Key Points
Intraosseous delivery of FVIII lentiviral vector led to long-term expression of FVIII in human platelets.
Transduced human hematopoietic stem cells showed polyclonal integration of FVIII lentiviral vector across the human genome.
Abstract
We previously showed that intraosseous (IO) delivery of factor VIII (FVIII, gene F8) lentiviral vector (LV) driven by the megakaryocyte-specific promoter Gp1bα (G-F8-LV) partially corrected the bleeding phenotype in hemophilia A (HemA) mice for up to 5 months. In this study, we further characterized and confirmed the successful transduction of self-regenerating hematopoietic stem and progenitor cells (HSPCs) in treated mice. In addition, secondary transplant of HSPCs isolated from G-F8-LV–treated mice corrected the bleeding phenotype of the recipient HemA mice, indicating the potential of long-term transgene expression following IO-LV therapy. To facilitate the translation of this technology to human applications, we evaluated the safety and efficacy of this gene transfer therapy into human HSPCs. In vitro transduction of human HSPCs by the platelet-targeted G-F8-LV confirmed megakaryocyte-specific gene expression after preferential differentiation of HSPCs to megakaryocyte lineages. Lentiviral integration analysis detected a polyclonal integration pattern in G-F8-LV–transduced human cells, profiling the clinical safety of hemophilia treatment. Most importantly, IO delivery of G-F8-LV to humanized NBSGW mice produced persistent FVIII expression in human platelets after gene therapy, and the megakaryocytes differentiated from human CD34+ HSPCs isolated from LV-treated humanized mice showed up to 10.2% FVIII expression, indicating efficient transduction of self-regenerating human HSPCs. Collectively, these results indicate the long-term safety and efficacy of the IO-LV gene therapy strategy for HemA in a humanized model, adding further evidence to the feasibility of translating this method for clinical applications.
Introduction
Hemophilia A (HemA) is a congenital disease that results from a deficiency of functional factor VIII protein (FVIII; gene F8) secreted into the circulatory system, hindering clot formation. The current mainstay of treatment for HemA entails prophylactic or on-demand infusions of recombinant FVIII protein. Not only is this current approach expensive and inconvenient for patients, but infusion therapy also induces a 30% risk of developing anti-FVIII inhibitory antibodies (inhibitors)1,2 in patients with severe hemophilia.3
Gene therapy, both using lentiviral vector (LV) and adeno-associated virus (AAV), holds significant potential to offer alternative long-term treatment of hemophilia and is currently undergoing clinical trials. Multiple clinical trials for FVIII-AAV therapies targeted to the liver are now in phase 2 or 3 and have shown promising outcomes in elevating FVIII activity to therapeutic degrees.4-6 However, a high prevalence of preexisting antibodies to AAVs in the pediatric and adult population raises eligibility concerns for this treatment.7-10 Furthermore, AAV vectors remain episomal within the target cell rather than integrating into the host genome, leading to a “dilution effect” of the transgene expression as transduced cells divide. HIV-derived LVs, on the other hand, resolve both of these issues, as HIV prevalence is much lower than that of AAV in the general population, and the retrovirus can integrate into the DNA of self-regenerating hematopoietic stem and progenitor cells (HSPCs) and be maintained for a prolonged period.11,12
Lentiviral therapies for hemophilia that have begun clinical trials so far have focused on ex vivo gene therapy. This method requires genotoxic and often myeloablative preconditioning to allow for the transplant of autologous HSPCs, which can be difficult to tolerate for many patients.13 Even nonmyeloablative procedures involving chemotherapy drugs cause cytotoxic damage.14,15 In addition, lentiviral therapy raises the concern of insertional mutagenesis, as monoclonal integration of retroviral vectors in oncogenic promoter regions can induce tumorigenesis.16 A cautious step to ensure safety in the development of LV therapies is to confirm the polyclonality of the LV integration profile in the human genome before use in humans.
Our previous studies have shown that direct intraosseous (IO) delivery of F8-LV (IO-LV gene therapy) can treat HemA while circumventing the need for preconditioning. We employed G-F8-LV, in which FVIII expression is driven by the megakaryocyte-specific Gp1bα (G) promoter in the in vivo gene therapy protocol. Ectopic localization within platelet α granules17 protects FVIII from detection by circulating antigen-presenting cells.18-21 Meanwhile, at the site of injury, platelets activate and release FVIII locally, allowing for amplification of the coagulation cascade while minimizing the risk of generating inhibitors. Evidence showed long-term partial correction of the bleeding phenotype using this strategy as well as efficacy in the presence of preexisting FVIII inhibitors.18,22,23 This method of gene therapy thus presents the opportunity to induce persistent endogenous production of FVIII after a single dose of vectors while avoiding inhibitor development as well as the complicated and toxic procedures required for HSPC transplant.
In this study, we confirmed the efficacy of the megakaryocyte-specific G-F8-LV to transduce human HSPCs both in vitro and in vivo by IO injection in humanized NBSGW mice.24-27 We also assessed the safety of this gene therapy approach by using modified genome sequencing (MGS)-polymerase chain reaction (PCR)28,29 to analyze the LV integration patterns in the human genome. The success of our approach both in inducing human platelet FVIII expression and polyclonal integration of our vector brings this gene therapy method closer to clinical trial.
Methods
Mice
Mice were housed according to the guidelines of the National Institutes of Health and SCRI (Seattle Children’s Research Institute). Protocols used in this study were approved by the Institutional Animal Care and Use Committee at SCRI. HemA mice with a C57/BL6 genetic background were generated as previously described.30 NBSGW24,31,32 mice were obtained from Jackson Laboratories.
Lentiviral vector construction and production
pRRLSIN·cPPT·MCS·WPRE and pRRLSIN·MND·eGFP·WPRE (MND-GFP construct) were obtained from the SCRI viral core. Promoters (the human Gp1bα promoter [from −274 to 54] amplified from genomic DNA) and transgenes (hFVIII/N633 and eGFP) were inserted into pRRLSIN·cPPT·MCS·WPRE at the NheI site, and between XhoI and SpeI sites, respectively, to generate pRRLSIN·Gp1bα·hFVIII/N6·WPRE (G-F8 construct) and pRRLSIN·Gp1bα·eGFP·WPRE (G-GFP construct). All reagents for cloning were purchased from Qiagen (Valencia, CA). The LVs (MND-GFP-LV, G-GFP-LV, and G-F8-LV) were produced by transient transfection of 3 plasmids using polyethyleneimine in 293T cells and concentrated by ultracentrifugation.34 The cocal envelope, which transduces human HSPCs with greater efficiency than VSV-G, was used to maximize the transduction of human cells in this study.35 Viral titers were determined by infection in HEK 293T cells, followed by quantitative PCR (qPCR) and expressed as ifu/mL.36
LV transduction of hCD34+ cells
Human CD34-enriched G-CSF–mobilized PBSCs were purchased from the Heimfeld Laboratory at Fred Hutchinson Cancer Research Center. Cells were plated at a density of 1 × 106 cells/mL and cultured in Serum-Free Expansion Medium II (SFEM II; Stemspan) supplemented with cytokine cocktail (CC110) to expand HSPCs for 48 days before LV transduction.37,38 For the generation of megakaryocytes, media was exchanged with SFEM II with megakaryocyte expansion supplement (MEG) and 40 μg/mL of low-density lipoprotein (LDL) following transduction to promote differentiation.39 Cells were subsequently analyzed by flow cytometry.
IO infusion of LVs into HemA and NBSGW mice
IO infusion procedure was modified from a previously reported protocol.22,40 A 27-gauge insulin needle was briefly inserted into the tibia of an anesthetized mouse. LVs were then infused into the mouse at a speed of 10 μL per minute for 2 minutes through a 30-gauge insulin needle.
NBSGW mice were treated with enrofloxacin (0.2 mg/mL) to prevent infection from the procedure starting 2 days before until 14 days after the transplantation or IO infusion procedure. Eight-week-old NSGBW mice were treated with busulfan (35 mg/kg)41,42 24 hours before transplant of 1 × 106 hCD34+ cells via IV injection to generate humanized NBSGW mice. IO infusion of LVs was performed at 8 weeks after transplantation. At week 20, mice were intraperitoneally injected with 100 μL clodronate liposomes (FormuMax) 3 times, each injection 3 days apart, to deplete mouse macrophages and elevate concentrations of human platelets.43,44 Viral copy numbers in BM cells in transduced mice were measured by the qPCR protocol as previously described.45
Isolation of bone marrow (BM) and human HSPCs
Femur and tibia of mice were collected, and bones were flushed with DMEM using a 27-gauge needle to obtain a single cell suspension. To maximize HSPC collection, bones were treated with collagenase II. Bones were cut using surgical scissors into 1 to 2 mm pieces and incubated in collagenase II buffer containing DMEM, 1% penicillin/streptomycin, and 2 mg/mL collagenase II (Sigma Aldrich) for 1 hour at 37°C. Cells treated with collagenase II were filtered through a 40 μm filter to isolate single cells and added to the flushed cell suspension. Human CD34+ cells were isolated from the BM cells using the human CD34 Microbead Kit and the autoMACS Pro Separator (Miltenyi Biotec).
Flow cytometry analysis
Platelets of mice treated with G-GFP-LV and G-F8-LV were isolated from PBMC using Pancoll Platelets (Pan Biotec Germany). Platelets were then stained for surface markers, mouse CD41, and human CD41a (eBioscience). To measure intracellular FVIII in platelets of mice treated with G-F8-LV, platelets were fixed and stained with a primary mouse anti-hFVIII monoclonal antibody (ESH8, American Diagnostica) and then rat anti-mouse IgG2a FITC (Thermofisher Scientific).
BM cells of mice treated with MND-GFP-LV (M-GFP-LV) were stained for LT-HSPC surface markers, LSK (Lineage, Sca1, C-kit), CD150, and CD48. Flow cytometry analysis was conducted using LSRII (BD Bioscience), and the data were analyzed using FlowJo 8.8.1.
Assessment of FVIII concentrations in platelets (enzyme-linked immunosorbent assay [ELISA])
Platelets were lysed in 0.5% CHAPS buffer and plated in a 96-well flat bottom plate, which was coated with the primary antibody (GMA-8020, 1 mg/mL). The FVIII standard curve was generated by serial dilutions (1:20-5120) of Human Normal Pooled Plasma in a blocking buffer. The secondary antibody (Biotinylated-GMA-8015, 0.33 mg/mL) was applied, followed by Avidin-HRP. K-blue was added to develop color, and the reaction was stopped with 2N H2SO4. The samples were read with a 450 nm filter using the Victor3 plate reader (PerkinElmer).
BM transplantation of LV-transduced BM cells
Antibody–drug conjugate (ADC) was generated according to previous reports.46,47 Briefly, biotinylated anti-mouse CD117 (BioLegend) was mixed with streptavidin-conjugated saponin (Advanced Targeting System, it27100) in a 1:1 molar ratio to form anti–CD117-ADC, and then diluted in PBS for retro-orbital injection. Each experimental mouse received 1.5 mg/kg anti–CD117-ADC (16.5 μg of anti-CD117 antibody and 13.5 μg of streptavidin-saponin) in a total volume of 200 μL PBS.
BM cells were harvested from all limbs, including the femur and tibia, by flushing with a sodium bicarbonate-buffered DMEM medium. Each recipient mouse was transplanted with around 1 × 106 BM cells by retro-orbital injection 7 days after treatment of anti–CD117-ADC. Coagulation function of BM-recipient HemA mice was examined 1 month after BM transplantation using whole blood and INTEM kit by rotational thromboelastography (ROTEM) assay48(ROTEM δ instrument; Instrumentation Laboratory).
Assessment of retroviral integration sites
Statistical analysis
Data were expressed as mean ± the standard deviation of the mean. The statistical significance was determined using a 2-sample assuming unequal variances t test or multiple comparison test with the Bonferroni correction between >2 samples. Differences were considered significant at P < 0.05.
For original data, please contact carol.miao@seattlechildrens.org.
Results
Transduction of LT-HSCs and HPCs using MND-GFP-LV
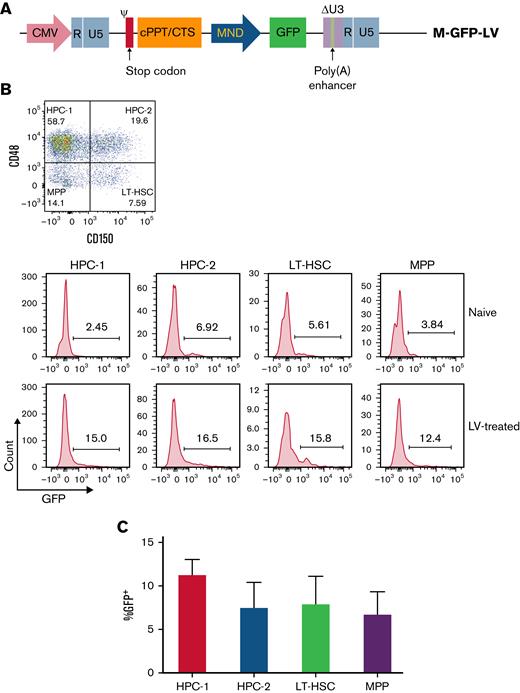
To evaluate if IO infusion of LVs can transduce primitive HSPCs to achieve long-term transgene expression for a persistent therapeutic effect, we performed 2 experiments. First, we assessed the ability of LVs to transduce the self-regenerating population of HSPCs, referred to as long-term hematopoietic stem cells (LT-HSCs), as well as restricted hematopoietic progenitor cells (HPC-1 and HPC-2), which are determined to differentiate into myeloid cells and megakaryocyte progenitor cells (MPP).49 C57BL/6 mice were IO injected with M-GFP-LV (1.44 × 109 ifu/mL) (Figure 1A). One week following IO infusion, BM was harvested and stained for Lineage, Sca1, and C-kit markers. LSK cells were then stained with anti-CD150 and anti-CD48 to differentiate between LT-HSC (LSK-CD150+CD48−), HPC-1 (LSK-CD150−CD48+), HPC-2 (LSK-CD150+CD48+), and MPP (LSK-CD150−CD48−) populations (Figure 1B). GFP expression was detected in all HPC subgroups (Figure 1C). These data show that LVs are capable of transducing LT-HSCs and hematopoietic progenitors, which presents the potential for lifelong maintenance of transgene copies in HSPCs.
M-GFP-LV transduces LT-HSCs and HPCs in C57BL/6 mice. MND-GFP-LV (2.88 × 107 ifu per animal) was IO-injected in C57BL/6 mice (n = 4 per group). Untreated naive mice were used as control samples. (A) BM was isolated 1 week after LV delivery and stained for LT-HSCs (LSK-CD150+CD48−), HPC-1 (LSK-CD150−CD48+), HPC-2 (LSK-CD150+CD48+), and MPPs (LSK-CD150−CD48−). (B) GFP expression was measured in each subtype of LSK cells in untreated naive (top) and LV-treated (bottom) mice. From left to right, HPC-1, HPC-2, LT-HSC, and MPP. (C) Summary plot of GFP expression after subtracting the control samples in different subpopulations of LSK cells. The data are presented as means with standard deviation.
M-GFP-LV transduces LT-HSCs and HPCs in C57BL/6 mice. MND-GFP-LV (2.88 × 107 ifu per animal) was IO-injected in C57BL/6 mice (n = 4 per group). Untreated naive mice were used as control samples. (A) BM was isolated 1 week after LV delivery and stained for LT-HSCs (LSK-CD150+CD48−), HPC-1 (LSK-CD150−CD48+), HPC-2 (LSK-CD150+CD48+), and MPPs (LSK-CD150−CD48−). (B) GFP expression was measured in each subtype of LSK cells in untreated naive (top) and LV-treated (bottom) mice. From left to right, HPC-1, HPC-2, LT-HSC, and MPP. (C) Summary plot of GFP expression after subtracting the control samples in different subpopulations of LSK cells. The data are presented as means with standard deviation.
Phenotypical correction in HemA mice after secondary transplant
While we confirmed that our LVs could transduce primitive HSPCs, we also aimed to confirm sustained correction of the bleeding phenotype in HemA mice after a secondary transplant of HSPCs from G-F8-LV–treated mice. Four weeks after IO injection of G-F8-LV (1.5 × 109 ifu/mL) in HemA mice, total BM cells were isolated and transplanted into recipient HemA mice (1 × 106 cells per mouse) preconditioned with CD117 antibody46,47 (Figure 2A). One month after secondary transplant, we performed the ROTEM assay using whole blood collected from HemA mice that had received a secondary transplant. Shorter median clotting time, shorter clot formation time, larger α angle, and larger maximum clot firmness were observed in recipient mice compared with the untreated control naïve HemA mice (Figure 2B-C). Overall, the mice that received transplanted cells showed significant correction of bleeding phenotype, indicating that IO infusion of LVs successfully transduced primitive HSPCs and enabled the production of factor VIII in the platelets of recipient mice.
Secondary transplant of HSPCs from HemA mice IO-treated with G-F8-LVs maintains phenotypical correction. HemA mice were treated with IO infusion of G-F8-LV (1.5 × 109 ifu/mL). Four weeks after treatment, total BM cells were isolated from LV-treated mice and transplanted into recipient HemA mice (1 × 106 BM cells per mouse). One month after the secondary transplant, we performed the ROTEM assay. (A) Schematic of self-inactivating LV genome encoding FVIII under the platelet-specific promoter, GpIbα (top panel), and the schedule of the secondary transplant experiments (bottom panel). (B) Representative ROTEM assays were shown for an untreated naive HemA mouse (HemA), a wild-type C57BL/6 mouse control (WT), a G-F8-LV–treated donor mouse (IO-LV), and a secondary BM transplant recipient mouse (BMT). (C) Summary of ROTEM assay results. The data are presented as means with standard deviation from 2 separate experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001.
Secondary transplant of HSPCs from HemA mice IO-treated with G-F8-LVs maintains phenotypical correction. HemA mice were treated with IO infusion of G-F8-LV (1.5 × 109 ifu/mL). Four weeks after treatment, total BM cells were isolated from LV-treated mice and transplanted into recipient HemA mice (1 × 106 BM cells per mouse). One month after the secondary transplant, we performed the ROTEM assay. (A) Schematic of self-inactivating LV genome encoding FVIII under the platelet-specific promoter, GpIbα (top panel), and the schedule of the secondary transplant experiments (bottom panel). (B) Representative ROTEM assays were shown for an untreated naive HemA mouse (HemA), a wild-type C57BL/6 mouse control (WT), a G-F8-LV–treated donor mouse (IO-LV), and a secondary BM transplant recipient mouse (BMT). (C) Summary of ROTEM assay results. The data are presented as means with standard deviation from 2 separate experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001.
Ex vivo expression of G-GFP-LV in human megakaryocytes
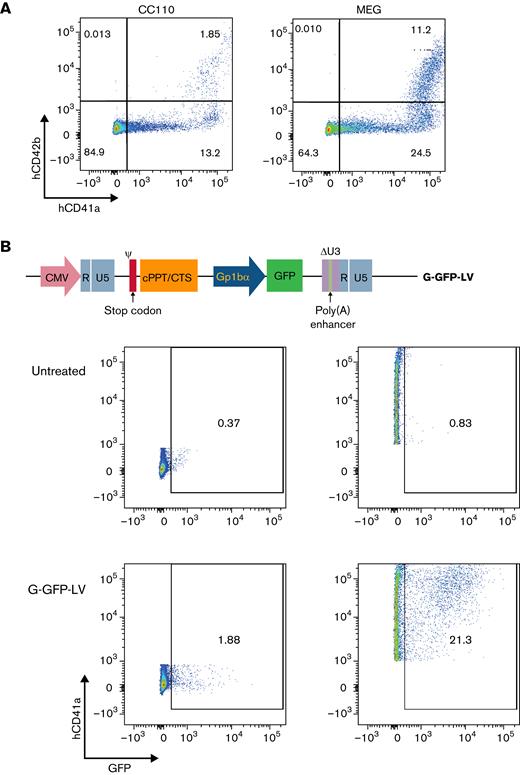
To determine whether our platelet-specific lentiviral constructs can transduce human cells, we first tested transduction of G-GFP-LV (7.1 × 108 ifu/mL) driven by a platelet-specific promoter GP1bα in human HSPCs ex vivo. CD41a (glycoprotein IIb) and CD42b (glycoprotein 1b) were used as platelet and megakaryocyte markers, with CD42b indicating later megakaryocyte differentiation.39,50 To assess the ability of the Gp1bα promoter in our LV constructs to drive expression in platelet progenitors, LV-transduced HSPCs (multiplicity of infection [MOI] = 1) were either expanded in media supplemented with CC110 or preferentially differentiated into megakaryocytes in media supplemented with MEG 24 hours after transduction. After 8 days of growth, the percentage of CD41a+CD42b+ cells increased from 1.85% in CC110-supplemented media to 11.2% in MEG (Figure 3A). Nine days after transduction, GFP expression was measured by flow cytometry. In cells cultured in MEG, CD41a+ cells showed higher GFP percentage expression than CD41a− cells. The GFP expression between CD41a+ and CD41a− cells after subtracting background was 20.47% and 1.51%, respectively. (Figure 3B). These results confirm that G-GFP-LV can efficiently transduce human HSPCs and promote megakaryocyte-specific expression in human cells.
G-GFP-LV transduction induces GFP expression in human megakaryocytes in vitro. (A) Human CD34+ cells isolated from G-CSF mobilized donors were cultured in SFEM II media supplemented with CC110 or MEG for 9 days. Preferential differentiation into megakaryocytes (CD41a+, CD42b+) was measured using flow cytometry. (B) Schematic of the self-inactivating LV genome encoding GFP under the platelet-specific promoter GpIbα is shown at the top. Human HSPCs were transduced with G-GFP-LV and cultured in MEG-supplemented SFEM media for 9 days before the expression was measured on flow. Top row of flow panels, untransduced cells; bottom row of flow panels, cells transduced at a multiplicity of infection (MOI) of 1. GFP expression was gated in CD41a− cells (left panels) and CD41a+ cells (right panels).
G-GFP-LV transduction induces GFP expression in human megakaryocytes in vitro. (A) Human CD34+ cells isolated from G-CSF mobilized donors were cultured in SFEM II media supplemented with CC110 or MEG for 9 days. Preferential differentiation into megakaryocytes (CD41a+, CD42b+) was measured using flow cytometry. (B) Schematic of the self-inactivating LV genome encoding GFP under the platelet-specific promoter GpIbα is shown at the top. Human HSPCs were transduced with G-GFP-LV and cultured in MEG-supplemented SFEM media for 9 days before the expression was measured on flow. Top row of flow panels, untransduced cells; bottom row of flow panels, cells transduced at a multiplicity of infection (MOI) of 1. GFP expression was gated in CD41a− cells (left panels) and CD41a+ cells (right panels).
Safety and efficacy of G-F8-LV in transducing human HSPCs ex vivo
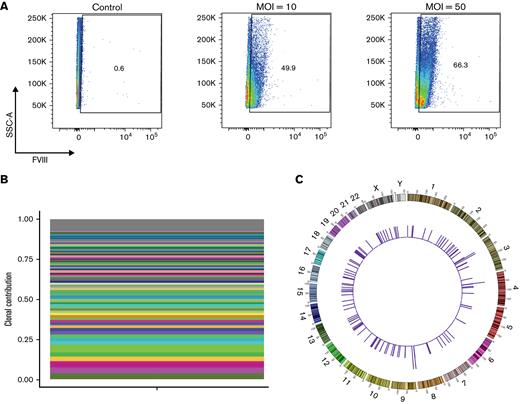
On confirming G-GFP-LV expression in human megakaryocytes, we assessed the efficacy of G-F8-LV (1.5 × 109 ifu/mL) to transduce human cells under similar conditions. HSPCs were treated at higher MOI of 10, 50, and 100 because of the increased size of the transgene, leading to less efficient transduction. After culturing in MEG-supplemented media for 14 days, LV-treated cells were analyzed by flow cytometry for intracellular FVIII expression. LV-treated cells showed that increasing MOIs led to increased percentage expression of FVIII, with percent expression increasing nearly twofold from an MOI of 10 to 50 (Figure 4A), indicating successful transduction of human HSPCs by G-F8-LV and subsequent expression of FVIII in human megakaryocytes.
G-GFP-LV transduction induces FVIII expression in human megakaryocytes in vitro and integrates polyclonally. Human CD34+ HSPCs were treated with G-F8-LVs (MOI = 5) and subsequently grown in MEG supplemented media for 14 days. (A) Intracellular FVIII expression was measured by flow cytometry. (B) MGS-PCR was performed on genomic DNA isolated from G-F8-LV transduced human HSPCs G-F8-LVfor RIS analysis. Clones contributing ≥1% of the detected pool are denoted by a colored bar. All remaining identified clones are grouped into the gray bar at the top of the plot. A total of 124 unique integration sites were found, none displaying clonal dominance. (C) Circle plot mapping RIS positions to the human genome (outer numbers indicate chromosome numbers). The heights of the histograms indicate the number of integrations found in a 1 Mbp bin.
G-GFP-LV transduction induces FVIII expression in human megakaryocytes in vitro and integrates polyclonally. Human CD34+ HSPCs were treated with G-F8-LVs (MOI = 5) and subsequently grown in MEG supplemented media for 14 days. (A) Intracellular FVIII expression was measured by flow cytometry. (B) MGS-PCR was performed on genomic DNA isolated from G-F8-LV transduced human HSPCs G-F8-LVfor RIS analysis. Clones contributing ≥1% of the detected pool are denoted by a colored bar. All remaining identified clones are grouped into the gray bar at the top of the plot. A total of 124 unique integration sites were found, none displaying clonal dominance. (C) Circle plot mapping RIS positions to the human genome (outer numbers indicate chromosome numbers). The heights of the histograms indicate the number of integrations found in a 1 Mbp bin.
To examine the safety of G-F8-LV integration in human HSPCs, we then used modified genome sequencing (MGS)-PCR to assess lentiviral integration sites (LIS) in the human genome.28 HSPCs were treated with G-F8-LV at MOI = 5 and expanded in CC110-supplemented media for 14 days, after which genomic DNA was isolated. The average marking number in gDNA from LV-treated cells was 0.252 integrations per cell, as measured by qPCR, and samples underwent LIS analysis. A total of 124 unique integration sites were identified, with the most frequently identified clone comprising 3.98% of total integration sites (Figure 4B). Mapped across the human genome, LISs were detected on nearly every human chromosome except chromosome 21 (Figure 4C). In addition, none of the integrations showed clonal dominance in the transduced HSPCs. In the top 20 abundant hits genes, only 2 of the integration sites were found within a tumor suppressor gene, OPCML and an oncogene (USP4), respectively. The integration site of OPCML was 960 kb downstream of the transcription start site and within intron 3. The integration site of USP4 was 25 kb downstream of the transcription start site and within intron 6. None of the integration sites were found in the promoter region. In addition, none of the mice demonstrated evidence of myelodysplastic syndrome or leukemia. These data indicate highly polyclonal integration in human HSPCs, implying the long-term safety of our vector in human HSPCs.
Optimizing human platelet concentrations in NSG mice
Next, we aimed to test the efficacy of our LV constructs in human cells in vivo using a humanized mouse model in immunodeficient mice. To optimize human cell engraftment, we compared engraftment in NOD SCID γ (NSG) and NOD, B6, SCID Il2rγ−/− Kit(W41/W41) (NBSGW) mice, the latter being characterized by the W-41J mutation in its Kit gene, conferring higher engraftment capability.24 Both strains were IV-transplanted with 1 × 106 human HSPCs per mouse via retro-orbital injection. Six weeks after transplant, we observed over fourfold greater efficiency of human leukocyte engraftment in the NSBGW strain compared with NSG, with percent hCD45+ of total PBMCs measuring 16% and 3.86%, respectively (Figure 5A).
NBSGW mice showed superior human HSPC engraftment compared with NSG mice. A total of 1 × 106 human CD34+ cells isolated from G-CSF mobilized donors were IV-transplanted to 8- to 10-week-old NSG (n = 16) and NBSGW (n = 9) mice. (A) Percentages of human CD45+ cells in total PBMCs were measured by flow cytometry to determine engraftment efficiency at 10 weeks after transplant. (B) Transplanted NSG (n = 6) and NBSGW (n = 9) mice were intraperitoneally injected 3 times with Clodronate liposomes (CL; 100 ul per injection) over a week. Human CD41+ platelet concentrations were evaluated by flow cytometry. The data are presented as means with standard deviation. ∗∗P < .01 and ∗∗∗P < .001. (C) Representative flow panels to show the proportion of human platelets and mouse platelets in NBSGW mice 1 day following CL treatment. Left, before CL treatment; right, after CL treatment.
NBSGW mice showed superior human HSPC engraftment compared with NSG mice. A total of 1 × 106 human CD34+ cells isolated from G-CSF mobilized donors were IV-transplanted to 8- to 10-week-old NSG (n = 16) and NBSGW (n = 9) mice. (A) Percentages of human CD45+ cells in total PBMCs were measured by flow cytometry to determine engraftment efficiency at 10 weeks after transplant. (B) Transplanted NSG (n = 6) and NBSGW (n = 9) mice were intraperitoneally injected 3 times with Clodronate liposomes (CL; 100 ul per injection) over a week. Human CD41+ platelet concentrations were evaluated by flow cytometry. The data are presented as means with standard deviation. ∗∗P < .01 and ∗∗∗P < .001. (C) Representative flow panels to show the proportion of human platelets and mouse platelets in NBSGW mice 1 day following CL treatment. Left, before CL treatment; right, after CL treatment.
Despite moderate concentrations of human lymphocytes in PBMC in NBSGW mice, human platelet (hCD41a) concentrations remained low, making detection of FVIII in human platelets difficult. To increase human platelet concentrations, both strains of mice were treated with clodronate liposomes via intraperitoneal injection to deplete mouse macrophages.43,44 Two days after the final clodronate liposome injection, platelets were isolated, stained intracellularly for FVIII, and analyzed by flow cytometry. Elevation in the percentage of human platelets was significantly more pronounced in NBSGW mice. Platelets positive for hCD41a in NBSGW mice averaged 10.7% of the chimera after clodronate liposome treatment, compared with 2.82% in the NSG strain (Figure 5B-C). Considering this data, we chose NBSGW mice as the strain in which to test the transduction efficiency and safety of our LVs in a humanized animal model.
In vivo expression of G-GFP-LV and G-F8-LV in human platelets in humanized NBSGW mice
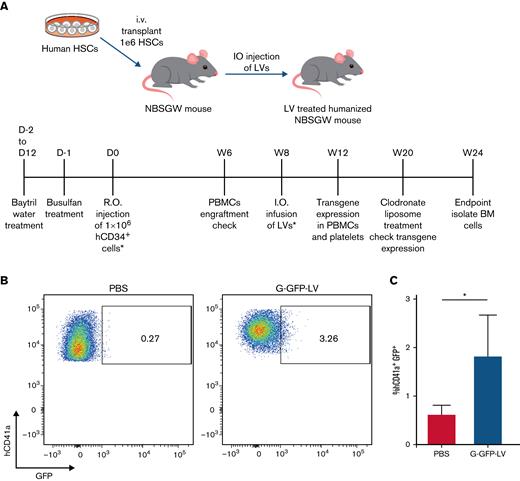
Having confirmed the megakaryocyte-specific expression of G-GFP-LV in vitro and optimized our humanized mouse model, we next tested the ability of G-GFP-LV to induce platelet-specific expression in the humanized model in vivo. PBS (20 ul per animal) or G-GFP-LVs (6.3 × 108 ifu per animal) were IO-injected into 8-week-old humanized NBSGW mice 8 weeks after human HSPC transplantation. Mice were treated with clodronate liposomes 12 weeks later to enhance our ability to detect GFP expression in human platelets (Figure 6A). GFP-expressing human platelets ranged from 0.8% to 3.26% (n = 4) of total human platelets in the LV-treated group 6 weeks after gene therapy (Figure 6B). These results show that G-GFP-LV can effectively transduce human HSPCs, and the transgene was expressed in human platelets up to 3 months after gene therapy (which was our experimental duration).
G-GFP-LV induces GFP expression in human platelets in vivo. (A) Schematics and schedule of IO-LV therapy in humanized NMSGW mice. A total of 1 × 106 human CD34+ cells were transplanted to 8- to 10-week-old NBSGW mice. Eight weeks after transplant, mice were IO-injected either with PBS (20 μL per animal) or G-GFP-LV (6.3 × 108 ifu per animal). Platelets from G-GFP-LV–injected humanized NBSGW mice were isolated after clodronate liposome treatment 12 weeks after LV infusion. (B) GFP expression was measured in human platelets by flow cytometry. (C) Summary plot of GFP expression in human platelets after treatment with clodronate liposomes (PBS, n = 4; G-GFP-LV, n = 5). The data are presented as means with standard deviation from 2 separate experiments. ∗P < .05.
G-GFP-LV induces GFP expression in human platelets in vivo. (A) Schematics and schedule of IO-LV therapy in humanized NMSGW mice. A total of 1 × 106 human CD34+ cells were transplanted to 8- to 10-week-old NBSGW mice. Eight weeks after transplant, mice were IO-injected either with PBS (20 μL per animal) or G-GFP-LV (6.3 × 108 ifu per animal). Platelets from G-GFP-LV–injected humanized NBSGW mice were isolated after clodronate liposome treatment 12 weeks after LV infusion. (B) GFP expression was measured in human platelets by flow cytometry. (C) Summary plot of GFP expression in human platelets after treatment with clodronate liposomes (PBS, n = 4; G-GFP-LV, n = 5). The data are presented as means with standard deviation from 2 separate experiments. ∗P < .05.
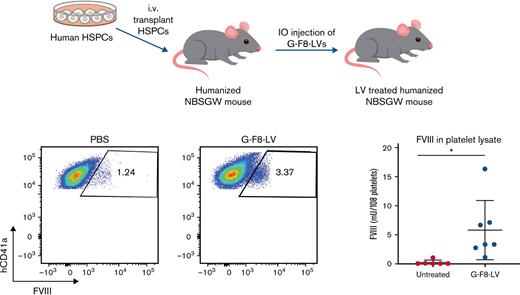
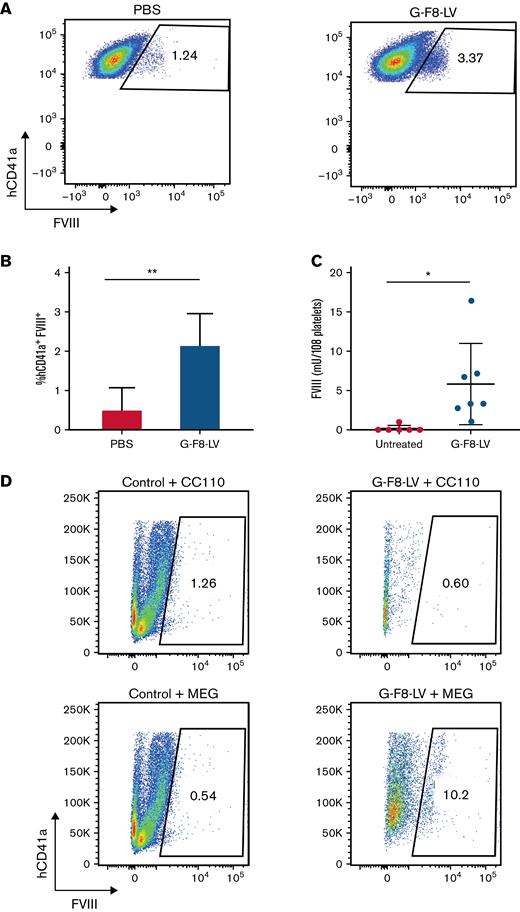
Next, we tested our disease-relevant G-F8-LV in the humanized mouse model. PBS or G-F8-LVs (5 × 107 ifu per animal) were IO-injected into humanized NBSGW mice. Twelve weeks later, the treated mice were administered clodronate liposomes to boost human platelet concentrations. Efficacy of expression in human platelets specifically was measured by flow cytometry (Figure 7A). The average FVIII expression, after subtracting background, was 1.70% (Figure 7B). FVIII concentrations in total platelet lysate, measured by ELISA, averaged 5.79 mU per 108 platelets 13 weeks after gene therapy (Figure 7C). Long-term expression of FVIII in human platelets implies successful transduction of human HSPCs in vivo by our gene therapy method.
IO delivery of G-F8-LV produced persistent FVIII expression in human megakaryocytes and platelets. Eight weeks after the transplant of 1 × 106 human CD34+ cells, humanized NBSGW mice were IO-injected either with PBS (20 μL per animal) or with G-F8-LV (5 × 107 ifu per animal). Twelve weeks following IO injection, platelets were collected from control mice injected with PBS (n = 5) and mice injected with G-F8-LV (n = 7) following CL treatment and lysed using 0.5% CHAPS. FVIII concentrations in platelets were measured by flow cytometry and ELISA. (A) Representative flow cytometry data from control and G-F8-LV–treated mice. The flow panels were gated for hCD41a+FVIII+ cells. Left, PBS injected; right, G-F8-LV injected. (B) Summary plot of FVIII+ cells in hCD41a+ platelets by flow cytometry (PBS, n = 4; G-F8-LV, n = 7). (C) Summary plot of FVIII concentrations measured in the supernatant of lysed platelets by ELISA. The data are presented as means with standard deviation from 3 separate experiments. ∗P < .05 and ∗∗P < .01. (D) BM of control or G-F8-LV–treated mice were harvested, and hCD34+ cells were isolated using autoMACS. The cells were grown in either CC110 or MEG-supplemented SFEM II media for 9 days, and intracellular expression of FVIII was measured by flow cytometry.
IO delivery of G-F8-LV produced persistent FVIII expression in human megakaryocytes and platelets. Eight weeks after the transplant of 1 × 106 human CD34+ cells, humanized NBSGW mice were IO-injected either with PBS (20 μL per animal) or with G-F8-LV (5 × 107 ifu per animal). Twelve weeks following IO injection, platelets were collected from control mice injected with PBS (n = 5) and mice injected with G-F8-LV (n = 7) following CL treatment and lysed using 0.5% CHAPS. FVIII concentrations in platelets were measured by flow cytometry and ELISA. (A) Representative flow cytometry data from control and G-F8-LV–treated mice. The flow panels were gated for hCD41a+FVIII+ cells. Left, PBS injected; right, G-F8-LV injected. (B) Summary plot of FVIII+ cells in hCD41a+ platelets by flow cytometry (PBS, n = 4; G-F8-LV, n = 7). (C) Summary plot of FVIII concentrations measured in the supernatant of lysed platelets by ELISA. The data are presented as means with standard deviation from 3 separate experiments. ∗P < .05 and ∗∗P < .01. (D) BM of control or G-F8-LV–treated mice were harvested, and hCD34+ cells were isolated using autoMACS. The cells were grown in either CC110 or MEG-supplemented SFEM II media for 9 days, and intracellular expression of FVIII was measured by flow cytometry.
To further confirm if human HSPCs, capable of self-renewal in the humanized mice, were LV-transduced, we next evaluated FVIII expression in megakaryocyte-differentiated HSPCs isolated from treated mice. Human CD34+ cells isolated from BM were cultured in CC110- or MEG-supplemented SFEM II for 6 days. Cells from G-F8-LV–treated mice and cultured in MEG showed 10.2% FVIII+ cells compared with 0.54% cultured in CC110, indicating megakaryocyte-specific expression (Figure 7D). These results indicate integration of the LV vector in human HSPCs, indicating the potential for long-lasting gene therapy.
Discussion
Our approach to direct long-term expression of FVIII in platelets is IO infusion of G-F8-LVs. We previously showed that a single IO infusion of G-F8-LV leads to phenotype correction in HemA mice. Here, we have further characterized and confirmed the transduction of primitive HSPCs by flow analysis of BM LSK cells. Transgene expression was detected in all 4 progenitor populations, including LT-HSC, HPC-1, HPC-2, and MPP, implying the potential for enduring transgene expression in LV-treated animals. This implication was further corroborated by sustained partial correction of the bleeding phenotype in HemA mice that received a secondary transplant of BM cells isolated from G-F8-LV–treated mice. Together these results indicate that primitive HSPCs were efficiently transduced by LVs in situ, resulting in FVIII expression in megakaryocytes and storage in platelet α granules, demonstrating the feasibility of IO-LV gene therapy to achieve a long-term therapeutic effect.
To facilitate translation of this novel technology, it is significant to optimize platelet FVIII gene expression in human megakaryocytes for future clinical applications. In this study, we showed efficient transduction of human HSPCs by both G-GFP-LV and G-F8-LV, leading to megakaryocyte-specific transgene expression in human cells. Moreover, the RIS profile of the LV integration in human HSPCs was confirmed to be polyclonal, implying the long-term safety of this LV in human cells. Next, we demonstrated for the first time successful transduction of human HSPCs by the megakaryocyte-specific G-F8-LV using the IO delivery method in a humanized mouse model. Since transgene expression could be limited by incomplete engraftment of human HSPCs in humanized mice, leading to low concentrations of human platelets, we used a humanized NBSGW mouse model that has higher engraftment efficiency and also supports much improved human erythropoiesis and platelet formation compared with humanized NSG mice.25 After IO delivery of G-F8-LV into the humanized NBSGW model, FVIII concentrations were comparable to our previous studies in hemophilia A mice.22 The proportion of cells expressing FVIII was similar between human and murine platelets, implying comparable transduction efficiencies in either species. Furthermore, human CD34+ cells isolated from the BM of LV-treated mice and cultured in MEG to differentiate into megakaryocyte lineages showed up to 11.2% FVIII expression, indicating efficient transduction of self-regenerating HSPCs in our humanized mouse model. Moreover, prior studies from other groups have shown that relatively low transduction, as low as 0.3% FVIII-expressing human platelets following ex vivo HSC gene therapy, can still lead to phenotypical correction in mice and thus carry significant clinical relevance.51
The issue of inhibitor development for patients with severe hemophilia remains the most significant obstacle to developing therapies for the disease.52 Many groups have attempted to understand and implement mechanisms of tolerance induction30,53-55 to enable patients to resume infusion therapy, but ITI therapy remains costly and clinically challenging.56 Delivery of FVIII to platelets, however, circumvents this issue. FVIII is stored intracellularly within the α granules17,19 of platelets, undetected by the immune system despite systemic circulation, and is released at the site of local injury upon platelet activation.18 Our laboratory and others have shown therapeutic efficacy and partial correction of the bleeding phenotype even in the presence of preexisting inhibitors via in vivo22 and ex vivo40 gene therapy, respectively, raising implications for the use of HSPC gene therapy targeting platelet expression of FVIII to treat patients who are widely considered the most challenging to treat today.
As gene therapy has advanced toward mainstream treatment of hemophilia, recent reports of clinical trials for AAV therapies for hemophilia have shown very promising results.6,57,58 However, the immune responses to viral vectors, transduced cells, transgene products, and potential tumor genesis are still major hurdles to overcome.59,60 In particular, this treatment is inaccessible to patients who have high-titer anti-AAV antibodies. Moreover, the significant loss of FVIII expression following AAV gene therapy year by year remains an unresolved problem.6 Currently, it is impossible to dose individuals with preexisting anti-AAV antibodies and to repeat the treatment because of the development of high-titer anti-AAV antibodies after gene therapy. Furthermore, this strategy may not be effective in children and adolescents because the episomal AAV vectors will be significantly diluted upon liver growth into adulthood. Lentiviral gene therapy targeting platelets, which uses the much less prevalent HIV integrating vector, presents a promising alternative strategy for gene therapy of hemophilia.
Lentiviral gene therapy targeting transduction of HSPCs has shown efficacy for genetic diseases such as sickle cell disease,61 β thalassemia,62 and Wiskott-Aldrich syndrome63,64 in numerous clinical trials. Recently, 4 phase 1 clinical trials using LVs for HemA and B have begun recruitment, all of which use an ex vivo strategy of gene therapy65 (supplemental Table 1 in the data supplement). Transplantation of transduced cells back into the patient’s BM requires a preconditioning regimen of cytotoxic drugs, which can lead to serious toxicities in various organ systems.13 More recently, a less toxic method of preconditioning, delivery of anti-CD117 antibody targeting the c-Kit gene, has been shown to lead to high engraftment.66 However, this new method does not avoid the elimination of many cell types, including myeloid and lymphoid cells and platelets, before engraftment in the BM is completely established. Considering the difficulty of tolerating ex vivo lentiviral-based gene therapy, direct delivery of LVs in vivo may be an attractive option to a wider array of patients. Preclinical testing for in vivo therapies for HemB is ongoing, such as in the investigation of hepatocyte-targeted FIX-LV delivery. IV delivery of LVs in canines and nonhuman primates elevated FIX serum concentrations, improved whole blood clotting time, and reduced frequency of bleeding for up to 90 days after gene therapy.67,68 Direct administration of LVs to patients thus presents as a promising and accessible treatment to HemA, as well.
Because of the ability of LVs to integrate into the host genome, they can achieve persistent transgene expression in cells. However, gene therapy involving retroviral vectors raises the concern of oncogenesis because of insertional mutagenesis.69,70 While checking the integration patterns of LVs in human cells is necessary, the only reported cases of insertional mutagenesis have occurred with γ-retroviral vectors, which have a proclivity to integrate near promoters, inducing clonal selection if integration affects oncogene expression.71 As expected in our transduced HSPCs, LIS analysis revealed polyclonal integration, with the site of integration most frequently observed comprising just 3.98% of total insertion sites. This demonstrates a safe integration profile of our LVs in human cells.
Conclusions
IO delivery of G-F8-LVs safely transduces human HSPCs and leads to FVIII expression in human megakaryocytes and platelets. Transduced HSPCs exhibited polyclonal insertion sites of the LVs across the human genome without clonal expansion. Our data demonstrate that this in vivo strategy harnessing platelet-specific F8-LV delivery is an effective gene therapy in a humanized model and presents a promising alternative therapy for HemA, especially for patients with inhibitory antibodies.
Acknowledgments
Financial support: This work was supported by grants from the National Heart, Lung, and Blood Institute (R01 HL134321 and R01 HL123326 [C.M.]).
Authorship
Contribution: J.H.J. designed and performed the experiments, collected the data, performed analysis, and wrote the paper; X.W. designed and performed the experiments, collected the data, and performed the analysis; S.S., C.-Y.C., and C.L. performed experiments; J.E.A., H.-P.K., and D.J.R. provided analysis tools and suggestions; and C.H.M. conceived the concepts, directed research, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carol H. Miao, Seattle Children’s Research Institute, 1900 9th Ave, Seattle, WA 98101; e-mail: carol.miao@seattlechildrens.org.
References
Author notes
Contact the corresponding author for data sharing: carol.miao@seattlechildrens.org.