Key Points
Consolidative SCT should be considered in patients with CD30+PTCL in a CR following frontline treatment with A+CHP.
Further studies are needed to establish the benefits of consolidative SCT in this setting.
Abstract
Peripheral T-cell lymphomas (PTCLs) are a heterogeneous group of aggressive non-Hodgkin lymphomas, the majority of which have high relapse rates following standard therapy. Despite use of consolidative stem cell transplant (SCT) following frontline therapy, there remains no consensus on its utility. The double-blind randomized phase 3 ECHELON-2 study (#NCT01777152; clinicaltrials.gov) demonstrated improved progression-free survival (PFS) and overall survival with frontline brentuximab vedotin plus cyclophosphamide, doxorubicin, and prednisone (A+CHP). Herein, we conducted an exploratory subgroups analysis of the impact of consolidative SCT on PFS in patients with previously untreated CD30+ PTCL (ALK− anaplastic large cell lymphoma [ALCL] and non-ALCL) who were in complete response (CR) after frontline treatment with A+CHP or cyclophosphamide, doxorubicin, vincristine, and prednisone. Median PFS follow-up was 47.57 months. The PFS hazard ratio was 0.36, equating to a 64% reduction in the risk of a PFS event in patients who underwent SCT. The median PFS in patients who underwent SCT was not reached, vs 55.66 months in patients who did not undergo SCT. PFS results favored the use of SCT in both ALK− ALCL and non-ALCL subgroups. These data support the consideration of consolidative SCT in patients with CD30+PTCL who achieve CR following treatment with A+CHP.
Introduction
Peripheral T-cell lymphomas (PTCLs) are a heterogeneous group of aggressive non-Hodgkin lymphomas (NHLs), accounting for ∼10% of all NHL cases in Western populations and 24% in Asia.1 The so-called “nodal” subtypes, PTCL not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma, and systemic anaplastic large cell lymphoma (sALCL), are the most common, comprising ∼60% of PTCLs. For decades, CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOP-like regimens have been considered the standard therapy2,3; however, outcomes are poor for most subtypes.4-6
Several PTCL subtypes express CD30, including sALCL, for which CD30 is a diagnostic criterion.7 Among the non-sALCL subtypes, CD30 expression is variable; CD30 expression has been documented in 30% to 64% of cases of PTCL-NOS, with varied frequencies reflecting different threshold cutoffs for positivity.8,9 Prior studies suggested that CD30 expression might be associated with inferior outcomes in PTCL-NOS.7,10
Given the historically high relapse rate in PTCLs, consolidative high-dose chemotherapy and autologous stem cell transplant (SCT) is commonly considered following frontline chemotherapy.2-4 However, in the absence of randomized studies, and the potential for selection bias in retrospective studies, there is no consensus on the role of SCT in this setting. The Nordic Lymphoma Group performed a prospective phase 2 study evaluating CHOP-like chemotherapy followed by high-dose chemotherapy/autologous SCT in newly diagnosed PTCL (excluding anaplastic lymphoma kinase [ALK]-positive ALCL) with chemoresponsive disease.11 The 5-year progression-free survival (PFS) was encouraging (44%) compared with historical estimates, but chemorefractory disease remained problematic. A registration data analysis by the Center for International Blood and Marrow Transplant Research12 suggested that post-SCT outcomes were improved in those who were in a complete remission (complete response, CR) at the time of transplantation, with a 3-year PFS from consolidative autologous SCT of 58% in CR patients vs 42% in non-CR patients. Other retrospective studies, however, have shown no difference in patients who achieve a CR regardless of receipt of subsequent consolidative SCT.13,14
The double-blind, randomized, phase 3 ECHELON-2 study (#NCT01777152; clinicaltrials.gov) demonstrated a significant improvement in PFS, overall survival, CR, and overall response rate with frontline brentuximab vedotin (Adcetris) plus cyclophosphamide, doxorubicin, and prednisone (A+CHP) compared with CHOP in CD30+ PTCLs, with sustained benefit at 5 years of follow-up, and has led to regulatory approval in many jurisdictions.15,16 With improvement of frontline therapy, the role of consolidative SCT requires reevaluation. Herein, we explored the impact of consolidative SCT in ECHELON-2 in a post hoc subgroup analysis of patients in CR at end of treatment (EOT) after frontline A+CHP.15 The primary focus of the present analysis was to evaluate the impact of consolidative SCT in the A+CHP group; however, the impact of SCT in the CHOP arm was also investigated.
Methods
ECHELON-2 is a double-dummy, placebo-controlled, active-comparator study.15 For these exploratory analyses, eligible patients were adults with previously untreated CD30+ (≥10% of cells) PTCL (ALCL or non-ALCL), with the exception of ALK+ sALCL, a subtype that tends to have more favorable outcomes7 and is less associated with SCT. Patients were randomly assigned 1:1 to receive either A+CHP or CHOP for 6 or 8 cycles. Consolidative SCT (autologous or allogeneic) or radiotherapy was permitted at the investigator’s discretion after treatment with their intent specified prior to the first cycle of treatment. The primary endpoint was PFS, defined as the time from randomization to the first of relapse/progressive disease, death due to any cause, or receipt of subsequent systemic chemotherapy to treat residual or progressive PTCL.
Patients who discontinued treatment due to adverse events were included in the analyses, provided they were in a CR at EOT. Response was determined by independent review per the Revised Response Criteria for Malignant Lymphoma.17 A univariate analysis of SCT vs no SCT and multivariate analyses adjusting for region and age were performed in both the A+CHP and CHOP arms. PFS was estimated by Kaplan-Meier methods. The study received institutional review board approval from multiple institutions and was conducted according to the Declaration of Helsinki.
Results and discussion
Use of SCT on the A+CHP arm
Among patients with ALK− sALCL or non-ALCL in the A+CHP arm, 114/177 (64%) were in a CR at the EOT including. Sixty-seven percent (76/113) with ALK− sALCL and 59% (38/64) of patients with non-sALCL. Thirty-six percent (27/76) of patients with ALK− sALCL and 29% (11/38) of patients with non-ALCL underwent consolidative SCT (Figure 1). The median age of patients who underwent SCT was lower compared with those who did not undergo SCT (either autologous [n = 36] or allogeneic [n = 2]), regardless of PTCL subtype (ALK− sALCL [50 years vs 59 years] or non-sALCL [57 years vs 66 years]) (supplemental Table 1). Among those for whom positive intent to transplant was indicated, patients who underwent SCT had no difference in adverse event profile vs those who did not undergo SCT (data not shown).
Patient flow diagram for ECHELON-2 exploratory analysis: A+CHP arm. There were no significant differences (P = .49) in the baseline international prognostic index score distribution between patients who received SCT and patients who did not receive SCT. Note that 12 transplanted patients were excluded from the analysis (4 patients with ALK+; 8 did not have CR at EOT [6 PR, 1 SD, and 1 not evaluable]), for a total analysis population of n = 38. Consistent criteria16 were applied per protocol for response assessments. PR, partial response.
Patient flow diagram for ECHELON-2 exploratory analysis: A+CHP arm. There were no significant differences (P = .49) in the baseline international prognostic index score distribution between patients who received SCT and patients who did not receive SCT. Note that 12 transplanted patients were excluded from the analysis (4 patients with ALK+; 8 did not have CR at EOT [6 PR, 1 SD, and 1 not evaluable]), for a total analysis population of n = 38. Consistent criteria16 were applied per protocol for response assessments. PR, partial response.
Prior to the start of treatment, the intent to transplant among ALK− sALCL and non-ALCL patients in Asian countries (13% and 29%, respectively) was less frequent than that in non-Asian countries (49% and 57%, respectively). The proportion of patients ultimately transplanted was also lower in Asian (ALK− sALCL, 13%; non-sALCL, 12%) than in non-Asian countries (32% and 23%, respectively), which may reflect regional differences in treatment practices (for patients in CR, see supplemental Tables 2 and 5).
PFS by use of consolidative SCT after A+CHP in patients with CR at EOT
Median PFS follow-up was 47.57 months (95% confidence interval [CI], 41.89-48.16). A summary of PFS by consolidative SCT after A+CHP is provided in supplemental Table 3.
All patients (ALK− sALCL and non-sALCL)
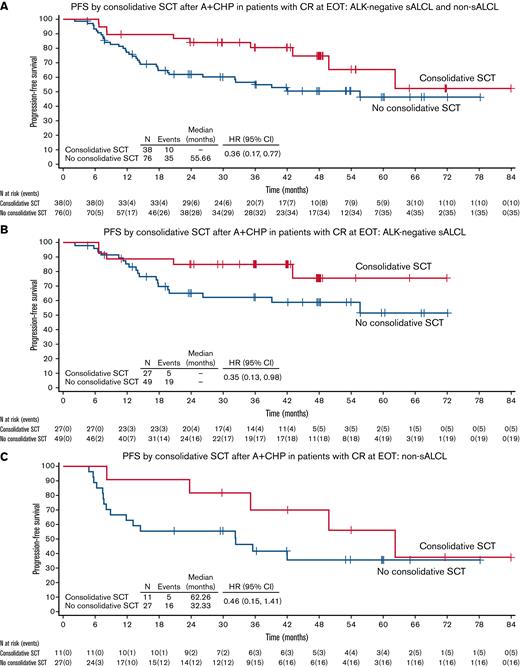
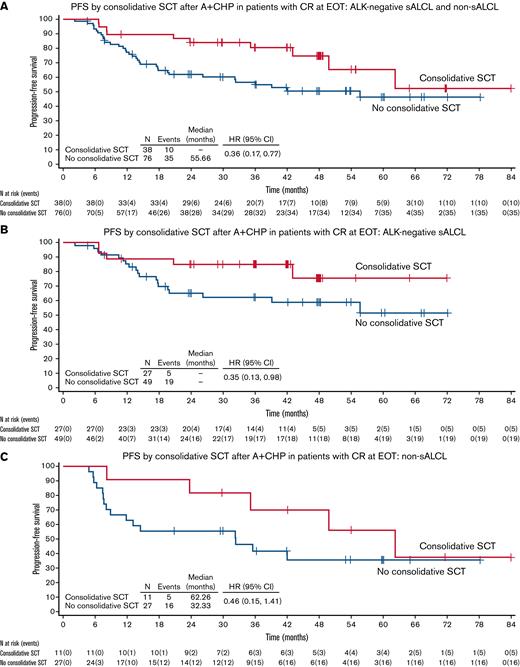
Across all patients (ALK− sALCL and non-sALCL) who achieved CR following A+CHP, patients who underwent SCT had a lower risk of experiencing a PFS event. The PFS hazard ratio (HR) was 0.36 (95% CI, 0.17-0.77), equating to a 64% reduction in the risk of a PFS event in patients who underwent SCT (Figure 2A). The estimated 3-year PFS in patients who underwent SCT was 80.4% vs 54.9% in patients who did not undergo SCT; at 5 years, the estimated PFS was 65.3% vs 46.4%, respectively (supplemental Table 3). A sensitivity analysis excluding patients ≥75 years of age (n = 10) demonstrated similar results (HR, 0.40; 95% CI, 0.18-0.89).
PFS by consolidative SCT: A+CHP arm. (A) PFS by consolidative SCT after A+CHP in patients with CR at EOT: ALK− sALCL and Non-sALCL. (B) PFS by consolidative SCT after A+CHP in patients with CR at EOT: ALK− sALCL. (C) PFS by consolidative SCT after A+CHP in patients with CR at EOT: Non-sALCL. ITT, intention to transplant.
PFS by consolidative SCT: A+CHP arm. (A) PFS by consolidative SCT after A+CHP in patients with CR at EOT: ALK− sALCL and Non-sALCL. (B) PFS by consolidative SCT after A+CHP in patients with CR at EOT: ALK− sALCL. (C) PFS by consolidative SCT after A+CHP in patients with CR at EOT: Non-sALCL. ITT, intention to transplant.
Patients with either ALK− sALCL or non-sALCL
Subgroup analyses in ALK− sALCL and non-sALCL subtypes were consistent with the overall combined PFS analyses. For those with ALK− sALCL, the HR for PFS was 0.35 (95% CI, 0.13-0.98), equating to a 65% reduction in the risk of PFS events in patients who underwent SCT (Figure 2B). The median PFS in patients was not reached regardless of SCT use among those with ALK− sALCL.
Patients with non-sALCL who underwent SCT had a 54% reduction in risk of a PFS event vs those who did not undergo SCT (HR, 0.46; 95% CI, 0.15-1.41) (Figure 2C). The median PFS in patients who underwent SCT was 62.26 months (95% CI, 23.72-NA) vs 32.33 months (95% CI, 8.08-NA) in patients who did not.
Adjusting for age and region, the multivariate proportional hazards regression analyses favored the use of SCT in PTCL patients (regardless of subtype) in a CR after A+CHP (supplemental Table 3).
Use of SCT in the CHOP arm
Of the patients with ALK− sALCL or non-ALCL on the CHOP arm, 97/177 (55%) were in a CR at EOT, including 50% (53/105) of patients with ALK− sALCL and 61% (44/72) of patients with non-sALCL. Twenty-five percent (13/53) of patients with ALK− sALCL and 36% (16/44) of patients with non-ALCL underwent consolidative SCT. Additional details on demographics and regional use of SCT on the CHOP arm can be found in supplemental Tables 4 and 5.
At a median follow-up of 53.72 months (95% CI, 47.54-59.37), there was a trend favoring consolidative SCT for ALK− sALCL and non-sALCL vs those who did not: HR, 0.63 (95% CI, 0.32-1.24). The estimated 3-year PFS in patients who underwent SCT was 67.2% vs 54.1% in patients who did not undergo SCT; at 5 years, the estimated PFS was 48.9% vs 50.9%, respectively, but it is noted that the latter estimates are based on very small patient numbers(supplemental Table 6). As with the A+CHP arm, a sensitivity analysis excluding patients ≥75 years of age (n = 9) demonstrated similar results (HR, 0.73; 95% CI, 0.36-1.49).
Subgroup analyses in ALK− sALCL and non-sALCL subtypes were consistent with the overall combined PFS results (supplemental Table 6). Adjusting for age and region, the multivariate proportional hazards regression analyses still favor the use of SCT; however, the benefit is less clear (supplemental Table 6).
Discussion
The overall impact of consolidative SCT, including after A+CHP or CHOP, remains unconfirmed given the lack of large, randomized, transplant-focused trials for this rare disease. The current analysis has limitations, including small patient numbers, thus limiting statistical power, and the potential for confounding variables (eg, regional differences in SCT use, older age in nontransplanted patients, and consolidative SCT performed at the discretion of the investigator). A significant difference in the CR rate with A+CHP (68%) vs CHOP (56%) should also be noted.
Analyses of SCT use among patients who received A+CHP appears to support the benefit of consolidative SCT; however, the benefit appeared less pronounced in the CHOP arm. Although these data provide additional context for the management of this rare patient population, the utility of consolidative SCT remains unanswered. Nevertheless, this exploratory analysis suggests that consolidative SCT should still be considered even with superior frontline therapy using A+CHP in CD30+ PTCL patients. Additional, larger studies are needed, particularly in DUSP22-rearranged and low International Prognostic Index ALK− ALCL, to determine whether there are favorable subgroups that could be treated with A+CHP alone.12,18,19
Acknowledgments
Medical writing assistance was provided by Michael Harrison (Seagen Inc.) and Lauren Angotti (BioBridges LLC) with funding from Seagen Inc.
This work was supported by Seagen Inc. and Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. This research was funded in part through National Institutes of Health National Cancer Institute cancer center support (grant P30CA008748).
Authorship
Contribution: K.J.S. and M.F. contributed to the analysis and interpretation of the data and wrote the manuscript; K.J.S., M.F., S.M.H., R.A., J.H.C., E.D.-D., G.R., F.M., O.A., C.S., K.T., A.S., M.T., S.Y., P.L.Z., L.T., T.I., O.A.O., and B.P. contributed to the acquisition of the data; and all authors contributed to the concept and design of the study, critically reviewed the manuscript, and approved the final version of the manuscript.
Conflict-of-interest disclosure: S.M.H. has consulted, received honorarium from, or participated in advisory boards for Acrotech Biopharma, C4 Therapeutics, Janssen, Kura Oncology, Kyowa Hakko Kirin, Myeloid Therapeutics, ONO Pharmaceuticals, Seagen Inc., Takeda, Trillium Therapeutics, and Vividion Therapeutics and has received research support for clinical trials from ADC Therapeutics, Affimed, Aileron, Celgene, Daiichi Sankyo, Forty Seven Inc., Kyowa Hakko Kirin, Millennium/Takeda, Portola Pharmaceuticals, Seagen Inc., Trillium Therapeutics, and Verastem. T.I. serves on the speakers bureau for Takeda. S.I. receives research funding from Arog, Bristol-Myers Squibb, Genentech/Roche, Incyte, and Seagen Inc. R.A. consults for Astra Zeneca, Bayer Healthcare Pharmaceuticals, Cell Medica, Celgene, Genentech/Roche, Gilead, KitePharma, Kyowa, Portola Pharmaceuticals, Sanofi, Seagen Inc., and Takeda. J.H.A. receives research funding from Seagen Inc. F.M. serves on the board of directors or advisory committees at Abbivie, Celgene, Epizyme, Gilead; receives honoraria from Janssen; consultants with and receives honoraria from and serves on the board of directors at Roche; and consultants for Servier. E.D.-D. consults for and receives travel, accommodation, expense, and research funding from Takeda; received travel and research funding from Bristol-Myers Squibb; receives travel, accommodation, and expense funding from Roche and Janssen; and receives research funding from Seagen Inc. G.R. consults for and receives honoraria from Daiichi Sankyo; serves on the advisory board for Roche, Janssen, Celgene, Amgen, Novartis, and Astellas; serves on the advisory board and receives travel expense funding from Gilead and Alexion; receives honoraria from and serves on the advisory board for Sandoz; receives honoraria from Incyte, Sanofi, Bristol-Myers Squibb, Mundipharma, and Novartis; is a member of the board of directors or advisory committees at Abbvie, Jazz, Pfizer, and Teva; receives research funding from Seagen Inc.; and travel expenses from Alexion. K.T. receives honoraria from Bristol-Myers Squibb, Eisai, and Solasia; consults and receives honoraria from Celgene, Chugai Pharma, Daiichi Sankyo, HUYA Bioscience, Kyowa Kirin, Mundipharma, Ono Pharma, Takeda, Yakult, and Zenyaku Kogyo; and consults for SymBio. A.R.S. receives research funding from Seagen Inc. K.J.S. consults and receives honoraria from Seagen Inc., Bristol Myers Squibb, Merck, Janssen, Kyowa; is a member of the advisory committees at Bristol-Myers Squibb, Steering Committee Beigene, and DSMC Regeneron; and receives institutional research funding from Roche and BMS. P.L.Z. receives honoraria and is a member of the board of directors or advisory committees and speakers bureau at Merck, AbbVie, Takeda, Incyte, Kirin Kyowa, and ADC Therapeutics; consults for, receives honoraria from, and is a member of the board of directors or advisory committees and speakers bureau at BMS, Gilead, Roche, Servier, EUSA Pharma, Verastem, Celltrion, Janssen-Cilag, Sandoz, MSD, Immune Design, and Celgene; receives honoraria from and is on the speakers bureau at TG Therapeutics Inc.; consults for and is a member of the board of directors or advisory committees at Sanofi; consults for and is a member of the board of directors or advisory committees, receives research funding, and is on the speakers bureau at Portola; consults for and is on the speakers bureau at EUSA Pharma and Kyowa Kirin; and is a member of the board of directors or advisory committees and speakers bureau at Immune Design. H.M. is a current equity holder at Takeda. V.B. receives research funding from Seagen Inc. and is currently employed at Takeda. K.F. is an employee of an has an equity interest in Seagen Inc. M.A.F. is an employee of and has an equity interest in Seagen Inc. M.P. is an employee of and has an equity interest in Seagen Inc. L.T. consults for Janssen and Nordic Nanovector; receives research funding from Mundipharma, Roche, and Seagen Inc.; and consults for and receives research funding from Takeda Europe. O.A. serves on the advisory board at Kyowa Kirin and Seagen Inc. and receives research funding from Seagen Inc. C.S. receives research funding from Seagen Inc. M.T. receives research funding from Seagen Inc. The remaining authors declare no competing financial interests.
Correspondence: Kerry J. Savage, Centre for Lymphoid Cancer and Division of Medical Oncology, British Columbia Cancer, 600 West 10th Ave, Vancouver, BC V5Z 4E6, Canada; e-mail: ksavage@bccancer.bc.ca.
References
Author notes
Data in this report was originally presented at the 61st meeting of the American Society of Hematology, Orlando, FL, 7-9 December 2019.
Requests for data sharing may be submitted to Kerry J. Savage (ksavage@bccancer.bc.ca.).
The full-text version of this article contains a data supplement.
The current affiliation for A.S. is Seagen Inc., Bothell, WA.

![Patient flow diagram for ECHELON-2 exploratory analysis: A+CHP arm. There were no significant differences (P = .49) in the baseline international prognostic index score distribution between patients who received SCT and patients who did not receive SCT. Note that 12 transplanted patients were excluded from the analysis (4 patients with ALK+; 8 did not have CR at EOT [6 PR, 1 SD, and 1 not evaluable]), for a total analysis population of n = 38. Consistent criteria16 were applied per protocol for response assessments. PR, partial response.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/6/19/10.1182_bloodadvances.2020003971/12/m_blooda_adv-2020-003971-gr1.jpeg?Expires=1767733503&Signature=zmWw1BqKIia2I~WOE3BsSto8LFu8aR5Ym-tJoQk5AR79oiiEtlqrSIfbQWwrLAAsmEid6buDPwd1yzYDkHfvvIFHH47EVONU0nX62n5HhYJLWa6h-OM8NzfLeCRQNU9CbsTsq-QAhEG6MjU1kn~HIdHG9w-gBqAWOQk13Q3N20fCwFUgTDCphh2FZT70EIZVcd1EPzWFfoW2KNUUOsBMiVgEiBpvGUa-55iJzumg5J~1D7ZWaPC5luw1l2K7N7qacXlZOGOQG2WAWthd~d0qpVQGvB70B1GgqeAkJhTRCQzdHQqquM086GsT9uMgaYF3wgobQsIhRDBh~a4Asw5~bg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Patient flow diagram for ECHELON-2 exploratory analysis: A+CHP arm. There were no significant differences (P = .49) in the baseline international prognostic index score distribution between patients who received SCT and patients who did not receive SCT. Note that 12 transplanted patients were excluded from the analysis (4 patients with ALK+; 8 did not have CR at EOT [6 PR, 1 SD, and 1 not evaluable]), for a total analysis population of n = 38. Consistent criteria16 were applied per protocol for response assessments. PR, partial response.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/6/19/10.1182_bloodadvances.2020003971/12/m_blooda_adv-2020-003971-gr1.jpeg?Expires=1767733504&Signature=4M93k46NxFviHEfORYycAzkl187E1BPiqf-Z5pJZU5iThfckn07txN2~FlL9bhdtFGcXZnUagsqDZDrdj07xqZFmwxhG~XI3-V-7cAzrht29cxU1gPSVeALkKQs8rpwI86EXdDSpNIGk81dXh94aOM4IDIgBAOXdcfhmuHmItsGE9PSX~eTT8~t-MnpslKge71sLwv1Y1Hx-Jhe7N12hhjnMrnboyXsFoxpPgfXNO1FhwClxNgZsuH5zjGXh1~cX0fK0nkr1ts4z3cqsQ7DgoYVSjs0j69Xgm~5xaFEneTiLatI3oxPmAakT6rkNXUgV2ra7kRfwYLJyaOaT7fhtkw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)