Key Points
PT-Cy after NMA matched alloHSCT will increase the proportion of patients that survive without severe GVHD.
PT-Cy could allow for implementation of early posttransplant chemo- and immunotherapy to further reduce the relapse risk after alloHSCT.
Abstract
Graft-versus-host disease (GVHD) is the most important complication of allogeneic hematopoietic stem cell transplantation (alloHSCT). We performed a prospective randomized, multicenter, phase 3 trial to study whether posttransplant cyclophosphamide (PT-Cy) combined with a short course of cyclosporine A (CsA) would result in a reduction of severe GVHD and improvement of GVHD-free, relapse-free survival (GRFS) as compared with the combination of CsA and mycophenolic acid (MPA) after nonmyeloablative (NMA) matched related and unrelated peripheral blood alloHSCT. Between October 2013 and June 2018, 160 patients diagnosed with a high-risk hematological malignancy and having a matched related or at least 8 out of 8 HLA-matched unrelated donor were randomized and allocated in a 1:2 ratio to CsA/MPA or PT-Cy/CsA; a total of 151 patients were transplanted (52 vs 99 patients, respectively). The cumulative incidence of grade 2 to 4 acute GVHD at 6 months was 48% in recipients of CsA/MPA vs 30% following PT-Cy/CsA (hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.29-0.82; P = .007). The 2-year cumulative incidence of extensive chronic GVHD was 48% vs 16% (HR, 0.36; 95% CI, 0.21-0.64; P < .001). The 1-year estimate of GRFS was 21% (11% to 32%) vs 45% (35% to 55%), P < .001. With a median follow-up of 56.4 months, relapse incidence, progression-free survival, and overall survival were not significantly different between the 2 treatment arms. PT-Cy combined with a short course of CsA after NMA matched alloHSCT significantly improves GRFS due to a significant reduction in severe acute and chronic GVHD.
Introduction
Severe graft-versus-host disease (GVHD) is associated with excess nonrelapse mortality (NRM) and reduced quality of life (Qol) after allogeneic hematopoietic stem cell transplantation (alloHSCT).1,2 The most widely used GVHD prophylaxis regimen after myeloablative transplantation consists of cyclosporine A (CsA) and methotrexate (MTX).3 Addition of antithymocyte globulin (ATG) to CsA/MTX has been shown to result in a reduction of chronic GVHD (cGVHD) following myeloablative conditioning (MAC).4-6 As a result, a recent European guideline recommended the use of ATG following MAC.7 In recipients of nonmyeloablative (NMA) conditioning using low-dose total body irradiation and fludarabine as developed by Storb et al, the combination of CsA and mycophenolate mofetil (MMF) has become an established immunosuppressive regimen resulting in an incidence of 30% to 50% acute GVHD (aGVHD) grade 2 to 4 and 40% to 60% of cGVHD.8-11
In recent years, increased use of haploidentical family donors has become apparent, greatly facilitated by effective GVHD prophylaxis using posttransplant cyclophosphamide (PT-Cy), as pioneered by Luznik et al.12 Following the favorable results in haploidentical transplantation, PT-Cy has also been successfully applied in recipients of matched related donor (MRD) and matched unrelated donor (MUD) transplants either as single GVHD prophylaxis after myeloablative bone marrow transplantation or combined with CsA after peripheral blood hematopoietic stem cell transplantation (HSCT).13-15 To study the effect of a more intensive immunosuppressive regimen using PT-Cy on the incidence of relapse and life-threatening opportunistic infections as compared with the ruling conventional regimens, a prospective randomized phase 2 trial was designed comparing 3 prophylactic GVHD regimens including a PT-Cy containing regimen with GVHD-free, relapse-free survival (GRFS) as the composite primary endpoint.16 Bolaños-Meade et al showed that the PT-Cy containing regimen was associated with a significantly better GRFS representing patients alive without relapse and no or only limited GVHD. A retrospective study performed by Kwon et al comparing PT-Cy vs CsA/MTX after MRD peripheral blood HSCT showed comparable results.17
Here we report the results of the first prospective, randomized, multicenter, phase 3 trial designed to study whether PT-Cy combined with a short course of CsA would result in less severe GVHD and better GRFS as compared with the combination of CsA and mycophenolic acid (MPA) after NMA matched alloHSCT.
Methods
Study design
Patients were randomly assigned in a 1:2 ratio to CsA/MPA or PT-Cy/CsA. Adults (age 18-70) with a World Health Organization performance status between 0 and 2, diagnosed with a high-risk hematological malignancy, and having an MRD or at least 8 out of 8 HLA (A, B, C, DRB1; DNA-based, 4 digits) MUD could participate in the trial. Patients were excluded in case of severe renal dysfunction, an active infection, progressive or refractory disease, and if ATG was part of the conditioning regimen. In recipients of CsA/MPA, the conditioning regimen was at the discretion of the treating physician. In recipients of PT-Cy/CsA, the conditioning regimen was specified and modified from the NMA Seattle protocol.12 In recipients of CsA/MPA, CsA was administered twice daily from 3 to 5 days before transplantation (depending on local procedures) at a dose of 4.5 mg/kg twice daily orally or 1.5 mg/kg IV twice daily aiming at trough levels in between 250 and 350 µg/L (immunoassay). MPA was administered from transplant at a dose of 16 mg/kg twice daily with a maximum daily dose of 2160 mg and discontinued at day +84. CsA was tapered with 10% per week from day +120 in patients without GVHD or from day +180 in patients with a history of GVHD. Patients allocated to PT-Cy/CsA received cyclophosphamide 50 mg/kg IV on days +3 and +4 combined with CsA from day +5 onward at a dose of 1.5 mg/kg IV twice daily aiming at trough levels in between 250 and 350 µg/L. In the absence of mucositis or as soon as mucositis had resolved, oral administration of CsA was allowed. In patients without any GVHD, CsA was discontinued without tapering at day +70.
Study oversight
The trial was registered as number NL2128 in the Dutch trial registry (www.trialregister.nl) and designed by the Stem Cell Transplantation Working Group of the Dutch-Belgian Cooperative Trial Group for Hemato-Oncology (HOVON).
Of note, the current study protocol is an amendment of the original HOVON-96 protocol. Explanation of the original design of the study and how the current design was established is described in the supplemental Appendix.
Data were collected at the HOVON Data Center, and HOVON statisticians conducted the analysis. The study protocol was approved by the ethics committee at each participating center and was conducted according to the principles of the Declaration of Helsinki. All patients gave written informed consent to enroll in the study.
Study endpoints
According to the original design of the study, the primary endpoint was defined as the proportion of patients with non-severe GVHD within 180 days posttransplantation (PG180). Non-severe GVHD was defined as aGVHD grade 1, aGVHD grade 2 without gut infiltration, or cGVHD not requiring systemic treatment within 180 days after randomization. Secondary endpoints included time from transplantation to aGVHD grade ≥1, ≥2, ≥3, and ≥4, time to limited/extensive and extensive cGVHD, incidence of relapse/progression, NRM, progression-free survival (PFS), overall survival (OS), GRFS, and adverse events (AEs). PFS was defined as the time from transplantation until relapse/progression or death, whichever came first. OS was defined as the time from transplantation until death, irrespective of the cause. GRFS was defined as survival without aGVHD grade 3 to 4, cGVHD requiring systemic immunosuppressive treatment, or relapse/progression (whichever came first).18 For GRFS, PFS, and OS, patients without an event were censored at the date last known to be alive. AEs were scored according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. aGVHD was graded according to the updated Glucksberg classification.19,20 cGVHD was graded according to the Seattle classification.21
Statistical analysis
The primary objective was to evaluate whether PT-Cy/CsA would result in a higher proportion of patients with non-severe GVHD PG180 compared with CsA/MPA. For the sample size calculation, it was assumed that PG180 would be about 35% with CsA/MPA. In order to detect with 80% power an increase of PG180 from 35% to 60% (2-sided significance level α = 0.05), 156 patients should be randomized 1:2 between CsA/MPA (n = 52) and PT-Cy/CsA (n = 104). Patients could be randomized 24/7 via the Internet using the randomization program TOP of the HOVON Data Center. Randomizations were balanced with a biased-coin minimization procedure, with the bias dependent on the average imbalance between the numbers of patients already assigned to each group overall, within the participating hospital, and within donor type (MRD vs MUD). All analyses were according to the intention-to-treat principle (ie, patients were analyzed according to the treatment arm they were assigned to). However, patients initially randomized but considered ineligible afterward based on information that should have been available before randomization were excluded from all analyses (modified-intention-to-treat), and data collection for these patients was discontinued. PG180 was determined per treatment arm with a 95% confidence interval (CI). As primary analysis, PG180 was compared between both arms using logistic regression with adjustment for donor type (MRD vs MUD), as specified in the protocol. Cumulative incidence curves for time to aGVHD and cGVHD were determined per treatment arm. The Fine and Gray model was used to assess the effect of the treatment arm on the cumulative incidence of GVHD in the presence of competing risks via regression on GVHD subdistribution hazard, adjusted for donor type. Kaplan-Meier curves for GRFS, PFS, and OS were constructed per treatment arm. A Cox proportional hazards model was used to assess the effect of the treatment arm on each of the survival endpoints, adjusted for donor type. Hazard ratios (HRs) with 95% CI were determined. The analyses of treatment toxicity were done by tabulation of the incidence of AEs with CTCAE grade 3 or more within 180 days posttransplant. All reported P values are 2-sided, and a significance level α = 0.05 was used. As there is one primary analysis, for endpoint PG180, all other analyses should be considered exploratory, and no correction for multiple testing was done. The data cutoff date was 19 January 2021. All analyses were performed using Stata software, version 16.1 (StataCorp, College Station, TX).
Results
Patient characteristics
A total of 160 patients were randomized between October 2013 and June 2018 at 6 centers. Eventually, 151 patients received an alloHSCT. The patient disposition flowchart is shown in supplemental Figure 1. As shown in Table 1, the 2 groups of patients were well balanced with respect to the baseline characteristics. Details of the diagnosis and disease status at transplantation are described in the supplemental Appendix. All but 4 patients received an NMA conditioning regimen. Details of the regimens used are shown in supplemental Table 3. The median follow-up of the 98 transplanted patients still alive was 56.4 months (range, 16.1-75.6) from transplantation, that is, median 54.3 months (range, 16.1-71.3) in the CsA/MPA arm and 56.4 months (range, 20.9-75.6) in the PT-Cy/CsA arm. Of note, follow-up data were only required until 5 years after randomization.
aGVHD and cGVHD
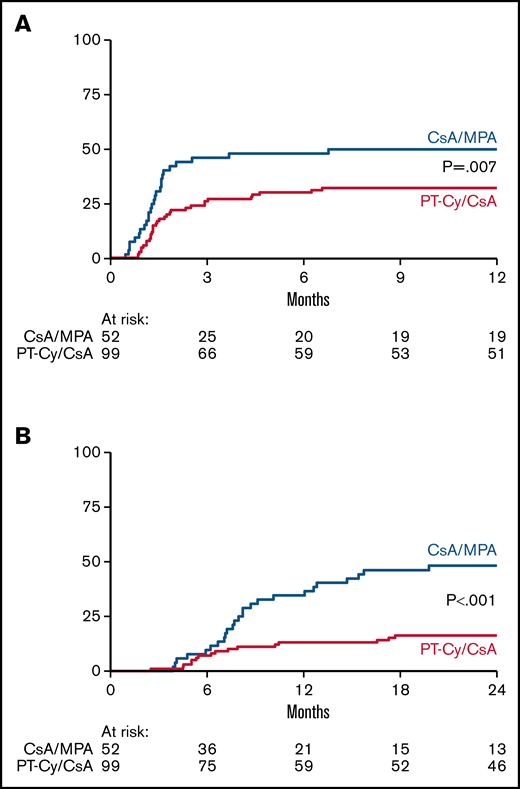
The proportion of patients with non-severe GVHD within 180 days posttransplant was not significantly different between recipients of CsA/MPA and PT-Cy/CsA (29% vs 38%; odds ratio, 1.53 [0.73-3.18]; P = .26, adjusted for donor type). Sixty-two percent of patients in both groups experienced aGVHD of any grade within 180 days posttransplant. The skin was involved in the majority of patients (30 vs 56 patients). In recipients of CsA/MPA, 11 out of 32 (34%) patients were diagnosed with aGVHD of the intestine as opposed to 10 out of 61 (16%) recipients of PT-Cy/CsA. The liver was affected in the minority of patients (2 vs 6 patients). Results are shown in Table 2. The cumulative incidence of aGVHD grade 2 to 4 at 6 months from transplantation was 48% (standard error [SE], 7%) in recipients of CsA/MPA vs 30% (SE, 5%) in recipients of PT-Cy/CsA (P = .007) (Figure 1A). The cumulative incidence of aGVHD grade 3 to 4 at 6 months was not significantly different between the two treatment arms: 12% (SE, 4%) vs 6% (SE, 2%), respectively (P = .14). In multivariate analysis, study arm (HR, 0.48 [0.29-0.82]; P = .007) and donor type (MUD vs MRD; HR, 1.90 [1.06-3.43]; P = .032) were significantly associated with aGVHD grade 2 to 4.
Acute and chronic GVHD. Cumulative incidence of grade 2-4 acute GVHD (A) and chronic extensive GVHD (B). GVHD denotes graft-versus-host disease, CsA cyclosporine A, MPA mycophenolic acid, and PT-Cy posttransplant cyclophosphamide.
Acute and chronic GVHD. Cumulative incidence of grade 2-4 acute GVHD (A) and chronic extensive GVHD (B). GVHD denotes graft-versus-host disease, CsA cyclosporine A, MPA mycophenolic acid, and PT-Cy posttransplant cyclophosphamide.
Overall, 56% of all patients developed cGVHD of any grade, including 69% of patients in the CsA/MPA group and 49% in the PT-Cy/CsA group. In recipients of CsA/MPA, 27 out of 52 (52%) patients were diagnosed with extensive cGVHD as opposed to 24 out of 99 (24%) recipients of PT-Cy/CsA. Results are shown in Table 2. The cumulative incidence of limited and extensive cGVHD at 2 years was 65% (SE, 7%) vs 43% (SE, 5%) in the CsA/MPA and PT-Cy/CsA arm, respectively. The cumulative incidence of extensive cGVHD at 2 years was 48% (SE, 7%) after CsA/MPA vs 16% (SE, 4%) after PT-Cy/CsA (P < .001) (Figure 1B). In multivariate analysis, the study arm exclusively was significantly associated with extensive cGVHD (HR, 0.36 [0.21-0.64]; P < .001).
Among 24 patients in the CsA/MPA arm with complete CsA data available, 10 (42%) patients were still receiving CsA at 6 months posttransplant. In contrast, among the 71 patients in the PT-Cy/CsA arm with available CsA data, all patients had discontinued CsA within 6 months posttransplant.
Toxicity, NRM, and relapse/progression
Forty-two percent of patients in the CsA/MPA arm experienced at least 1 CTCAE grade 3 to 5 AE within 6 months posttransplant vs 61% of patients in the PT-Cy/CsA arm. CTCAE grade 3 to 5 infections were observed in 21% vs 41% in the respective study arms. The proportion of patients with at least 1 episode of febrile neutropenia was higher in recipients of PT-Cy/CsA (25% vs 15%). Invasive pulmonary aspergillosis was diagnosed in 2 recipients of CsA/MPA as opposed to 4 recipients of PT-Cy/CsA. The number of patients experiencing at least 1 cytomegalovirus (CMV) reactivation was not different between the 2 groups (19% and 20%, respectively). One patient in the PT-Cy/CsA group developed CMV disease. Three patients experienced a cardiac event. Two patients experienced a graft failure, 1 in each study arm. Results are shown in Table 2. A full list of all CTCAE grade 3 to 5 events is available in supplemental Table 4.
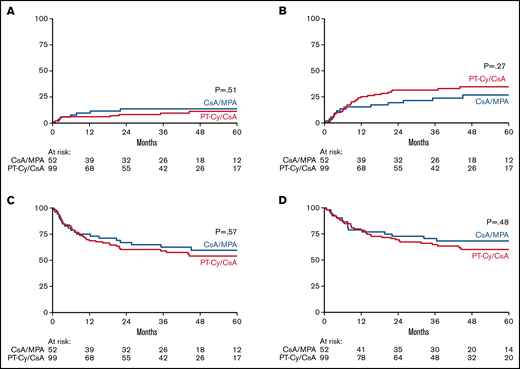
NRM estimated 14% (SE, 5%) vs 10% (SE, 3%) in the respective study arms at 3 years posttransplant (P = .51) (Figure 2A). The cumulative incidence of relapse at 3 years posttransplant was 24% (SE, 6%) after CsA/MPA vs 32% (SE, 5%) following PT-Cy/CsA (P = .27) (Figure 2B).
Non-relapse mortality (NRM), relapse/progression, progression-free survival (PFS) and overall survival (OS). Cumulative incidence of NRM (A) and relapse/progression (B). Kaplan Meier estimates of PFS (C) and OS (D) CsA denotes cyclosporine A, MPA mycophenolic acid, and PT-Cy posttransplant cyclophosphamide.
Non-relapse mortality (NRM), relapse/progression, progression-free survival (PFS) and overall survival (OS). Cumulative incidence of NRM (A) and relapse/progression (B). Kaplan Meier estimates of PFS (C) and OS (D) CsA denotes cyclosporine A, MPA mycophenolic acid, and PT-Cy posttransplant cyclophosphamide.
Survival
The 3-year estimate of PFS was 63% (95% CI, 48-74) and 59% (95% CI, 48-68) in recipients of CsA/MPA and PT-Cy/CsA, respectively. The 3-year estimate of OS was also similar in both treatment arms being 71% (95% CI, 56-81) vs 65% (95% CI, 54-73). Results are shown in Figure 2C-D. Causes of death are shown in supplemental Table 5.
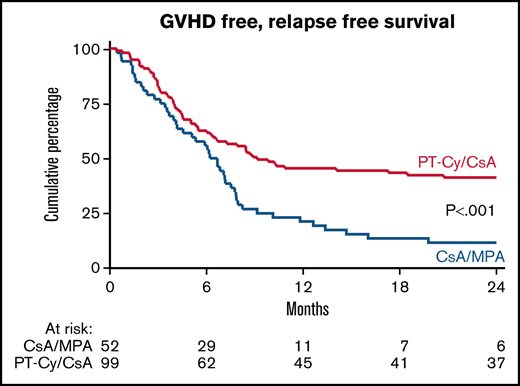
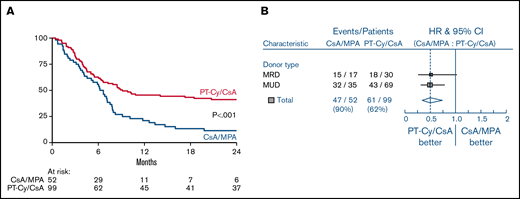
The 1-year estimate of the GRFS was 21% (95% CI, 11-33) after CsA/MPA vs 45% (95% CI, 35-55) following PT-Cy/CsA (HR, 0.50 [0.34-0.74]; P < .001, adjusted for donor type) (Figure 3A). As shown in the forest plot, improvement of GRFS was irrespective of donor type (Figure 3B).
GVHD free, relapse free survival (GRFS). Kaplan Meier estimate of GRFS (A) and forest plot of GRFS by donor type (B) GVHD denotes graft-versus-host disease, CsA cyclosporine A, MPA mycophenolic acid, and PT-Cy posttransplant cyclophosphamide.
GVHD free, relapse free survival (GRFS). Kaplan Meier estimate of GRFS (A) and forest plot of GRFS by donor type (B) GVHD denotes graft-versus-host disease, CsA cyclosporine A, MPA mycophenolic acid, and PT-Cy posttransplant cyclophosphamide.
Discussion
This randomized prospective phase 3 trial was designed to study the efficacy of PT-Cy after NMA matched related and unrelated alloHSCT as compared with conventional immunosuppression (IS) with CsA and MPA. We show that the use of PT-Cy combined with a short course of CsA results in significantly improved GRFS as a result of a significant reduction in severe aGVHD and cGVHD without significantly affecting the cumulative relapse incidence. This study compares well to the results of the prospective phase 2 randomized bone marrow transplantation CTN trial performed by Bolaños-Meade et al comparing 3 different prophylactic GVHD regimens. The combination of tacrolimus, MMF, and PT-Cy was the only regimen associated with a significantly better GRFS in their study.16 GRFS has become an important composite endpoint that is increasingly incorporated in current transplant studies. Although GRFS was a secondary endpoint in our study, the significant reduction is important as it represents the percentage of surviving patients without relapse and without GVHD necessitating ongoing systemic immunosuppressive treatment, thereby most likely contributing to an increase in the Qol. Severe cGVHD and its treatment, in particular, are known to be associated with a diminished Qol, and improved prevention has been shown to increase the Qol score.22-24 In future studies concerning PT-Cy, it is of great interest to consider the patient’s perspective by using so-called patient-reported outcome measures to be able to show that improved GRFS is indeed associated with improved Qol.25
Other lymphocyte-depleting strategies include the application of ATG. Recently published guidelines by the European Society for Blood and Marrow Transplantation (EBMT) recommended the use of ATG in addition to conventional IS in recipients of MUD grafts after MAC.7 One might argue that the difference in the incidence of GVHD observed with PT-Cy is due to comparison with an insufficient prophylactic strategy as ATG was not included. However, the recommendation is based on 3 randomized controlled trials, including 2 trials concerning only MAC4,5 and 1 trial concerning recipients of MUD grafts after both myeloablative and NMA or reduced-intensity conditioning (RIC).6 Except for 2 patients in the CsA/MPA arm receiving MAC, all patients in our study received an NMA conditioning (n = 147) or RIC (n = 2). Apart from the aforementioned randomized study in which only a minor subset of patients received an NMA or RIC regimen,6 large prospective randomized trials assessing the efficacy of ATG in the NMA/RIC setting are lacking. Nevertheless, a comparison of ATG to PT-Cy as GVHD prophylaxis might be of interest. So far, only retrospective comparisons have been reported.26-28 Results, however, need confirmation in prospective randomized controlled trials. Monitoring and comparing all posttransplant infections might be of particular interest in such trials. We observed a relatively high incidence of infections within the first 6 months posttransplant in recipients of PT-Cy, although the difference was mainly determined by a higher incidence of febrile neutropenia, explained by the more prolonged and profound neutropenia because of a more intensive conditioning regimen as compared with recipients of conventional IS. In addition, an accurate cost-effectiveness analysis of PT-Cy vs ATG may be performed, especially since ATG (Grafalon) is more expensive than cyclophosphamide.
Apart from the clinical perspective, insight into the mechanism by which cyclophosphamide protects against GVHD while preserving the graft-versus-leukemia effect is of interest. Selective elimination of proliferating alloreactive T cells was considered to be the dominant underlying mechanism.29,30 However, evidence from murine studies that the persistence and expansion of donor FoxP3+CD4+ regulatory T cells are critical for the action of PT-Cy is accumulating.31-33 In addition, timing and dosing of PT-Cy appear to be crucial for its protective effect. As shown in a murine haploidentical HSCT model, only optimal dosing of PT-Cy will result in the successful prevention of GVHD.34 Future research is needed to further improve our understanding and elucidate the mechanism underlying the effects of PT-Cy as well as defining the most optimal schedule of administration.
This study might have some limitations.
Firstly, the application of PT-Cy was added as a third treatment arm to an initially designed two-armed study concerning the comparison of standard-duration IS vs time-restricted IS with CsA and MPA (de Jong et al ASH 2019; abstract# 371). After the introduction of PT-Cy as the third arm, the primary endpoint was not adapted. Sample size and power calculation were performed according to the expected difference in the primary endpoint. However, in retrospect, it might have been more appropriate to define an additional primary endpoint that would have suited the comparison with PT-Cy. For example, the composite endpoint GRFS as a measure of cure without ongoing morbidity.18
Secondly, different conditioning regimens were used. In recipients of CsA/MPA, the conditioning was at the discretion of the treating physician, with most patients (79%) treated according to the NMA Seattle protocol. The more intensified conditioning used in recipients of PT-Cy/CsA was specified and modified from the NMA Seattle protocol. One might argue that the difference in conditioning intensity might have contributed to a difference in GVHD. Both regimens, however, are classified as NMA. In addition, one would expect a higher incidence of GVHD in the PT–Cy-treated patients as a consequence of a more intensified conditioning regimen which, however, was not the case.
Thirdly, the incidence of cGVHD was reported according to the Seattle classification. At the time of initiation of the study, the National Institutes of Health classification was already published but not yet standard for the classification of cGVHD in European transplantation programs.35,36
Finally, the application of PT-Cy combined with a short course of CsA was studied in patients receiving an NMA conditioning regimen. Therefore, translation of our positive findings to alloHSCT using RIC or MAC should be performed with caution.
In conclusion, PT-Cy combined with a short course of CsA effectively prevents the occurrence of severe aGVHD and cGVHD in patients after NMA-matched alloHSCT with an acceptable toxicity profile. Without significantly affecting the cumulative relapse incidence, PT-Cy results in a significantly improved GRFS, whereas PFS and OS are similar compared with conventional IS. Hence, a more intensified immunosuppressive regimen with PT-Cy might be preferred in the setting of NMA peripheral blood HSCT from both MRD and MUD. In addition, as relapse after transplantation remains a major concern, PT-Cy is highly attractive, as it enables the implementation of early posttransplant chemo- and immunotherapy without facing possible drug interactions.37 Although the relapse rate was not statistically different between the two treatment arms, the result should be interpreted with caution, and further study through retrospective registry analyses (EBMT/CIBMTR) or additional prospective trials seems warranted.38
Acknowledgments
The authors thank all local data managers and the HOVON Data Center trial team for trial management and central data management; the members of the Data and Safety Monitoring Board: J.H.F. Falkenburg (Leiden, The Netherlands), N. Kröger (Hamburg, Germany), and A. Benner (Heidelberg, Germany) for their contribution to the conduct of the study; and all collaborators and patients who participated in the study.
Supported by the Dutch Cancer Society (grant 2008-4331) and Novartis.
Authorship
Contribution: A.E.C.B., B.v.d.H., E.M., and J.J.C. designed the study; A.E.C.B., C.N.d.J., K.B., B.v.d.H., E.M., and J.J.C. were involved in analyzing and interpreting the data and writing the report; A.E.C.B., C.N.d.J., M.D.H., M.v.M.K., M.R.d.G., M.v.G., J.K., E.M., and J.J.C. were responsible for patient recruitment and collection and assembly of the clinical data; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Annoek E. C. Broers, Department of Hematology, Erasmus MC Cancer Institute, Doctor Molewaterplein 40, 3015 GD Rotterdam, The Netherlands; e-mail: a.broers@erasmusmc.nl.
References
Author notes
Requests for data sharing may be submitted to Annoek E.C. Broers (a.broers@erasmusmc.nl).
The full-text version of this article contains a data supplement.