Key Points
Blacks/African Americans have a higher incidence, whereas Asians/Pacific Islanders have a lower incidence, of CAT than White Americans.
The disparity is driven by the incidence of pulmonary embolism.
Abstract
Race and ethnicity are associated with risk of venous thromboembolism in population-based studies. Blacks/African Americans have a higher incidence, whereas Asians/Pacific Islanders and Hispanics have a lower incidence of venous thromboembolism compared with non-Hispanic Whites. The impact of race/ethnicity on the incidence of cancer-associated thrombosis (CAT), a common complication in patients with malignancy, has not been well defined. Using the California Cancer Registry linked to the California Patient Discharge Dataset and Emergency Department Utilization database, we studied a large, diverse cohort of patients (n = 942 109) from 2005 to 2017 with the 13 most common, first primary malignancies to determine the association between race/ethnicity and incidence of incident and recurrent CAT. Multivariable Cox proportional hazards regression models were performed to determine the effect of race/ethnicity on the risk of overall CAT, specific CAT by location, and recurrent CAT. Blacks/African Americans had a higher incidence of CAT for all tumor types except myeloma, whereas Asians/Pacific Islanders had a consistently lower incidence of CAT compared with non-Hispanic Whites, after adjusting for potential confounders. The main driver for the racial/ethnic differences was incidence of pulmonary embolism. We speculate the association of race/ethnicity with incidence of CAT may be partially because of underlying thrombotic predisposition that varies by ancestry, but we also must consider the impact of social determinants of health on our results.
Introduction
Cancer-associated thrombosis (CAT), which is primarily venous thromboembolism (VTE), is common and associated with significant morbidity and mortality.1 Overall, patients with malignancy have an estimated four- to sevenfold increased risk of developing VTE compared with the general population.2 The incidence of CAT varies by tumor type, stage at diagnosis, type of therapy, and patient comorbidities.3-5
Race and ethnicity, generally self-identified in research studies, are associated with the risk of VTE in population-based studies. Prior work using the California Patient Discharge Dataset showed that Blacks/African Americans had higher rates of VTE compared with non-Hispanic Whites, whereas Hispanics and Asians/Pacific Islanders had significantly lower incidence of VTE.6 Blacks/African Americans have higher rates of pulmonary embolism (PE) and PE-related mortality.7-9 Blacks/African Americans also have higher rates of comorbidities such as diabetes, chronic kidney disease, sickle cell trait/disease, and obesity compared with non-Hispanic White patients, all of which may affect the incidence of VTE.7,8,10 Most of the known genetic predisposing factors for VTE are more prevalent in non-Hispanic White populations, suggesting that other factors, including unidentified genetic risk factors, access to health care, quality of care received, discrimination, and environmental exposures may be contributing to these differences.9,11 The 2020 American Society of Clinical Oncology Clinical Practice Guideline update regarding VTE prophylaxis and treatment in patients with cancer also highlights that racial/ethnic minorities have disproportionate access to care, are more likely to be uninsured, and have a greater number of comorbidities. These racial/ethnic disparities in health care may in turn contribute to differences seen in rates of VTE.12
The effect of race/ethnicity on incidence of CAT has not been well defined. Prior analyses have focused on relatively homogeneous racial/ethnic populations. However, our previous study of Californians with colorectal cancer showed that Asians/Pacific Islanders had a lower incidence of VTE compared with non-Hispanic Whites.13 In addition, the risk of recurrent VTE is higher in patients with cancer, especially those receiving chemotherapy, compared with patients without malignancy, despite anticoagulation.14-17 To our knowledge, there are no studies in the current literature examining potential racial differences in the incidence of recurrent CAT. Therefore, we examined the association of race/ethnicity on the incidence of initial and recurrent CAT in a large, diverse population of patients with cancer, because this may have important implications for prophylaxis and prognosis. Because cancer is considered a strong provoking factor for VTE, we had hypothesized that race/ethnicity would have less association with incident and recurrent CAT than previously described in the general noncancer population.
Methods
Databases
We performed a retrospective observational cohort study using data from the California Cancer Registry (CCR) linked to the California Patient Discharge Dataset (PDD) and Emergency Department Utilization (EDU) database. The CCR is a statewide cancer surveillance program that has reported cancer incidence and mortality information since 1988, capturing >98% of all cancer diagnoses in the state, in both the inpatient and outpatient setting. We obtained information from the CCR including primary cancer site, stage at diagnosis, date of diagnosis, type of treatment, and demographic information including age, sex, race/ethnicity, marital status, neighborhood socioeconomic status (nSES), and type of health insurance.18,19 The PDD includes information regarding all inpatient discharges from nonfederal California hospitals since 1991. The EDU database includes data from all hospital-associated emergency departments since 2005. Both PDD and EDU include up to 25 diagnoses and 21 procedures associated with each hospitalization. The diagnoses/procedures were coded using International Classification of Diseases, 9th revision (ICD-9) and 10th revision (ICD-10) codes in the PDD and Current Procedural Terminology (CPT) codes in the EDU. Each procedure code has an associated date. These databases were linked using unique patient identifiers, that is social security number, date of birth, sex, and zip code. All patients with cancer who did not have a PDD or EDU record (n = 155 075) or who received care through the Department of Veterans Affairs, which does not send data to the PDD or EDU, were excluded. The unlinked cases reflect those who never hospitalized or visited an emergency department between 1991 and 2018 or did not have a social security number for linkage. This is typical when doing linkages with the CCR data and likely reflects many undocumented residents in our state. Patients with an unknown date of cancer diagnosis or unknown follow-up date were also excluded.
Patient selection criteria
We identified a cohort of patients of all ages with first primary diagnosis of the 13 most common cancers in California between 2005 and 2017, including breast, prostate, lung, colorectal, bladder, uterine, kidney, pancreatic, stomach, ovarian, brain cancer, non-Hodgkin lymphoma (NHL), and multiple myeloma, with CAT follow-up through 2018, using Surveillance, Epidemiology, and End Results (SEER) Site recode.20 Patients with cancer in situ or nonmalignant cases were excluded.
Exposure/outcomes
Race/ethnicity was identified using CCR data and classified as non-Hispanic White (White), non-Hispanic Black/African American (Black/AA), Hispanic, Asian/Pacific Islander (API), American Indian, and other/unknown, based on the North American Association of Central Cancer Registries’ Hispanic and Asian/Pacific Islander Identification Algorithm.21 Patients were followed for diagnoses of CAT in PDD or EDU, using specific ICD-9-CM/ICD-10-CM codes (supplemental Table 1).22 CAT locations included PE without deep venous thrombosis (DVT; PE only), PE with DVT (PE+DVT), proximal lower extremity (LE) DVT alone (pDVT), isolated LE distal DVT (iDDVT), or LE DVT, not otherwise specified. Patients diagnosed with VTE before cancer diagnosis were excluded. Recurrent VTE (rVTE) was defined as a subsequent PDD admission meeting 1 of the following criteria: (1) a principal diagnosis of acute VTE, (2) principal diagnosis of cancer and second position VTE code, or (3) any secondary position of hospital acquired VTE code (present on admission-No).23,24
Covariate descriptions
Cancer stage at diagnosis was defined using American Joint Commission on Cancer (AJCC) staging for all cancers except brain and myeloma, where no staging data were available. Initial course of therapy was obtained from CCR and included chemotherapy (yes, no/unknown), radiation (yes, no/unknown), and surgery (yes, no/unknown). nSES was divided into low SES (first, second, or third quintiles) and high SES (fourth or fifth quintiles). nSES is a composite measure comprising Census 2000 variables at the block group level: education index,25 proportion with a blue-collar job, proportion older than age 16 years without a job, median household income, proportion below 200% of the poverty line, median rent, and median house value.26 Type of health insurance at cancer diagnosis or initial treatment was categorized as private insurance, Medicaid, Medicare, no insurance/self-pay, or unknown. Comorbidities were identified using the Elixhauser comorbidity index and were captured up to 2 years prior and including the date of cancer diagnosis.27 Comorbidities were classified based on admissions in PDD as no admission (and thus no information), or 0, 1 to 2, or ≥3 comorbidities.
Statistical analysis
Descriptive statistics were used to describe the baseline characteristics of the cohort with CAT by race/ethnicity groups. All analyses were stratified by cancer site. The cumulative incidence and 95% confidence intervals (CIs) of overall CAT, PE only, PE+DVT, pDVT, iDDVT, and rVTE were determined from initial cancer diagnosis (or incident CAT discharge date for rVTE) to event date of interest, death date, last known date of contact, or study cutoff (31 December 2018), whichever occurred first, accounting for competing risk of death. Median potential follow-up time defined as months from cancer diagnosis to death or last known contact date was calculated using reverse Kaplan-Meier methods.28,29 Multivariable Cox proportional hazards regression models were performed to determine the effect of race/ethnicity on the risk of overall CAT, each specific CAT location, and recurrent CAT, using the methods of Fine and Gray to account for competing risk of death.30 Proportional hazard assumptions for all Cox models were evaluated using the Schoenfeld residuals test.31 Variables violating proportional hazards assumptions were included as a stratification variable. P < .05 was considered statistically significant. All analyses were performed using SAS 9.4. This study was approved by the California Health and Human Services Agency Committee for the Protection of Human Subjects, and the University of California, Davis Institutional Review Boards. It was conducted in accordance with the Declaration of Helsinki.
Results
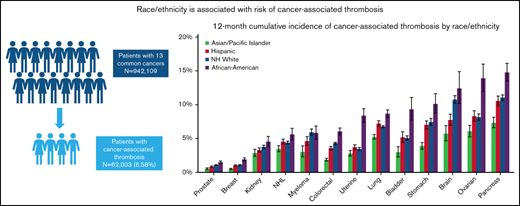
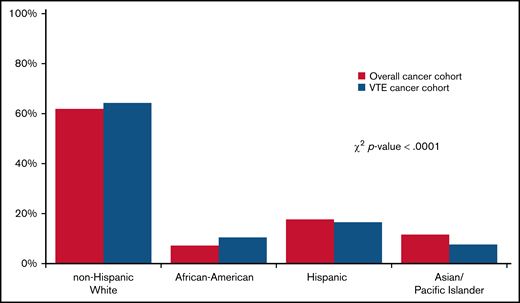
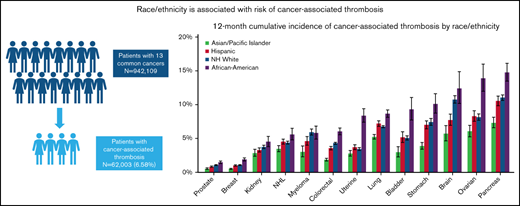
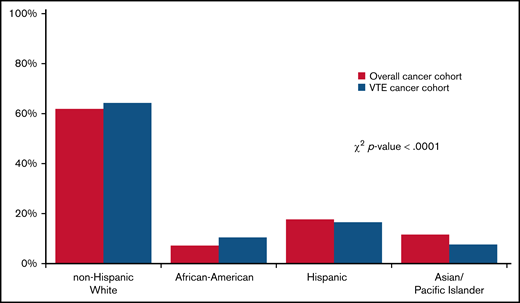
A total of 942 109 cancer patients were identified, of which 62 003 (6.6%) developed a CAT. Table 1 shows baseline characteristics of the overall cohort. Most patients were age 50 and older. Stage 4 disease was most common, accounting for 33.4% of the overall stage distribution. Racial/ethnic differences were seen by nSES, with 71.9% of Black/AAs and 74.9% of Hispanics residing in lower SES neighborhoods compared with 46.1% of Whites and 50.4% of APIs. The distribution of the sites of VTE was 43.7% PE only, 15% PE+DVT, 22.1% pDVT, and 11.0% iDDVT. Among the patients who developed CAT, 63.1% were White, 17.4% were Hispanic, 10.6% were Black/AA, 8.0% were API, and 0.9% were other/unknown race/ethnicity (Figure 1).
Distribution of race/ethnicity among California cancer patients with 13 common cancers, 2005 to 2017. χ2 test was used to compare the race/ethnicity distribution of patients with cancer with and without CAT.
Distribution of race/ethnicity among California cancer patients with 13 common cancers, 2005 to 2017. χ2 test was used to compare the race/ethnicity distribution of patients with cancer with and without CAT.
Cumulative incidence of CAT varies by race/ethnicity
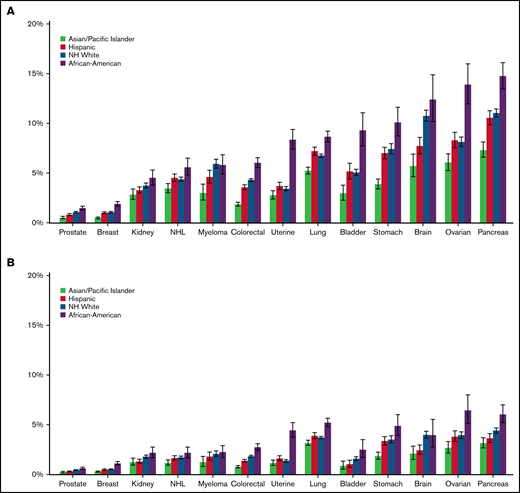
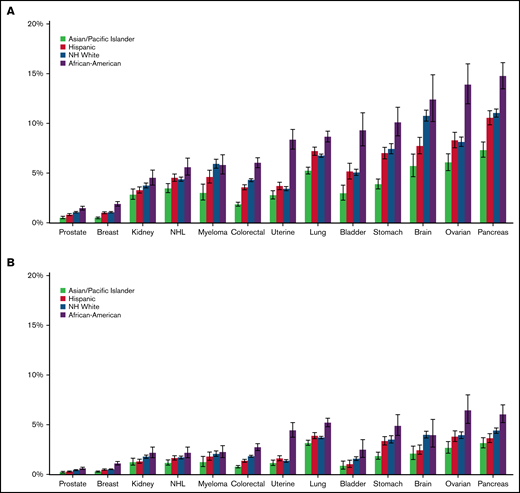
Compared with the other racial/ethnic groups, Black/AAs had the highest 12-month cumulative incidence of CAT for all cancer types, except for myeloma (range, 1.90%-15.00%). The difference was most striking in ovarian cancer, where the 12-month cumulative incidence for Black/AAs was 14.00% (95% CI, 12.00-16.00) compared with 9.14% in Whites (95% CI, 7.68-8.62), 8.30% in Hispanics (95% CI, 7.55-9.10), and 6.06% in APIs (95% CI, 5.24-6.95; Figure 2A; supplemental Figure 1A-M). When looking at PE only, Black/AAs had the highest 12-month cumulative incidence compared with other racial groups for all cancer types except for brain cancer. The most prominent difference was seen in uterine cancer, where Black/AAs had a cumulative incidence of 4.44% (95% CI, 3.75-5.22) compared with 1.36% in Whites (95% CI, 1.23-1.50), 1.60% in Hispanics (95% CI, 1.37-1.86), and 1.14% in APIs (95% CI, 0.90-1.44; Figure 2B). Black/AAs had the highest cumulative incidence of PE+DVT for all cancer types except bladder cancer (0.86%; 95% CI, 0.44-1.53) and myeloma (0.70%; 95% CI, 0.42-1.12), where Whites had the highest incidence (0.88%; 95% CI, 0.75-1.02, and 1.05%; 95% CI, 0.86-1.26, respectively), and in lung cancer (1.06%; 95% CI, 0.88-1.28), where Hispanics had the highest incidence (1.26%; 95% CI, 1.09-1.44). Black/AAs also had the highest 12-month cumulative incidence of pDVT among all cancer types, with the highest incidence in bladder cancer (3.59%; 95% CI, 2.63-4.77). For iDDVT, Black/AAs had the highest 12-month cumulative incidence among all cancer types except for in pancreatic cancer (1.29%; 95% CI, 0.92-1.77), where Hispanics had the highest incidence (1.33%; 95% CI, 1.09-1.63), and kidney cancer (0.27%; 95% CI, 0.13-0.53), where Whites had the highest rate (0.42%; 95% CI, 0.34-0.50; supplemental Figure 2A-C).
Twelve-month cumulative incidence of cancer associated thrombosis. Twelve-month cumulative incidence, accounting for the competing risk of death, of overall CAT (A) and PE only (B) among California patients with cancer with 13 common cancers, 2005 to 2017. NH, non-Hispanic.
Twelve-month cumulative incidence of cancer associated thrombosis. Twelve-month cumulative incidence, accounting for the competing risk of death, of overall CAT (A) and PE only (B) among California patients with cancer with 13 common cancers, 2005 to 2017. NH, non-Hispanic.
APIs had the lowest 12-month cumulative incidence of CAT for all cancer types among all racial/ethnic groups and statistically lower incidence than other racial/ethnic groups in 7 of the 13 cancer sites, ranging from 0.50% to 7.33% (Figure 2A). Focusing on PE-only events, APIs again had the lowest 12-month cumulative incidence among all racial groups for all 13 cancer types, with the lowest incidence in prostate cancer (0.22%; 95% CI, 0.15-0.31) and the highest in lung cancer (3.16%; 95% CI, 2.91-3.43; Figure 2B). For PE+DVT events, APIs had the lowest cumulative incidence among all cancer types, with the lowest incidence in breast (0.04%; 95% CI, 0.02-0.07) and prostate cancer (0.04%; 95% CI, 0.02-0.09). APIs had the lowest 12-month cumulative incidence of pDVT, except in ovarian cancer (1.51%; 95% CI, 1.12-1.99) and kidney cancer (1.03%; 95% CI, 0.75-1.38), where Whites had the lowest cumulative incidence (1.41%; 95% CI, 1.22-1.62, and 0.95%; 95% CI, 0.84-1.08, respectively; supplemental Figure 2A-C).
Race/ethnicity is an independent predictor for incident CAT
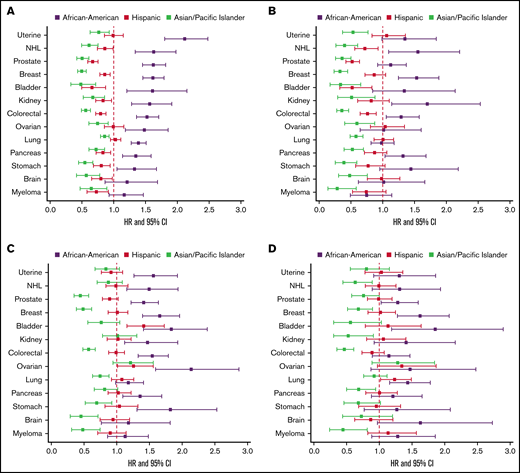
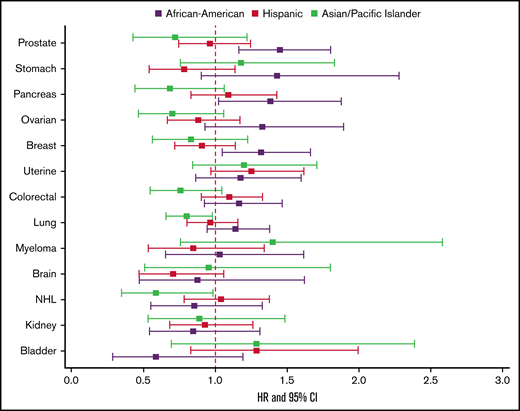
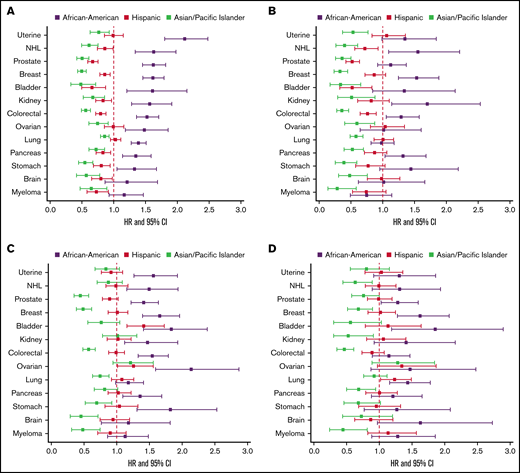
In multivariable models of overall CAT, Black/AAs had the highest risk of CAT across all cancer types except for myeloma, with hazard ratios (HRs) ranging from 1.27 to 1.69 compared with Whites, adjusted for covariates including stage, type of treatment, number of comorbidities, nSES, and type of health insurance, and stratified by tumor type (supplemental Figure 3). When examining PE only, Black/AAs had significantly higher risk of PE compared with Whites in all cancer types except for brain cancer and myeloma (HR range, 1.32-2.11). The difference was most notable in uterine cancer (HR, 2.11; 95% CI, 1.80-2.48; Figure 3A). For PE+DVT, Black/AAs had higher risk compared with Whites with breast (HR, 1.53; 95% CI, 1.25-1.88), colorectal (HR, 1.29; 95% CI, 1.06-1.58), kidney (HR, 1.70; 95% CI, 1.15-2.54), and pancreatic (HR, 1.32; 95% CI, 1.04-1.68) cancers and NHL (HR, 1.56; 95% CI, 1.10-2.21; Figure 3B). For pDVT only, Black/AAs had higher risk compared with Whites for all cancer types except lung, brain, and myeloma, with an approximate twofold increased risk in stomach (HR, 1.82; 95% CI, 1.31-2.53) and ovarian cancer (HR, 2.14; 95% CI, 1.59-2.87; Figure 3C). For iDDVT events, Black/AAs had higher risk compared with Whites in breast, prostate, lung, bladder, pancreatic, and ovarian cancers, with the highest risk in breast cancer (HR, 1.88; 95% CI, 1.40-2.53; Figure 3D).
The effect of race/ethnicity on the risk of cancer associated thrombosis. Effect of race/ethnicity compared with non-Hispanic Whites on the incidence of PE only (A), PE+DVT (B), proximal DVT (C), and isolated distal DVT (D), accounting for the competing risk of death and other known risk factors, among California patients with cancer with 13 common cancers, 2005 to 2017. Cox proportional hazard regressions models, using Fine and Gray methodology to account for the competing risk of death, were stratified by cancer type and adjusted for sex, age at diagnosis, stage at diagnosis, initial treatment (chemotherapy, radiation, and surgery), neighborhood sociodemographic status at diagnosis, and health insurance at diagnosis or initial treatment.
The effect of race/ethnicity on the risk of cancer associated thrombosis. Effect of race/ethnicity compared with non-Hispanic Whites on the incidence of PE only (A), PE+DVT (B), proximal DVT (C), and isolated distal DVT (D), accounting for the competing risk of death and other known risk factors, among California patients with cancer with 13 common cancers, 2005 to 2017. Cox proportional hazard regressions models, using Fine and Gray methodology to account for the competing risk of death, were stratified by cancer type and adjusted for sex, age at diagnosis, stage at diagnosis, initial treatment (chemotherapy, radiation, and surgery), neighborhood sociodemographic status at diagnosis, and health insurance at diagnosis or initial treatment.
APIs had significantly lower risk of CAT than Whites across all subgroups, with HRs ranging from 0.48 to 0.84 (supplemental Figure 3). APIs had significantly lower risk of PE only in all cancer types (15%-52% decreased risk), with the lowest risk in bladder cancer (HR, 0.48; 95% CI, 0.33-0.71; Figure 3A), and this was true for PE+DVT as well, with HRs ranging from 0.29 to 0.60 (Figure 3B). For pDVT, APIs had lower risk in all cancer types except NHL, bladder, uterine, kidney, and ovarian cancers, with the lowest comparative risk in brain cancer (HR, 0.33; 95% CI, 0.18-0.62; Figure 3C). APIs had lower risk of iDDVT compared with Whites in myeloma, breast, colorectal, kidney, pancreatic, and stomach cancers. The risk was lowest in myeloma (HR, 0.40; 95% CI, 0.18-0.91; Figure 3D).
Hispanics had significantly lower risk of CAT compared with Whites in breast, prostate, colorectal, NHL, stomach, brain, and myeloma cancers, with HRs ranging from 0.75 to 0.92 (supplemental Figure 3). Hispanics had lower risk of PE than Whites in all cancer types except lung, uterine, and ovarian cancers, with the largest difference seen in bladder cancer (HR, 0.66; 95% CI, 0.50-0.87; Figure 3A). In PE+DVT, Hispanics had lower risk than Whites in prostate (HR, 0.52; 95% CI, 0.42-0.64), colorectal (HR, 0.76; 95% CI, 0.65-0.90), NHL (HR, 0.72; 95% CI, 0.56-0.93), and bladder cancers (HR, 0.52; 95% CI, 0.33-0.83; Figure 3B). For pDVT, Hispanics differed significantly compared with Whites only in bladder cancer (HR, 1.40; 95% CI, 1.09-1.80; Figure 3C). Hispanics did not differ significantly from Whites for iDDVT events in any of the cancer types except for increased risk in lung cancer (HR, 1.23; 95% CI, 1.02-1.49; Figure 3D).
Race/ethnicity and recurrent CAT
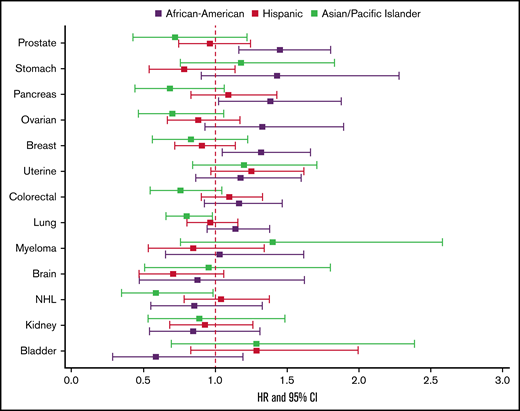
When examining the risk of recurrent CAT, Black/AAs had a significantly higher risk compared with Whites only in breast (HR, 1.36; 95% CI, 1.08-1.72), prostate (HR, 1.37; 95% CI, 1.09-1.72), and pancreatic cancers (HR, 1.37; 95% CI, 1.01-1.86). APIs and Hispanics did not differ significantly from Whites for risk of recurrent CAT in any of the cancer types (Figure 4).
Effect of race/ethnicity compared with non-Hispanic Whites on subsequent VTE, accounting for the competing risk of death and other known risk factors, among California patients with cancer with 13 common cancers, 2005 to 2017. Cox proportional hazard regressions models, using Fine and Gray methodology to account for the competing risk of death, were stratified by cancer type and adjusted for sex, age at diagnosis, stage at diagnosis, initial treatment (chemotherapy, radiation, and surgery), neighborhood sociodemographic status at diagnosis, health insurance at diagnosis or initial treatment, and initial CAT location.
Effect of race/ethnicity compared with non-Hispanic Whites on subsequent VTE, accounting for the competing risk of death and other known risk factors, among California patients with cancer with 13 common cancers, 2005 to 2017. Cox proportional hazard regressions models, using Fine and Gray methodology to account for the competing risk of death, were stratified by cancer type and adjusted for sex, age at diagnosis, stage at diagnosis, initial treatment (chemotherapy, radiation, and surgery), neighborhood sociodemographic status at diagnosis, health insurance at diagnosis or initial treatment, and initial CAT location.
Discussion
In this population-based study of a large, diverse cohort of patients with common cancers and at least 1 hospital admission or emergency department visit before or after their cancer diagnosis (1991-2018), race/ethnicity was associated with the risk of CAT. Patients with cancer of Black/AA race/ethnicity had consistently higher and those of API race/ethnicity had consistently lower CAT incidence, particularly PE, compared with Whites, mirroring trends found in the general population.6 Because the presence of active cancer itself is such a strong risk factor for VTE, we had hypothesized these racial/ethnic differences would be less pronounced than in the general population. However, race/ethnicity was strongly associated with risk of CAT even after adjusting for covariates such as age, stage, cancer type, nSES, insurance, and comorbidities, which are known to be associated with increased risk of CAT. Our results are consistent with smaller studies from Asia that showed lower rates of VTE in Asian patients with advanced gastrointestinal malignancies32,33 and prior work in California showing lower incidence of VTE in API patients with colorectal cancer.13 To our knowledge, this is the largest cohort studied to confirm racial/ethnic disparities in CAT.
A prior study of US Medicaid enrollees who developed VTE showed higher prevalence of VTE in Black/AAs and lower prevalence of VTE in Hispanics compared with Whites.34 White et al6 previously used the California Patient Discharge Dataset to show significantly higher incidence rate of VTE in Black/AAs (141/100 000 adults/y) compared with Whites (103/100 000 adults/y), whereas Hispanics and APIs had significantly lower incidence rates of VTE (61.5/100 000 and 29/100 000 adults/y, respectively) compared with Whites. Similarly, in an analysis of a diverse population in Oklahoma, age-adjusted incidence of VTE was highest in Blacks and lower in APIs and Hispanics compared with Whites.35 An analysis of 3 prospective study databases was done to examine association of race with incidence of VTE.36 In the Cardiovascular Health Study, Blacks had an 81% higher rate of VTE compared with Whites. In the Atherosclerosis Risk in Communities study, which was the only 1 of the 3 to include patients with cancer, Blacks had a 21% higher rate of VTE than Whites.36,37 There were geographic variations in rates of VTE observed in the Reasons for Geographic and Racial Differences in Stroke study; specifically, Black/AAs in the Southeast were found to have higher relative risk of VTE compared with Black/Aas in the rest of the United States.36
The racial/ethnic differences in the incidence of CAT for each tumor type was largely driven by the differences in the incidence of PE. This is similar to what has been found in the general population. Heit et al8 analyzed patients from multiple Centers for Disease Control Thrombosis and Hemostasis Centers and showed significantly higher proportion of PE only events in Black/AAs compared with Whites (27.6% vs 14.4%, respectively). It was uncertain why a higher proportion of VTE presented as PE only in Black/AAs. White et al6 also found Black/AAs had significantly higher odds of PE (odds ratio, 1.2), whereas Hispanics had significantly lower odds of PE compared with Whites (odds ratio, 0.8).6
The reason(s) underlying the observed racial/ethnic differences in CAT is unknown. The Genetic Attributes and Thrombosis Epidemiology study examined the epidemiology of VTE cases in Black/AAs compared with Whites and found higher prevalence of hypertension, chronic kidney disease, and diabetes in Black/AAs, whereas cancer, immobilization, and recent surgery were more common in Whites with VTE.10 Family history of VTE was similar between both groups.10 As race/ethnicity is a sociopolitical, not biological, construct in the United States,38,39 it is speculated that potential differences in sociodemographic factors, such as access to primary care and differences in health care delivery, may affect the rates of VTE.7-10 An effect of institutional and structural racism that may affect the access to, intensity, and quality of care may also contribute.38,39 In the present study, the models were adjusted for recorded comorbidities, broad categories of cancer treatments, nSES, and health insurance, but measures of care access, intensity, and quality were not available and should be the focus of future research.
Thrombophilia genes are differentially prevalent depending on ancestry. The most well-known genetic risk factors for the development of VTE, factor V Leiden G1691A and prothrombin G20210A, are more prevalent in populations of Northern European ancestry. Levels of procoagulant and anticoagulant factors may also vary by ancestry and affect incidence of VTE.9,11 There are likely additional underlying genetic variants associated with other ancestries not yet identified because most people enrolled in genetic predisposition studies have been of European descent. It is imperative that more diverse populations be included in these types of studies.
Despite patients with malignancy being at increased risk of recurrent VTE compared with patients without cancer,16 rates of recurrent CAT by race/ethnicity have not been previously described. The Ottawa score was developed as a predictive model for risk of recurrent VTE within 6 months of an index CAT event while patients were on anticoagulation, but the racial/ethnic distribution of the population used to develop the model was not described.40-42 Our data showed Black/AAs had significantly higher risk of recurrent CAT compared with Whites in breast, prostate, and pancreatic cancers, whereas APIs and Hispanics did not differ significantly from Whites in any of the cancer types. In the general population, White et al17 previously showed Black/AA and Hispanic women had higher rates of recurrent VTE (relative risk, 1.6 and 1.7, respectively) compared with White women. Although men had higher rates of recurrent VTE compared with women overall, there was no significant difference by race/ethnicity seen in men.17 We speculate that in patients who have already had CAT, the risk of a recurrent event is not determined by the same risk factors as the incident event, including race/ethnicity and other social determinants of health. These factors likely include the status of the underlying cancer (complete response, recurrence, or progression), ongoing therapy with variable VTE risk (immunotherapy, targeted therapy, antiangiogenic therapy), overall performance status, and the intensity and duration of therapeutic anticoagulation for the incident event. Incident event bias, which leads to a redistribution of baseline risk factors for an outcome compared with the parent cohort, also likely plays a role.43
Risk assessment scores have been developed to help predict the risk of incident VTE in patients with cancer44-47 and consider primary thromboprophylaxis. One of the most well-known risk models for CAT is the Khorana score, which was validated as a predictive model for VTE in patients with cancer receiving chemotherapy. The racial/ethnic characteristics of the study population used to develop the Khorana score are not specifically described.44 There are additional risk assessment models, including the Prophylaxis of Thromboembolism during Chemotherapy (PROTECHT), Charité Onkologie (CONKO), and Vienna scores, but none of the risk models include race/ethnicity as a predictive variable.45-47 Racial/ethnic minorities have historically been underrepresented in prior clinical trials looking at treatment of unprovoked VTE,48 which may be why this parameter has not been included in predictive models.
SAVE-ONCO was a clinical trial comparing semuloparin thromboprophylaxis with placebo for ambulatory patients with cancer receiving chemotherapy.49 An analysis of the placebo arm of this trial showed Black/AA patients were at more than a threefold risk of VTE compared with White patients after adjusting for baseline characteristics, including Khorana score and chemotherapy agents, whereas there was no difference in risk between White and Asian patients.50 It would be interesting to perform the same analysis on the experimental arm of the trial determine whether there was an effect modification of primary thromboprophylaxis by race/ethnicity. Further research would also be needed to determine differences in thromboprophylaxis recommendations in hospitalized vs ambulatory patients with cancer, because most studies have focused on the ambulatory setting.
There were limitations of our study. Incident VTE was only ascertained if the patient was seen in an emergency department or hospitalized and therefore may have been underestimated. The Reasons for Geographic and Racial Differences in Stroke study showed 28% of patients with DVT received outpatient treatment, although patients with cancer were excluded in this study.51 VTE was ascertained using ICD-9-CM/ICD-10-CM codes, which have inherent limitations. However, previous work has shown good validity using an algorithm with more specific codes.22 We do not have information regarding underlying thrombophilia, smoking, obesity, mobility, or primary thromboprophylaxis (although this is not widely practiced in ambulatory patients with cancer). Although we adjusted for number of comorbidities, we do not have information on severity that likely alters VTE risk. There are also no data on some social determinants of health such as level of education, transportation, access to care, toxic exposures, personal income, and medication adherence that likely affect risk of outcomes. The incidence of some cancers varies by race/ethnicity, potentially confounding our analysis. Granular data on types of cancer treatment (eg, immunotherapy and hormonal therapy), anticoagulation (for other indications or as primary prophylaxis), antiplatelet therapy, and use of statins was also not available. Despite these limitations, we analyzed a very large cohort of patients with cancer with the most common types of malignancy. The population of California is quite racially and ethnically diverse, so there was good representation among all cancer types in all racial/ethnic groups. The trends we observed are consistent with data from prior smaller studies.31,32
Our data suggest that race/ethnicity might be considered, along with other predictive tools, in decisions regarding primary prophylaxis for CAT. However, this is contrary to the current trend to eliminate race-based adjustments in medicine, because of questions about validity, application (assignment of race is not consistent within or between locales), and downstream consequences that may further exacerbate health inequities and disparities.52-54 Vyas et al52 examined race/ethnicity-adjusted algorithms across various fields of medicine and highlighted questions to consider when factoring race/ethnicity into clinical decision making. These include whether there is strong evidence for race/ethnicity correction after considering potential confounders and bias, whether a causal mechanism exists for the racial/ethnic difference, and whether inclusion of race/ethnic correction would help or exacerbate health disparities. In our study, race/ethnicity is likely a surrogate for many factors that are not traditionally measured in biomedical studies and should stimulate further research into the reasons for the association of race/ethnicity with this common and serious complication of cancer, with the goal of reducing potential health inequities.
Acknowledgments
The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885, Centers for Disease Control and Prevention’s National Program of Cancer Registries, under cooperative agreement 5NU58DP006344, the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute. T.W. is supported by grant UL1TR001860 from the National Center for Advancing Translational Sciences, National Institutes of Health.
The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors.
Authorship
Contribution: T.D., A.B., A.M., T.K., and T.W. designed the study, acquired and analyzed the data, drafted the manuscript, made revisions, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ted Wun, Division of Hematology and Oncology, UC Davis Comprehensive Cancer Center, 4501 X St, Sacramento, CA 95817; e-mail: twun@ucdavis.edu.
References
Author notes
Requests for data sharing may be submitted to Ted Wun (twun@ucdavis.edu). Re-disclosure of data is limited by the data use agreement with the State of California.
The full-text version of this article contains a data supplement.