elderly” in the 2008 WHO classification and restriction to elderly patients until 2016 may certainly contribute to bias this prevalence. Interestingly, we observed a bimodal distribution among both histological pattern groups that might be in part explained by EBV-primo infection occurring in young adulthood in Western coutrnies.47
The EBER cutoff of 80% set by the WHO in 2016 suggests that in EBV+ DLBCL-NOS, all neoplastic B cells are infected with EBV, with a slight deviation that may take into account technical limitations such as RNA degradation in highly proliferative and necrotic cells. In fact, during the review, we observed that EBER positivity was either low or encompassed the vast majority of tumor cells (data not shown).
We identified a majority of polymorphic cases (69%) among all age groups, with a polymorphic/monomorphic ratio similar to the one reported in other series.3,19,21,22,36 This classification of “monomorphic” vs “polymorphic” histological patterns, first described by Oyama et al in 2007,30 has largely been used in the literature since then and was introduced in the latest WHO classification.6 We found a positive expression of CD30 in almost all EBV+ DLBCL-NOS cases (98%), in contrast with the literature, where CD30 positivity range from 29% to 89% in EBV+ DLBCL-NOS,3,5,7,19,29,39 a discrepancy that could be explained by a lower EBER positivity cutoff used in these studies. Association between CD30 and EBV infection might be due to specific viral mechanisms, as it has been shown in vitro that lymphoblastoid cell lines derived from circulating B lymphocytes infected with EBV expressed high levels of CD30.48 The expression of CD30 by malignant B cells is of therapeutic relevance, with promising results of the anti-CD30 antibody coupled to a microtubule inhibitor (brentuximab vedotin) in the treatment of CD30+ lymphoma,49,50 and brentuximab vedotin is currently being evaluated in EBV+ DLBCL-NOS (NCT01805037). PD-L1 expression was described in 95% of the patients within this cohort, in accordance with other studies finding PD-L1 positivity on EBV+ malignant cells between 76% and 100%.3,51 In comparison, Xing et al reported PD-L1 positivity in only 16% among 86 cases of EBV- DLBCL.52 Different mechanisms for PD-L1 overexpression have been described in several EBV-associated lymphoma, especially through LMP1, which activates the transcription factor AP-1, JAK-STAT, and NF-κB pathways,19,53-56 or through structural variations, including PD-L1/PD-L2 gene.57 PD-L1 appears as an interesting target in these lymphomas, and several clinical trials with immune checkpoint inhibitors (nivolumab or pembrolizumab) are ongoing.58
We report here a type II EBV latency in 88% of the cases, and only 12% had a latency III, mostly described in posttransplant lymphoproliferative disease.48,59,60 Similar to Nicolae et al’s finding,3 there was no significant age difference between patients with type II vs type III latency. Aging of the immune system has initially been implicated in the development of EBV+ DLBCL-NOS, as most cases occur in elderly patients and share pathological features with posttransplant lymphoproliferative disease.55,61,62 However, other factors may play a role, especially in young patients, such as alteration of the immune microenvironment.
We provide here a very exhaustive clinical description of these EBV+ DLBCL-NOS cases and describe for the first time in this lymphoma subtype a high rate of HLH at diagnosis or under treatment in 14% of the cases, with an extremely poor outcome (median OS of 1 month). In the absence of treatment recommendation, we can highlight the importance of an early screening of HLH in EBV+ DLBCL-NOS in order to rapidly initiate immunochemotherapy. Secondary HLH (sHLH), as opposed to primary or hereditary HLH, occurs in adults and is mainly triggered by infections (50%, with EBV in first place) or malignancies (45%).63-66 In hematologic malignancies, sHLH occurs in ∽1%, a prevalence that could increase to 20% for EBV-related natural killer/T-cell lymphoma or intravascular lymphoma,67 with cases mostly described in Asia.68-71 In Western countries, small series have reported sHLH in T-cell lymphoma,72 DLBCL,64,72,73 T-cell/histiocyte-rich large B-cell lymphoma,74,75 primary effusion lymphoma,76 or Hodgkin lymphoma.77 The role of EBV as an additional trigger for sHLH has been discussed in natural killer/T-cell lymphoma,110 but to our knowledge, there are no data in the literature on sHLH and EBV+ DLBCL-NOS.
Importantly, 19% of the patients in this cohort could not benefit from curative-intent therapy, mainly due to their advanced age (median age of 86 years) and altered ECOG-PS. In this cohort, half of the patients >75 years (52%) were not treated with a curative intent. This is significantly greater than in EBV− DLBCL, as reported in a large Danish study, where 20% of the 1011 newly diagnosed DLBCLs patients aged ≥75 years received palliative care.81 However, data in frail patients with DLBCL assessing palliative care treatment are rare in the literature and may be underreported. Our data suggest that very elderly EBV+ DLBCL-NOS patients frequently present a poor ECOG-PS and/or comorbidity at diagnosis, precluding them from curative therapeutic options.
In this study, prognostic impact of EBV status varied among age groups and was negative only within the elderly population, who presented shorter OS, but not PFS, compared with EBV− DLBCL-NOS. In the literature, 3 series described similar results, with shorter OS and PFS in elderly EBV+ vs EBV− DLBCL-NOS patients, but not young patients,11,27,28 and 2 other studies in elderly patients (>50 years) showed a worse prognosis for EBV+ compared with EBV−.18,31 Elderly EBV+ DLBCL-NOS patients presented with very close PFS and OS probabilities and with similar rate of R/R disease compared with EBV− DLBCL-NOS. Therefore, the difference observed in OS, but not PFS, could suggest failure of salvage therapy in this elderly population and/or rapidly PD precluding efficient salvage therapies. Furthermore, 77% of the patients in our cohort died of lymphoma and 13% of infection after chemotherapy. Compared with a large study of 18 047 DLBCL cases,82 the death rate from lymphoma was similar (76%), but infection-related death was lower (3.2%) than in our EBV+ DLBCL-NOS patients. This strengthened the necessity to develop targeted treatment options, driving lower toxicities for EBV+ DLBCL-NOS patients.
Our series includes both exhaustive pathological and treatment data with outcome correlates not previously published in the literature. We also describe sHLH in EBV+ DLBCL-NOS, occurring in 14% of the patients and associated with an extremely poor outcome. This unreported observation requires a specific attention at diagnosis, as it might partially explain the poor prognosis related to age, with HLH being particularly challenging to cure in elderly patients. Finally, we provide additional data regarding the differential prognosis impact of EBV positivity according to age group with a negative impact restricted to the elderly patients, and we highlight the challenges of treatment in this population.
Given its retrospective and monocentric nature, and due to the limited size of the cohort, our study has some limits, and our data need to be interpreted with caution. In addition, the prevalence may have been biased due to the lack of systemic EBER ISH performed before 2016 or overrepresentation of EBV+ DLBCL-NOS in our expert center; multiple comparisons were performed, and treatment regimens were heterogenous. Other studies will be necessary to confront our survival data.
In conclusion, we report here a global characterization of EBV+ DLBCL-NOS with clinical, pathological, and outcome data. EBV+ DLBCL-NOS appears as a heterogenous entity, particularly in regard to its histological presentation with 2 distinct patterns, but also clinically, with a more aggressive course in elderly patients than young patients compared with EBV− DLBCL-NOS. These data reinforce the necessity to decipher the molecular landscape of EBV+ DLBCL-NOS and develop EBV-specific treatment strategies.
Acknowledgments
The authors thank the Lymphopath Consortium for sending their samples. The authors thank technicians from the Pathology Department, including Aurélie Gaultier, Garance Tondeur, and Beatrice Bancel.
Authorship
Contribution: E. Bourbon performed experiments; E. Bourbon, D.M.-B., and C.S. analyzed the data; A.T.-G., J.F., and C.M. participated in the histological review; E. Bourbon, C.S., and A.T.-G. wrote the paper; P.S., V.S., E.F., C.G., D.G., L.K., A.L., F.B., G.M.P., F.O.-P., R.G., C. Rocher, N.M., A.D., C. Rossi, H.G., and E. Bachy were in charge of the patients; and all authors reviewed the final version of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Clémentine Sarkozy, Département d’Innovation thérapeutique, Institut Gustave Roussy, Villejuif, France; e-mail: clementine.sarkozy@gustaveroussy.fr.
References
Author notes
A.T.-G. and C.S. contributed equally to this study.
For original data, please contact the corresponding author (clementine.SARKOZY@gustaveroussy.fr).
The full-text version of this article contains a data supplement.