Key Points
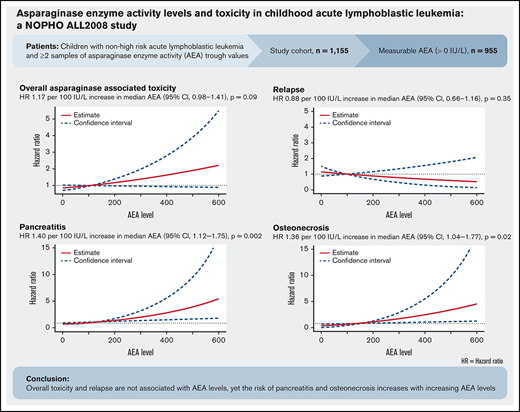
Overall asparaginase-associated toxicity and relapse were not significantly associated with increased asparaginase enzyme activity levels.
The risk of pancreatitis and osteonecrosis were significantly associated with increasing asparaginase enzyme activity.
Abstract
Asparaginase treatment is a mainstay in contemporary treatment of acute lymphoblastic leukemia (ALL), but substantial asparaginase-related toxicity may lead to jeopardized protocol compliance and compromises survival. We investigated the association between risk of asparaginase-associated toxicities (AspTox) and asparaginase enzyme activity (AEA) levels in 1155 children aged 1.0 to 17.9 years, diagnosed with ALL between July 2008 and March 2016, and treated according to the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL2008 protocol. Patients with ≥2 blood samples for AEA measurement drawn 14 ± 2 days after asparaginase administration were included (6944 trough values). AEA was measurable (or >0 IU/L) in 955 patients, whereas 200 patients (17.3%) had asparaginase inactivation and few AspTox recorded. A time-dependent multiple Cox model of time to any first asparaginase-associated toxicity adjusted for sex and age was used. For patients with measurable AEA, we found a hazard ratio (HR) of 1.17 per 100 IU/L increase in median AEA (95% confidence interval [CI], 0.98-1.41; P = .09). For pancreatitis, thromboembolism, and osteonecrosis, the HRs were 1.40 (95% CI, 1.12-1.75; P = .002), 0.99 (95% CI, 0.70-1.40; P = .96), and 1.36 (95% CI, 1.04-1.77; P = .02) per 100 IU/L increase in median AEA, respectively. No significant decrease in the risk of leukemic relapse was found: HR 0.88 per 100 IU/L increase in AEA (95% CI, 0.66-1.16; P = .35). In conclusion, these results emphasize that overall AspTox and relapse are not associated with AEA levels, yet the risk of pancreatitis and osteonecrosis increases with increasing AEA levels.
Introduction
Survival rates in childhood acute lymphoblastic leukemia (ALL) now exceed 90%.1-7 However, serious treatment-related toxicities challenge protocol compliance and cure rates. Thus, the balance between antileukemic effect and toxicity has become critical. Asparaginase is essential in the treatment of ALL in children, and truncated asparaginase therapy has been associated with inferior survival.8-11 Unfortunately, asparaginase is also associated with a significant burden of serious toxicity that often prevents further treatment.11-13 The most common asparaginase-associated toxicities (AspTox) in children are hypersensitivity (clinical allergy, 13%-15%8,11,14 or silent inactivation, 3%-8%11,15,16 ), pancreatitis (5%-11%8,9,16-19 ), and thromboembolism (5%-8%20-23 ). Asparaginase, at least when administered concomitantly with glucocorticosteroids, has also been suggested to contribute to the development of osteonecrosis (6%-11%).24,25 Hypersensitivity is accompanied by immunologic inactivation of asparaginase enzyme activity (AEA) preventing an effective antileukemic effect.15,26
The treatment efficacy of asparaginase is based on adequate depletion of the nonessential amino acid, asparagine, because lymphoblasts are dependent on exogenous sources of asparagine.27 Because of continued asparagine depletion ex vivo after sampling, it is difficult to measure asparagine levels in the blood during asparaginase therapy.28-30 Measurement of AEA is considered the best way to monitor clinical effectiveness. Therapeutic drug monitoring (TDM) is therefore widely used to identify patients with no AEA as a result of silent inactivation or in association with hypersensitivity reactions because absence of AEA has been associated with inferior outcome.16,27,31 However, AEA is not commonly used to identify patients with levels above the therapeutic target or to assess the risk of developing AspTox owing to limited knowledge regarding the association between toxicities and AEA levels.
We evaluated the association between AEA levels and the risk of AspTox and relapse in a large Nordic/Baltic cohort of children with ALL.
Patients and methods
Study population
Participants were children aged 1.0 to 17.9 years and diagnosed with non-high-risk, Philadelphia chromosome-negative B-cell precursor or T-cell ALL.
Patients were diagnosed between 1 July 2008 and 1 March 2016 and treated according to the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL2008 protocol in Denmark, Estonia, Finland, Iceland, Lithuania, Norway, and Sweden.
NOPHO ALL2008 and asparaginase treatment
In the NOPHO ALL2008 protocol, patients are stratified into 3 risk groups: standard risk, intermediate risk, and high risk. Polyethylene glycol conjugated Escherichia coli–derived asparaginase (PEG-asparaginase) was used as standard preparation in the protocol; 1000 IU/m2/dose was administered intramuscularly from treatment day 30 from diagnosis. All non-high-risk patients received 5 doses of PEG-asparaginase at 2-week intervals during consolidation therapy. Subsequently, patients were invited to participate in a randomized asparaginase study in which they were randomly assigned to receive either 10 additional doses of PEG-asparaginase at 2-week intervals (standard arm) or 3 additional doses at 6-week intervals (experimental arm). Patients not included in the randomization were scheduled for 15 doses of PEG-asparaginase. The randomization was prematurely truncated on 1 March 2016 because an interim analysis showed no difference in disease-free survival between the 2 arms but did show a significantly reduced incidence of AspTox in the experimental arm.13 After closure of the randomization and end of this study, all patients received 8 doses as standard of care. Details about the NOPHO ALL2008 protocol and asparaginase treatment are presented in the supplemental Data.
The NOPHO ALL2008 protocol was approved by the National Medicines Agencies (EudraCT no. 2008-003235-20) and the relevant national or regional ethics committees in each participating country. The study was registered at clinicaltrials.gov (NCT03987542). Informed consent was obtained from patients and/or legal guardians according to national guidelines, and the study complied with the Declaration of Helsinki (version 2008).
Asparaginase enzyme activity measurements
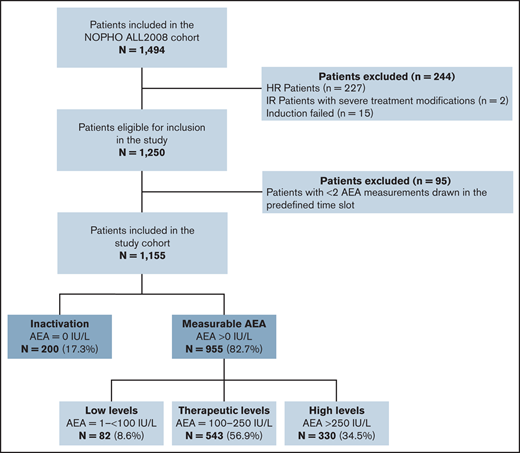
Blood samples for AEA measurements were scheduled before each PEG-asparaginase administration. Asparaginase trough levels were defined as samples drawn 14 ± 2 days after administration. Patients with ≥2 samples drawn during this period or early inactivation were included in the study and divided into 2 main groups based on AEA measurements: (1) asparaginase inactivation (AEA = 0 IU/L) and (2) measurable AEA (>0 IU/L). Patients with measurable AEA were subdivided into 3 predefined groups based on the median AEA level (AEAmed): (A) low level (1-<100 IU/L), (B) therapeutic level (100-250 IU/L), and (C) high level (>250 IU/L). Patients with clinical allergy and only 1 AEA sample drawn less than 16 days after an administration without measurable AEA (n = 32) were presumed to have no AEA, and thus assigned to the inactivation group because clinical allergy typically appeared after the second or third dose of PEG-asparaginase.13 Patients with inactivation are underexposed to PEG-asparaginase, and these patients were therefore not included in the main statistical analyses. All samples were analyzed retrospectively and not reported continuously to the clinicians. Samples were analyzed by Nessler’s reagent or aspartic acid β-hydroximate assay.31,32
Definition and registration of asparaginase-associated toxicities
Hypersensitivity, pancreatitis, thromboembolism, and osteonecrosis were defined and graded according to the Ponte di Legno Toxicity Working Group consensus definitions.33 Only symptomatic events fulfilling diagnostic criteria were included. Toxicities were defined as AspTox if they occurred within 18 days after last PEG-asparaginase administration, when most patients have been shown to have enzyme activity levels >100 IU/L after intramuscular administration.27,32 However, osteonecrosis was defined as an asparaginase-associated toxicity if it occurred any time after start of asparaginase treatment. Clinical characteristics were collected to describe the severity of each toxicity. Toxicities were prospectively included in the NOPHO registry through online mandatory toxicity registration quarterly, with a compliance rate of 98.9%.25,34 Detailed data were subsequently retrieved from the treating centers, NOPHO Working Group coordinators, and involved researchers who have published data of the individual AspTox. Data on patient demographics, ALL characteristics, and treatment were retrieved from the NOPHO ALL2008 study database.
Statistical analyses
All AEA measurements done in the defined time slot were included in the analyses.
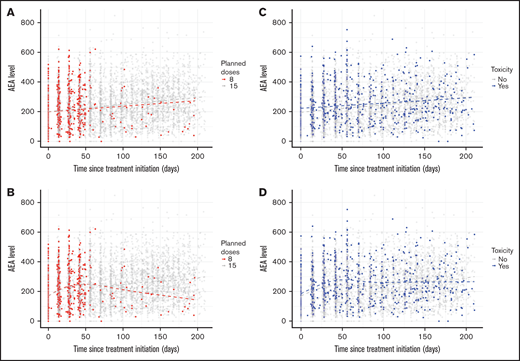
To assess whether trends over time in AEA levels depended on the number of planned asparaginase treatments (8 vs 15), and whether the patients experienced any AspTox within the follow-up period (yes vs no), we performed a linear regression analysis of individual AEA measurements against time since treatment initiation stratified by the number of doses and toxicity. Trends in AEA levels over time were also assessed visually by plotting the median AEA level on each day since treatment initiation with addition of a nonparametric smoother to describe the trend over time in median AEA level for each group.
In the primary analysis, we considered any asparaginase-associated toxicity as the outcome parameter. In this analysis, all patients were followed from first AEA measurement until the date of first asparaginase-associated toxicity (pancreatitis, thromboembolism, or osteonecrosis), relapse, death, secondary malignancy, or 18 days after the last asparaginase administration, whichever occurred first. Death was considered a competing event, and secondary malignancy was treated as censoring event. For the analysis of any asparaginase-associated toxicity, pancreatitis, thromboembolism, and asparaginase treatment truncation, the AEA level throughout the study period was modeled as a time-dependent covariate that was updated at every AEA measurement and defined as the median of the current AEA measurement and any AEA measurements done in the past 30 days. Individuals were furthermore censored if more than 30 days had elapsed without a new AEA measurement. Patients reentered the analysis if a new AEA measurement was registered.
For the analysis of osteonecrosis and relapse as outcomes, patients were followed from the date of the last treatment until the date of event, death, secondary malignancy, or end of follow-up on 18 September 2018, whichever occurred first. Death was considered a competing event, and secondary malignancy was treated as censoring event. For these outcomes, the median AEA level throughout the entire treatment period was used as covariate.
For all outcomes, separate Cox proportional hazards models were fitted with median AEA level as the primary covariate and adjusted for age and sex. The analysis of relapse was further adjusted for the ALL risk group.
To describe the AEA levels of the entire treatment period for each patient and clinical characteristics of toxicity, the AEAmed was calculated. Effect modification by number of doses was assessed in a subgroup analysis.
Two-sided P values < .05 were considered statistically significant. All statistical analyses were carried out using the statistical computing software R version 4.0.3 (R Core Team).
Results
Patient characteristics
During the study period, 1494 patients with ALL were treated according to the NOPHO ALL2008 protocol. Patients with high-risk ALL (n = 227), severe treatment modifications (n = 2), and induction failure (n = 15) were excluded. Additionally, 95 patients with <2 AEA measurements were excluded; 22 of these patients had 1 AEA measurement in the defined time slot and 73 patients had none. In total, 1155 patients were included; 955 patients (82.7%) had measurable AEA and 200 (17.3%) had asparaginase inactivation. Thirty-two patients were included in the inactivation group based on evidence of clinical allergy and 1 available sample; they were presumed to have early inactivation. In this study cohort, 868 patients were scheduled for 15 doses (315 of whom were included in the randomization) and 287 patients were randomized to 8 doses of PEG-asparaginase.
Patients with measurable AEA were divided into 3 predefined subgroups: (A) low level; n = 82 (8.6%), median AEAmed level: 80 IU/L (interquartile range [IQR], 58-91 IU/L); (B) therapeutic level; n = 543 (56.9%), median AEAmed level: 182 IU/L (IQR, 148-213 IU/L); and (C) high level; n = 330 (34.5%), median AEAmed level: 311 IU/L (IQR, 282-369). Flowchart is shown in Figure 1.
In the study cohort, 324 cases of asparaginase-associated toxicity were registered in 308 patients (308/1155; 26.7%) including 161 cases of clinical allergy (13.9%), 69 cases of pancreatitis (6.0%), 43 cases of thromboembolism (3.7%), and 51 cases of osteonecrosis (4.4%). The median age at diagnosis for the total cohort was 4 years (IQR, 4-7 years). Median age for patients with inactivation was 3 years (IQR, 2-6 years) compared with 4 years (IQR, 2-8) for patients with AEA >0 IU/L (P < .001). Sex, risk group, immunophenotype, and white blood cell count at diagnosis were equally distributed between patients with inactivation and measurable AEA and between patients with AspTox and no AspTox. Baseline characteristics can be found in Table 1.
Of 200 patients with inactivation, 149 had clinical allergy (74.5%) and 51 had silent inactivation (25.5%). No prophylactic treatment to avoid clinical allergic reactions were used as standard of care in the NOPHO ALL2008 protocol. Four cases of other toxicities (2%) were registered in this group: 1 with pancreatitis, 1 with thromboembolism, and 2 with osteonecrosis compared with 16.8% of AspTox in the group with measurable AEA.
Asparaginase enzyme activity levels
The total number of samples with AEA trough values was 6944, and the median number of samples per patient was 5 (range, 2-15). The median AEA trough level for all patients with measurable AEA was 221 IU/L (IQR, 138-316). Overall, the study illustrated large intra- and inter-individual variation for all patients (supplementary Data; supplemental Figure 1). Figure 2 shows AEA levels over time since first sample, stratified by the number of planned doses and by toxicity. The relationship between median AEA level and time since first sample showed a somewhat different pattern for patients receiving asparaginase at 2-week intervals (15 doses total) and asparaginase at 6-week intervals (8 doses) (Figure 2A). In a linear regression model of all individual AEA measurements, the AEA level increased with time, but we found no significant difference between the increase in patients scheduled for 8 doses (slope = 0.40 IU/L; 95% confidence interval [CI], 0.02-0.62) compared with 15 doses (slope = 0.49 IU/L; 95% CI, 0.43-0.54) (P = .44); Figure 2B.
Individual asparaginase enzyme activity measurements over time stratified by number of planned doses and toxicity (n = 6944).
Individual asparaginase enzyme activity measurements over time stratified by number of planned doses and toxicity (n = 6944).
Asparaginase-associated toxicities
In all, 159 events, pancreatitis, thromboembolism, or osteonecrosis, were registered in 955 patients with measurable AEA. Twelve patients were registered with clinical allergy without having inactivation of asparaginase. Median age for patients with AspTox was 8 years (IQR, 4-12) compared with 4 years (IQR, 2-6) for patients with no AspTox (P < .001). Other baseline characteristics were equally distributed. The median AEA level for patients with measurable AEA who experienced AspTox was 224 IU/L (IQR, 149-333) compared with 221 IU/L (IQR, 138-316) for patients with no AspTox (P = .1).
Individual AEA measurements stratified by toxicity over time are shown in Figure 2C; a flexible curve describing median levels showed a similar pattern for patients with and without toxicity. A linear regression model of all individual measurements was used to show how AEA levels change with time for patients with and without toxicity (Figure 2D). The slope describes increases in AEA over time, and we found no significant difference between the increase in AEA in patients with AspTox (slope = 0.36 IU/L; 95% CI, 0.21-0.51) compared with patients without AspTox (slope = 0.51 IU/L; 95% CI, 0.45-0.56) was found (P = .07) (Figure 2D). Most patients with AspTox had AEAmed at a therapeutic level (98/159, 61.6%); 32.7% had AEA at a high level, and 5.7% had AEA at a low level (Table 2).
In an age- and sex-adjusted time-dependent multiple Cox proportional hazards model of time to any first asparaginase-associated toxicity, we found no significant association between AEA levels and the hazard ratio (HR) of asparaginase-associated toxicity (HR, 1.17 per 100 IU/L increase in median AEA; 95% CI, 0.98-1.41; P = .09) (Figure 3). In a subgroup analysis for patients scheduled for 8 (n = 269) and 15 doses (n = 686) of PEG-asparaginase the HR of asparaginase-associated toxicity per 100 IU/L increase in median AEA were 0.61 (95% CI, 0.24-1.50; P = .296) and 1.23 (95% CI, 1.02-1.49; P = .03), respectively (supplemental Table 1). We found no significant difference in the association between the groups (P = .995)
Adjusted hazard ratio of any asparaginase-associated toxicity by median asparaginase enzyme activity level. Hazard ratio of 1.17 per 100 IU/L increase in median AEA (95% CI, 0.98-1.41; P = .09).
Adjusted hazard ratio of any asparaginase-associated toxicity by median asparaginase enzyme activity level. Hazard ratio of 1.17 per 100 IU/L increase in median AEA (95% CI, 0.98-1.41; P = .09).
Pancreatitis
In total, 68 cases of pancreatitis in 955 patients were identified, among these, 58 (85.3%) had severe pancreatitis. Four patients (5.9%) had an AEAmed in the low level, 39 (57.4%) in the therapeutic level, and 25 (36.8%) the high level.
In an age- and sex-adjusted time-dependent Cox proportional hazards model, we found a significantly increased risk of pancreatitis with increasing AEA levels (HR, 1.40 per 100 IU/L increase in AEA; 95% CI, 1.12-1.75; P = .002) (supplemental Figure 2). A subgroup analysis for patients scheduled for 8 or 15 doses was not feasible for this outcome because of a low number of events among patients scheduled for 8 doses.
We did not observe a higher number of patients diagnosed with severe pancreatitis (according to Ponte di Legno Toxicity Working Group consensus definitions) in the group with high levels of AEA (20/25, 80%) compared with the group with AEA at therapeutic levels (33/39, 85%). Clinical characteristics of AspTox can be found in Table 2.
Thromboembolism
Among 955 patients, 42 cases of thromboembolism were identified, of these 23 (54.8%) had grade 3 thromboembolism or higher. Two patients (9.5%) had a low level AEAmed, 31 (73.8%) a therapeutic level, and 9 (21.4%) a high level. No significant association between increasing levels of AEA and hazard of thromboembolism was found (HR, 0.99 per 100 IU/L increase in AEA; 95% CI, 0.70-1.40); P = .96) in a time-dependent Cox proportional hazards model (supplemental Figure 3). We found no difference in the association between AEA and thromboembolism in the groups scheduled for 15 or 8 doses, P = .580 (supplemental Table 1).
Three patients were registered with grade 5 thromboembolism (thrombosis-related death); all had a AEAmed at a therapeutic level (Table 2).
Osteonecrosis
Forty-nine of 955 patients developed osteonecrosis. Grade ≥3 osteonecrosis was found in 24 patients (49.0%). Three patients (6.1%) had a AEAmed in the low level, 28 (57.1%) in the therapeutic level, and 18 (36.7%) in the high level.
In an age- and sex-adjusted Cox proportional hazards model, a significantly increased hazard of osteonecrosis with increasing AEA levels was found (HR, 1.36 per 100 IU/L increase in AEA; 95% CI, 1.04-1.77; P = .02) (supplemental Figure 4).
The subgroup analysis for patients scheduled for 8 and 15 doses of PEG-asparaginase showed significant difference between the 2 groups (P = .034). HR of asparaginase-associated toxicity were 0.83 (95% CI, 0.47-1.46; P = .52) and 1.66 (95% CI, 1.22-2.26; P = .001), respectively (supplemental Table 1). Patients with grade 4 osteonecrosis had low levels (1/3, 33.3%), therapeutic levels (13/28, 46%), and high levels (8/18, 44%) of AEA (Table 2).
PEG-asparaginase treatment and leukemia-specific outcomes
Of the study cohort, 268 (23.2%) patients were registered with premature discontinuation of PEG-asparaginase therapy. The most frequent causes of truncation were clinical hypersensitivity (n = 161, 60.1%, 149/161 with inactivation), pancreatitis (n = 57, 21.3%), and thromboembolism (n = 15, 5.6%). None of the patients experienced truncation of PEG-asparaginase therapy because of osteonecrosis. All truncation characteristics are shown in supplemental Data and supplemental Table 2.
We found no association between increasing AEA levels and hazard of asparaginase truncation (HR, 0.99 per 100 IU/L increase in AEA; 95% CI, 0.81-1.19; P = .85) for patients with measurable AEA (supplemental Figure 5).
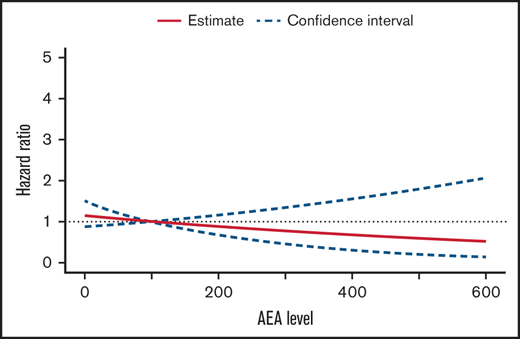
Of 955 patients with measurable AEA, 57 experienced leukemic relapse after first complete remission, 9 developed a second malignant neoplasm, and 9 patients died during first remission. In a Cox proportional hazards model adjusted for sex, age, and risk group, we found no significant association between increasing AEA levels and hazard of relapse (HR, 0.88 per 100 IU/L increase in AEA; 95% CI, 0.66-1.16; P = .35) (Figure 4). No difference in the association between AEA and relapse in the groups scheduled for 15 or 8 doses was found, P = .30 (supplemental Table 1).
Adjusted hazard ratio of relapse by median asparaginase enzyme activity level. Hazard ratio 0.88 per 100 IU/L increase in median AEA (95% CI,0.66-1.16; P = 0.35).
Adjusted hazard ratio of relapse by median asparaginase enzyme activity level. Hazard ratio 0.88 per 100 IU/L increase in median AEA (95% CI,0.66-1.16; P = 0.35).
Discussion
Measurements of AEA are considered the best way to monitor clinical efficacy of asparaginase.9,26,27,31 However, TDM is not commonly used to identify patients with high levels of AEA because there is limited evidence assessing its clinical importance. In this study, we illuminate the association between risk of AspTox and leukemic relapse for different AEA levels during treatment in childhood ALL.
This study demonstrated no significant increase in the risk of any type of asparaginase-associated toxicity with increasing AEA levels. Based on these results, we do not think there is any clinical advantage of using TDM to identify patients with high levels of AEA to enable dose reduction with the aim of avoiding overall toxicity associated with asparaginase. This is consistent with a recent study demonstrating a limited effect on AspTox after reducing the dose of PEG-asparaginase (starting at 1500 IU/m2) in patients with AEA >250 IU/L.16 However, it is important to note that Figure 3 shows a trend of increasing risk of any toxicity with increasing AEA levels. Furthermore, the association between any AspTox and increasing AEA levels was statistical significance for patients scheduled for 15 doses, illuminating that number of doses and frequency in administration affect the association. Therefore, we speculate whether the association between AEA levels and AspTox is more apparent in patients with even higher AEA levels than shown in this study, because it is well-known that a high cumulative dose of asparaginase causes higher AEA levels, and this has previously been associated with an increased burden of toxicity.13,15,35,36 Because our conclusions based on children treated with a relatively low dose of PEG-asparaginase, this should be confirmed in a cohort receiving higher asparaginase doses than those used in the present study and in patients older than age 18 years. Furthermore, the intervals between asparaginase doses might affect this as well because we observed higher AEA levels in patients with continuous PEG-asparaginase exposure at 2-week intervals compared with patients with intermittent asparaginase dosing at 6-week intervals. Results of the separate toxicity analyses showed that each type of asparaginase-associated toxicity might affect this association in different directions and that it is important to investigate each toxicity separately.
This study reports a very limited incidence of toxicity in patients with inactivation of asparaginase, emphasizing that these patients are underexposed to asparaginase.
For pancreatitis, we found an increasing risk with increasing AEA levels. A high cumulative dose of PEG-asparaginase has been suggested to be a risk factor for pancreatitis,13,37 supporting the importance of both total asparaginase exposure and AEA trough levels. Using TDM to identify patients with high AEA levels might be useful to detect patients assumed to have an increased risk of pancreatitis, especially patients older than 10 years of age because these patients have been shown to have an increased risk of pancreatitis.18 Dose reduction might be beneficial for these patients. However, a recent study found no significant correlation between pancreatitis and AEA levels, and dose reduction in patients with high AEA levels had a limited effect on the risk of toxicity when comparing the results with a previous treatment protocol; however, the nonrandomized study included a smaller cohort, fewer AEA measurements, and fewer events than ours.16
Moreover, we found an association between increasing AEA levels and osteonecrosis. This was somewhat unexpected because the association between osteonecrosis and asparaginase treatment is still being debated,38 but we interpret the results as confirming asparaginase as a contributing factor in the risk for developing osteonecrosis during or after ALL treatment. Osteonecrosis is associated with exposure to glucocorticoids.39 In the NOPHO ALL2008 protocol, dexamethasone is scheduled in the same time periods as PEG-asparaginase treatment of patients receiving 15 doses. The subgroup analysis showed a significant difference in the association between AEA levels and osteonecrosis in patients scheduled for 8 or 15 doses. This illustrates that the number of scheduled doses is an effect modifier for the association. Concomitant treatment with dexamethasone might affect the association as well. The use of TDM to identify patients with high AEA levels to prevent osteonecrosis might be relevant in a clinical setting, especially in patients with additional risk factors such as hyperlipidemia,24,36,40,41 being female, and age older than 10 years.24 Finally, it might be beneficial to avoid concurrent therapy with glucocorticoids39 in patients with high AEA levels. Whether dose reduction is beneficial must be investigated further.
We demonstrated no association between increasing AEA levels and risk of thromboembolism, which is supported by a report by Kloos et al.16 This consolidates the notion that asparagine depletion causes a prothrombotic state regardless of AEA level. Furthermore, several other factors are known to increase the risk of thromboembolism in children with leukemia (eg, presence of indwelling central venous lines, immobilization, leukemic burden, and corticosteroids).42-44 These factors likely contribute to the risk of thromboembolism to a greater extent than the AEA level.
We did not observe an association between grade and severity of the clinical characteristics of each asparaginase-associated toxicity and AEA level. Especially, we did not find any evidence of an association between high levels of AEAmed and increased severity compared with lower levels. However, the small number of events in each AEA group precludes conclusions regarding the association between clinical characteristics and the grading of AspTox. Thus, future studies are needed to further explore this.
In our patients, premature asparaginase discontinuation because of toxicity was not associated with increasing AEA levels. The most frequent causes of truncation were clinical allergy, pancreatitis, and thromboembolism. It is important to explore further how to avoid asparaginase truncation because truncation has been associated with inferior event-free survival in several studies.8-11
The risk of leukemic relapse did not decrease significantly with increasing AEA levels, which confirms the idea that if patients have measurable AEA levels and thus asparagine depletion, this might be sufficient to ensure an antileukemic effect. This also indicates that dose reduction might not increase risk of relapse if asparagine depletion is maintained.
Overall, we consider that the association between high AEA levels and increasing risk of pancreatitis and osteonecrosis justifies the use of TDM to identify patients with high AEA levels, but the benefit of dose reduction in relation to prevention of pancreatitis and osteonecrosis should be validated in another cohort. The trend of increasing risk of overall AspTox with increasing AEA must be investigated further, especially in patients with even higher AEA levels or for patients receiving higher doses of asparaginase. These patients might benefit from dose reduction to a greater extent.
We showed that asparaginase levels change over time, which consolidates the use of TDM during the whole asparaginase treatment period for identification of emerging inactivation.
The main strengths of this study are the large number of patients included and the systematic, prospective toxicity registration with a very high compliance rate of 98.9%.25,34 This provides a unique platform for exploring AspTox. Furthermore, the high number of AEA measurements is a strength; only a small number of patients (95/1,250, 7.6%) were excluded because of too few AEA measurements in the defined time period. Many single AEA measurements were noninformative because of a prolonged interval between PEG-asparaginase and sampling; these measurements were thus excluded from the analysis, which is a limitation.
In conclusion, our study demonstrated no significant increase in the risk of overall AspTox, asparaginase truncation, or leukemic relapse with increasing AEA levels, but a significant association between increasing AEA levels and pancreatitis and osteonecrosis was found, which should be taken into consideration when future treatment protocols are designed. TDM of AEA is indicated, mainly to detect inactivation, but may be further explored for dose reduction to reduce some specific toxicities.
Acknowledgments
The authors kindly thank all clinicians at the ALL centers for reporting data to the NOPHO ALL registry, for providing missing data, and sending samples for AEA analyses. Furthermore, the authors sincerely thank laboratory technician, Jane Hagelskjaer Knudsen, at the Paediatric Research Laboratory, Aarhus University Hospital, for excellent work conducting the asparaginase enzyme activity analyses.
This work was supported by grants from the Danish Childhood Cancer Foundation (B.K.A.), the Swedish Childhood Cancer Foundation (B.K.A.), the Dagmar Marshall’s foundation (L.S.L.), the Arvid Nilsson’s foundation (L.S.L.), Fabrikant Einar Willumsen’s foundation (L.S.L.), Eva and Henry Fraenkel’s foundation (L.S.L.), Jens and Maren Thestrups grant for cancer research (L.S.L.), and Holm’s memorial grant (L.S.L.).
Authorship
Contribution: L.S.L. designed the study, collected, analyzed, and interpreted data, wrote, and edited the manuscript; B.K.A. designed the study, collected, and interpreted data and critically reviewed the statistical analyses and the manuscript; S.N.H. analyzed and interpreted the data and reviewed the manuscript; C.U.R. contributed to data collection and interpretation and critically reviewed statistical analyses and the manuscript; K.S. was responsible for NOPHO ALL2008 protocol designed and critically reviewed the manuscript; and S.G.H., L.T.H., K.B.J., S.R., R.N., A.H.-S., B.O.W., T.L.F., and M.H. collected data and edited the manuscript.
Conflict-of-interest disclosure: B.K.A.: sponsor of the investigator-initiated trial NOR-GRASPALL2016. K.S.: speaker and/or Advisory Board Honoraria from Jazz Pharmaceuticals (2020, 2021) and Servier (2020); speaker fee from Amgen (2020) and Medscape (2020, 2021); educational grant from Servier (2020, 2021). The remaining authors declare no competing financial interests.
Correspondence: Birgitte Klug Albertsen, Aarhus University Hospital, Palle Juul-Jensens Blvd 99, 8200 Aarhus N, Denmark; e-mail: biralber@rm.dk.
References
Author notes
Requests for data sharing may be submitted to Birgitte Klug Albertsen (biralber@rm.dk).
The full-text version of this article contains a data supplement.