Key Points
NRM increases without apparent plateau in patients with cGVHD.
The most common reported causes of death were cGVHD and infection.
Abstract
Chronic graft-versus-host disease (cGVHD) is the leading cause of late morbidity and mortality after allogeneic hematopoietic cell transplantation. To better understand patients at highest risk for nonrelapse mortality (NRM), we analyzed patient-, transplant-, and cGVHD-related variables, risk factors, and causes of nonrelapse deaths in an updated cohort of 937 patients enrolled on 2 prospective, longitudinal observational studies through the Chronic GVHD Consortium. The median follow-up of survivors was 4 years (range, 0.1 months to 12.5 years). Relapse accounted for 25% of the 333 deaths. The cumulative incidence of NRM was 22% at 5 years, and it increased over time at a projected 40% (95% confidence interval, 30%-50%) at 12 years. Centers reported that cGVHD (37.8%) was the most common cause of NRM and was associated with organ failure, infection, or additional causes not otherwise specified. The next most frequent causes without mention of cGVHD were infection (17%) and respiratory failure (10%). In multivariable analysis, an increased risk for NRM was significantly associated with the use of reduced intensity conditioning, higher total bilirubin, National Institutes of Health (NIH) skin score of 2 to 3, NIH lung score of 1 to 3, worse modified Human Activity Profile adjusted activity score, and decreased distance on walk test. To summarize, cGVHD NRM does not plateau but increases over time and is most commonly attributed to GVHD or infection, presumably associated with immunocompromised status. Severe skin and lung cGVHD remain challenging manifestations associated with increased NRM, for which novel therapeutic options that do not predispose patients to infections are needed.
Introduction
Chronic graft-versus-host disease (cGVHD) is common after allogeneic hematopoietic cell transplantation (allo-HCT). It is a major cause of late morbidity and impaired quality of life,1-3 because of direct target organ involvement and immune dysfunction, as well as immune compromise from ongoing therapy.4 cGVHD remains the main driver of late nonrelapse mortality (NRM) after HCT,5-7 but few recent studies have examined the impact of cGVHD on NRM8 with attention to potential mitigating interventions. A large US registry study reported an increasing incidence of cGVHD between 1995 and 2007 (odds ratio [OR], 1.19; P < .0001) over 3 intervals (1995-1999; 2000-2003; 2004-2007). NRM rates at 1 and 3 years among patients with cGVHD decreased over this same time period; however, at 5 years posttransplant, no significant differences among the different time periods analyzed suggested that ongoing late NRM was not improved.9
Recent advances in the management of allo-HCT recipients have led to increased transplant survivorship.10 Major strides have been taken by expanding our knowledge of cGVHD pathophysiology with an increased number of novel treatment trials for patients.11-13 However, it is unclear whether these advances have reduced cGVHD-associated NRM; detailed explorations into the association between cGVHD-related factors and NRM are lacking.
In this study, we sought to determine the cumulative incidence of cGVHD and NRM in a more recent patient cohort. In addition, we evaluated causes of death in patients with GVHD and analyzed patient-, transplant-, and cGVHD-related clinical risk factors for NRM.
Methods
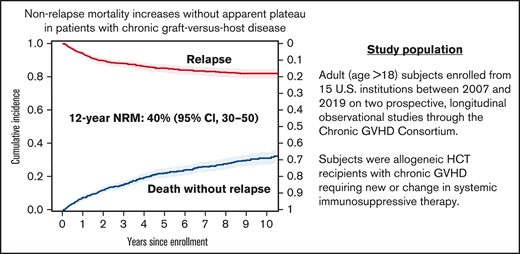
Adult patients (age older than 18 years) enrolled from 15 US institutions between 2007 and 2019 on 2 prospective, longitudinal observational studies through the Chronic GVHD Consortium were included for analysis.14,15 Patients had received allo-HCT with cGVHD requiring new or change in systemic immunosuppressive therapy. cGVHD patients were classified at enrollment as incident (<3 months after diagnosis) or prevalent (≥3 months but <3 years after diagnosis). This study analyzes only baseline (time of enrollment) data and was approved by the institutional review boards of all participating centers; all participants provided signed informed consent.
Data
Data used in this analysis include provider, patient, and laboratory measures at enrollment recommended for collection by the National Institutes of Health (NIH) Chronic GVHD Consensus conferences.16 These include the 8 provider-reported NIH organ severity scored from 0 to 3 (skin, eye, mouth, vulvo-vaginal, gastrointestinal, liver, lung, and joint). Available parameters for pulmonary function testing were considered first, and if they were unavailable, pulmonary symptoms as reported by physicians were used. Vulvo-vaginal scoring was also clinically assessed and reported by the provider, and gynecologic examinations were not mandated within the observational trials. cGVHD was graded according to the 2014 NIH Consensus Criteria.17 Also tested for their relationship with NRM were year of transplantation, time from transplantation to cGVHD diagnosis, time from diagnosis to enrollment, incident vs prevalent case, and parent clinical study. We did not include global NIH severity scoring in the analysis because that is calculated from the individual organ scores, but we present results according to global NIH mild, moderate, and severe groups.
Patient-reported outcomes (PROs) and functional measures included the Lee Symptom Scale, the Human Activity Profile (HAP), and the Short Form Health Survey (SF-36). Patients reported their cGVHD symptoms using the Lee Symptom Scale (30 items, captured as a 5-point Likert scale from “no symptoms” to “extremely bothered”).18 The summary score was calculated according to the recommendations of the developer, with higher scores reflecting worse symptoms. The HAP is a patient-reported measure of physical activity in adults, consisting of 94 questions.19 Higher scores represent greater physical activity. A modified activity score excludes activities that are restricted after HCT.20 The SF-36 is a 36-item self-report questionnaire that measures patient-reported health and functioning.21 The physical component score is a sum of individual measures of physical functioning, role limitations because of physical health, bodily pain, and general health. Mental component score is the composite of mental health, role limitations because of emotional problems, social functioning, and vitality. Higher scores represent better functioning and are normalized relative to healthy individuals. Functional measures examined in this analysis were standardized hand grip strength and the 2-minute walk test, as previously described.22
Cause of death was collected for participants who died. In one study, it was categorized with options of GVHD-related relapse, infection, other, and unknown, and with a specification of other causes. In the other study, it was free-form text. Two independent reviewers used the available information as reported by investigators to categorize the cause of death. Nine categories were created: cGVHD not otherwise specified, cGVHD and infection, cGVHD and organ failure, infection, respiratory failure, organ failure (excluding respiratory failure), bleeding, other, and unknown.
Statistical analysis
Descriptive statistics were used to summarize baseline transplantation and cGVHD features, as well as PROs. The cumulative incidence of NRM starting from the time of enrollment was estimated by the Kaplan-Meier method, with relapse and relapse deaths as competing risks. Cox regression models were used to examine associations between NRM and transplantation factors, cGVHD factors, and PROs, first univariably and then multivariably for factors found to be associated at a significance level of 0.1, using stepwise regression with entrance into and exit from the model based on a significance level of 0.1. A sensitivity analysis of the incident cGVHD patients alone was performed to investigate whether the results differed. All analyses were performed using SAS/STAT software, version 9.4 (SAS Institute, Cary, NC).
Results
Patient, transplant, and cGVHD characteristics
Patient and transplant characteristics are provided in Table 1. The median age at transplantation was 52 years (range, 18-78 years) and 60% of the patients were male. The median year of transplantation was 2010 (interquartile range [IQR], 2007-2013). Transplantations were predominately performed for hematologic malignancies (95%), most commonly acute myeloid leukemia. Conditioning was 51% myeloablative, and grafts were mostly peripheral blood (90%) and HLA-matched (85%). Half the patients had previous grade 2 to 4 acute GVHD.
cGVHD characteristics are provided in Table 2. The median time from transplantation to cGVHD diagnosis was 8 months (IQR, 7-23 months). NIH cGVHD global severity scores at enrollment were mild (13%), moderate (49%), or severe (37%). The most commonly involved organs at enrollment were skin (68%), mouth (60%), and eyes (55%). Of note, skin scores were most commonly either 2 (26%) or 3 (24%). For patients with lung involvement (29%), scoring was 1 (21%), 2 (6%), or 3 (2%) based on pulmonary function tests (if available) or clinical symptoms (if pulmonary function tests were absent). High-risk laboratory features at enrollment were rare (platelet count <100 × 109/L, 15%; total bilirubin >2 mg/dL, 4%). Functional parameters at enrollment included a median walk test of 484 feet (IQR, 400-552 feet) and median grip strength of 61 lbs (IQR, 43-81 lbs). Median summary scores at enrollment for SF-36, modified HAP adjusted activity score and Lee Symptom Scale scores are further detailed in Table 2.
NRM and cause of death
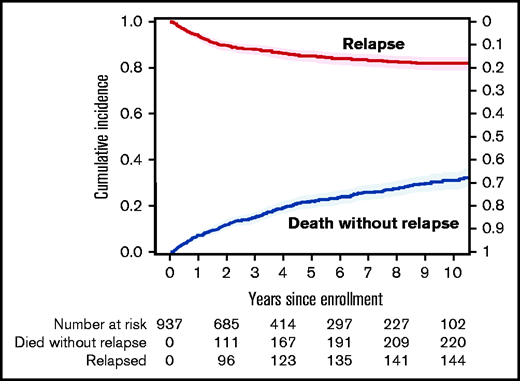
The median follow-up for survivors was 4 years (range, 0.1 months to 12.5 years). There were 333 deaths reported during the follow-up. Disease relapse accounted for 25% of the deaths. The cumulative incidence of NRM at 1 year was 7% (95% confidence interval [CI], 6%-9%), at 3 years it was 15% (95% CI, 13%-17%), and at 5 years, it was 22% (95% CI, 19%-25%) after enrollment. The cumulative incidence of NRM continued to increase over time without apparent plateau and is predicted to reach 40% (95% CI, 30%-50%) at 12 years (Figure 1).
The cumulative incidence of NRM among patients diagnosed with cGVHD. Relapse shown as a competing risk.
The cumulative incidence of NRM among patients diagnosed with cGVHD. Relapse shown as a competing risk.
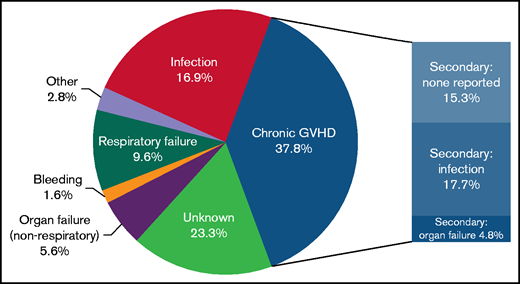
The most common identified cause of nonrelapse death was cGVHD (37.8%) associated with organ failure, infection, or additional causes not otherwise specified. The next most frequent identified causes without mention of cGVHD were infection (17%), respiratory failure (10%), and non-respiratory organ failure (6%) (Figure 2). Of note, for 23% of deaths, the cause was unknown. There was no difference in cause of death between incident and prevalent cases (data not shown).
Multivariable analysis of risk factors for NRM
Multiple variables were associated with an increased risk in univariable analysis (data not shown), including patient (age, performance status, HCT comorbidity index), transplantation (donor, conditioning intensity), cGVHD-related (NIH stage, platelet count, total bilirubin, skin, gastrointestinal, liver, lung scores), and functional and PRO (walk and grip test, SF-36 scores, modified HAP score, Lee Symptom Scale skin, energy, lung, eye, and summary scores) variables (supplemental Table 1). In multivariable analysis, an increased risk for NRM was significantly associated with the use of reduced intensity conditioning (RIC) (hazard ratio [HR], 1.5; P = .024), total bilirubin >2 mg/dL (HR, 2.24; P = .017), skin scores 2 to 3 (HR, 1.9; P = .002), lung score of 1 (HR, 1.68; P = .009), and lung score of 2 or 3 (HR, 2.25; P = .002), worse modified HAP-adjusted activity score (HR, 0.80 per 10 points; P = .01), and decreased distance on walk test (HR, 0.97 per 10 feet; P = .001) (Table 3).
Discussion
In this analysis, we sought to characterize the incidence and nature of NRM over time among patients with cGVHD enrolled on 2 large longitudinal observational studies with standardized data assessments and follow-up. NRM for patients with cGVHD in this cohort was lower compared with what has been previously reported. In the aforementioned registry analysis, the cumulative incidence NRM at 1 year, 3 years, and 5 years was 18%, 30%, and 37%, respectively.9 Regardless, the absence of an apparent plateau in NRM remains a significant long-term concern for patients living with cGVHD. Although the incidence of death is expected to increase with time in any population, the 12-year NRM of 40% estimated in this cohort of patients with cGVHD is higher than what has been previously observed in the long-term follow-up of the general allo-HCT population.7 Furthermore, the most common identified causes of death were related to cGVHD and infection, suggesting that cGVHD and its ongoing treatment are largely responsible for the sustained risk for death in this population.23
Although the association between cGVHD and NRM has long been appreciated, this study provides further evaluation of cGVHD-related factors and their association with NRM. Previous studies have identified patient- and transplantation-related factors associated with the development of cGVHD, such as patient age, donor type, graft source, and previous acute GVHD.24 The detailed cGVHD assessments collected through the Consortium allowed us to evaluate many variables which, to our knowledge, have never been investigated in the context of evaluating NRM. In this study, we found that RIC was associated with increased NRM. We hypothesize that RIC is likely a surrogate for factors such as older patient age, worse performance status, or increased comorbidities, although none of these additional factors were independently associated with NRM in multivariable analysis. We identified high-risk biochemical assessments (total bilirubin >2 mg/dL) and organ involvement (score 2 to 3 for skin, any lung involvement) associated with an increased risk for NRM. Lung or score 2 to 3 for skin involvement are known to be manifestations of cGVHD that are more difficult to treat, and these results suggest that novel approaches to either prevent or treat these challenging manifestations without exacerbating immunodeficiency should be of high importance. We also identified several enrollment functional and patient-reported assessments that are associated with a higher risk for NRM. We believe that continued investigations into how organ-specific severity, functional assessments, and PROs potentially impact clinical outcomes is of key importance for future studies.
We specifically analyzed causes of death among patients with cGVHD. As expected, most NRM deaths were attributed, at least in part, to ongoing cGVHD. Death related to cGVHD can be multifactorial. Organ failure related to GVHD was reported in 4.8% of patients. Although some patients will experience organ failure directly because of ongoing cGVHD involvement, this is less likely to happen with the most commonly involved organs such as skin, mouth, and eyes and may be related to additional complications of GVHD and therapy. Direct organ involvement is most prominent for patients with advanced lung involvement, which was previously associated with poor outcomes and is confirmed in our analysis. cGVHD can also be associated with increased risk for infection because of immune dysregulation and ongoing immunosuppression. Infection was listed as a primary contributing cause of death in 35% of NRM deaths in this study, further emphasizing the need for continued vigilance in this population, and it justifies prolonged infection prophylaxis measures. The anticipated future use of targeted therapies with less systemic immunosuppressive effects might also decrease the risk for serious infectious complications. Finally, it is important to note that relapsed malignancy accounted for 25% of the deaths in this study cohort. Although studies have suggested a lower risk of relapse in patients with cGVHD,8 our study demonstrates that this risk remains significant, even years after transplantation.
This study has several limitations. First, because the eligibility criteria was cGVHD at new or at change of systemic immunosuppressive therapy regimen, there was a higher proportion of patients with moderate or severe disease. Thus, the study population may not be representative of cGVHD patients as a whole. Second, the analysis was performed by using data collected at the time of study entry. Longitudinal evaluations of cGVHD-related factors over time may be able to more accurately identify changes that signal a new or increasing risk for NRM over the natural history of cGVHD and its treatment. Next, adjudication of cause of death is a challenge in many clinical studies. In these longitudinal studies, cause of death was reported by the participating center with no specific guidelines to follow. Whether cGVHD was active or thought to be a contributing factor in death was not standardized and therefore may be underestimated. In addition, the impact of systemic therapy, including response to therapy and duration of treatment, could not be evaluated in this analysis. Finally, the majority of the follow-up in this cohort took place before the recent introduction of novel approaches for the prevention (posttransplant cyclophosphamide) and treatment (ibrutinib, ruxolitinib) of cGVHD.12,13 Thus, it remains unknown whether using these agents could reduce NRM because of their less immunosuppressive nature and potential to induce sustained cGVHD responses.
In conclusion, NRM continues to increase over time in patients with cGVHD without an apparent plateau and is most commonly attributed to GVHD or infection, presumably associated with immunocompromised status. More advanced skin cGVHD and the presence of lung cGVHD remain challenging manifestations associated with increased NRM, for which novel therapeutic options are needed.
Acknowledgement
This work was supported by a grant from the National Cancer Institute, National Institutes of Health (CA118953).
Authorship
Contribution: Z.D., L.E.O., S.J.L., and B.K.H. designed the study; A.M.A., J.A.P., P.A.C., S.A., M.A., C.S.C., M.E.D.F., C.L.K., G.L.C., S.J.L., and B.K.H. contributed clinical data; Z.D., L.E.O., S.J.L., and B.K.H. designed and performed the analyses; Z.D. and B.K.H. wrote the manuscript; and all authors approved the final version of the manuscript and submission of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zachariah DeFilipp, Hematopoietic Cell Transplant and Cellular Therapy Program, Massachusetts General Hospital, 55 Fruit St, Zero Emerson, Suite 118, Office 134, Boston, MA 02114; e-mail: zdefilipp@mgh.harvard.edu.
References
Author notes
The full-text version of this article contains a data supplement.