TO THE EDITOR:
CD19 CAR T cells became a breakthrough therapy in pediatric relapsed and refractory B-lineage acute lymphoblastic leukemia.1,2 Standard usage of autologous T cells as a starting population for CAR-T manufacturing is often limited by the functional state of a patient's T cells and negatively affected by previous chemotherapy and posttransplant immune suppression. Healthy donor-derived T cells for CAR-T production may solve the issue of poor T-cell quality but is restricted by the risks of graft-versus-host disease (GVHD) and rejection of the CAR-T product, especially in the setting of haploidentical family donors.3-5 Use of virus-specific T cells for CAR-T manufacturing was proposed as an approach to limit the alloreactivity of donor-derived CAR T cells after allogeneic hematopoietic stem cell transplantation (HSCT).6 Depletion of naïve (CD45RA+) T cells reduces the frequency of alloreactive T cells and is used to process hematopoietic stem cell grafts and donor lymphocyte infusions to lower the risk of GVHD.7,8 The potential mechanisms of reduced alloreactivity of the CD45RA-depleted T cells include limited diversity of the T-cell receptor repertoire, diminished proliferative capacity, and differential tissue homing.9-11 Recently, the possibility of large-scale bioreactor-based manufacturing of the memory T-cell–derived CAR-T product was demonstrated.12 The animal studies suggest that CD45RA-depleted fraction can be used to produce CAR T cells that are equipotent to conventionally generated CAR T cells in vivo and do not induce xenogeneic GVHD.13 Encouraged by these reports, we validated the manufacturing of healthy donor-derived memory CAR T cells (CAR-Tm) based on automatic large-scale bioreactor processing.
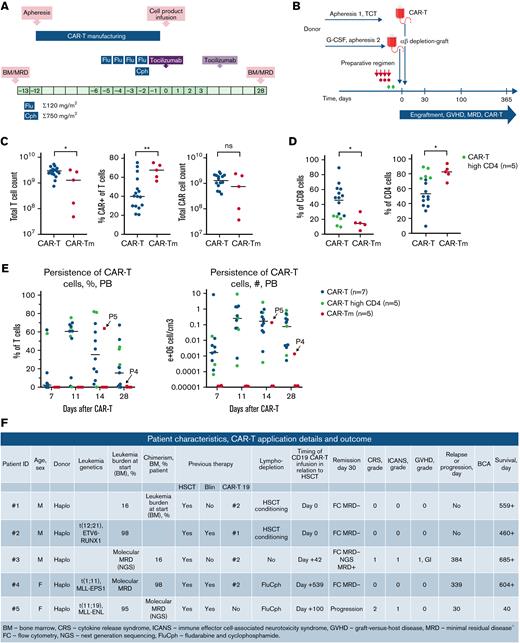
Five pediatric patients with relapsed and refractory B-lineage acute lymphoblastic leukemia were offered the therapy with CAR-Tm on a compassionate-use basis. In each case, an Institutional Review Board approval for named-patient use was provided. All 5 cases had B-ALL, relapsing after multiple lines of treatment, including HSCT (n = 5), autologous CD19 CAR-T (n = 4), and blinatumomab (n = 3) therapy (Figure 1F). Median age at treatment was 9 years old. At the time of allogeneic CAR-Tm application, disease burden was either overt leukemia (n = 3) or minimal residual disease-level disease (n = 2). CAR-Tm were derived from haploidentical familial HSCT donors, and in all cases, the same donor as for HSC graft was used. CAR-Tm products were applied after HSCT on days plus 42, plus 100, and plus 539 in 3 cases and simultaneously with the HSC grafts on day 0 in 2 cases (Figure 1A). In all cases, HSCT from haploidentical donors was performed based on the ex vivo αβ T-cell depletion platform as described before (Figure 1B).8,14 In 2 cases, standard fludarabine (120 mg/m2) and cyclophosphamide (750 mg/m2) lymphodepletion was used (Figure 1A; supplemental Figure 1). In 2 cases, lymphodepletion was represented by the pre-HSCT conditioning as shown in Figure 1F. Specifically, the use of antithymocyte globulin was omitted, creating the opportunity for CAR T-cell expansion early after HSCT. One patient had not received any lymphodepletion (day plus 42).
Manufacturing of CD19 CAR T-cell products based on CD4/CD8-selected (CAR-T) or CD45RA-depleted T cells (CAR-Tm). (A) Overall CAR-T manufacturing and lymphodepletion plan. (B) Schema of simultaneous donor-derived CAR-T plus haplo HSCT. (C) Transduction efficacy and numbers of T-cell products after the CAR-T or CAR-Tm (CD45RA-depleted) manufacturing. (D) Analysis of frequency of CD4+ and CD8+ T cells in CAR-Tm and CAR-T products. Dots represent individual patients in panels A, B, and C. Green dots represent CAR-T patients with dominance of CD4+ cells. (E) Peripheral blood CAR-Tm and CAR-T persistence. Green dots represent CAR-T patients with dominance of CD4+ cells. (F) Patient characteristics, CD19 CAR T-cells (CAR-Tm) application, details, and outcome. For panels C and D, statistical analysis was performed using unpaired t tests. For all panels, ∗P < .05, ∗∗P < .01, ∗∗∗P < .005.
Manufacturing of CD19 CAR T-cell products based on CD4/CD8-selected (CAR-T) or CD45RA-depleted T cells (CAR-Tm). (A) Overall CAR-T manufacturing and lymphodepletion plan. (B) Schema of simultaneous donor-derived CAR-T plus haplo HSCT. (C) Transduction efficacy and numbers of T-cell products after the CAR-T or CAR-Tm (CD45RA-depleted) manufacturing. (D) Analysis of frequency of CD4+ and CD8+ T cells in CAR-Tm and CAR-T products. Dots represent individual patients in panels A, B, and C. Green dots represent CAR-T patients with dominance of CD4+ cells. (E) Peripheral blood CAR-Tm and CAR-T persistence. Green dots represent CAR-T patients with dominance of CD4+ cells. (F) Patient characteristics, CD19 CAR T-cells (CAR-Tm) application, details, and outcome. For panels C and D, statistical analysis was performed using unpaired t tests. For all panels, ∗P < .05, ∗∗P < .01, ∗∗∗P < .005.
Unstimulated leukapheresis was used to obtain the starting population for CAR-T manufacturing (Figure 1A-B). CliniMACS-based CD45RA depletion procedure was used to produce memory T-cell fraction. CD45RA− population was used to initiate a CliniMACS Prodigy bioreactor-based CD19 CAR-T manufacturing according to the previously reported protocol.15,16 Briefly, 1 × 108 T cells were CD4/CD8 enriched prior to CD3/CD28 stimulation, lentiviral transduction, and expansion in the presence of interleukin-7 (IL-7) and IL-15 over 7 days. Lentiviral vector encoding the second-generation CD19-4-1BBz CAR was used. CD19 CAR T cells manufactured according to identical protocol from allogeneic donors (n = 16) without prior CD45RA depletion were used for the comparison of product characteristics. At the end of manufacturing, the median yield of T cells was 1.3 × 109 (0.48-3.2) (Figure 1C). Median transduction efficiency was 67% (56% to 75%) for CAR19-Tm and 40% (20% to 75%) for CAR19-T, P < .01 (Figure 1C). The predominant phenotype of CAR19-Tm was effector memory (CD45RA−CD197−), compared with the predominant central memory (CD45RA−CD197+) phenotype of conventional CAR-T, P < .005 (supplemental Figure 2A-B). CAR19-Tm were also significantly enriched with CD4+ population (median, 82% vs 50% in the conventional CAR-T product, P = .019) (Figure 1D; supplemental Figure 7A).
CAR19-T product was harvested, formulated, and infused after fulfilling the release criteria. The dose of CAR19-Tm was 5 × 105/kg when infused after stable engraftment (n = 3) and 1 × 105/kg when infused simultaneously with the HSC graft (n = 2). Tocilizumab was used prophylactically at 8 mg/kg, adopted from the concurrent clinical trial, and overall clinical and laboratory monitoring was performed as described.16,17 After CAR19-Tm infusion, 2 patients developed grade 1 and 2 cytokine release syndrome and grade 1 Immune effector cell-associated neurotoxicity syndrome (Figure 1F). In one case, grade 1 GVHD (skin plus upper gastrointestinal) developed. Among the 2 cases of concurrent CAR19-Tm and HSC graft infusion, engraftment of neutrophils and platelets was registered as expected, and graft function was not compromised. In one case, testicular relapse was registered at day 384, and in one case, CD19+ bone marrow relapse developed at day 339 after allogeneic CAR19-Tm application and 180 days after consolidative HSCT from a new unrelated donor. Two patients, who received coinfusion of CAR19-Tm with the HSCT, remain in continuous complete remission for 538 and 439 days, respectively (Figure 1F; supplemental Table 2).
CAR19-Tm cells were detected after infusion by flow cytometry in 3 cases, and CAR19-Tm persistence in the peripheral blood was documented for a maximum of 60 days (Figure 1E; supplemental Table 1), compared with a persistence of 6 months in 90% of the recipients of conventional CAR T cells.16 Leukemia response occurred in 4 cases as flow-based minimal residual disease was not detectable at day 30 in 4 cases. According to Figure 1E and supplemental Table 1, the persistence of CAR19-Tm cells was significantly shorter in comparison with conventional CAR19 T cells. Only in the case of patient #5 at day 14 postinfusion was marked expansion of CAR19-Tm observed (paradoxically, this patient deceased from rapid leukemia progression) (Figure 1F).
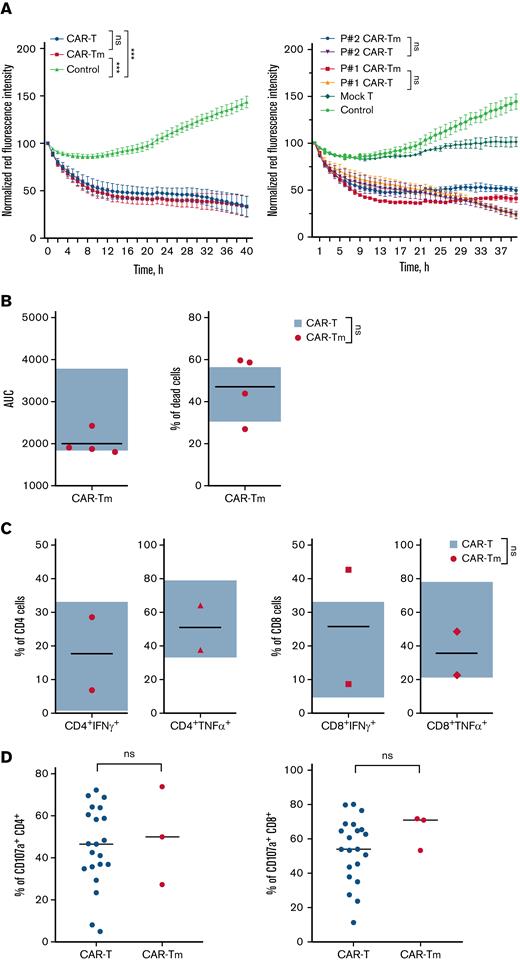
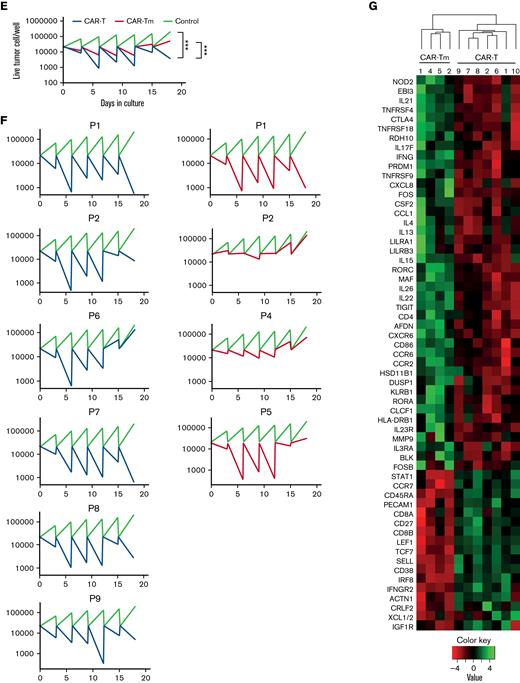
Unexpectedly inefficient expansion and persistence of the CAR19-Tm made us wonder how CAR19-Tm functionally differ from conventional CAR19-T. Firstly, we compared cytotoxicity and capacity of CAR-T and CAR-Tm to degranulate and secrete proinflammatory cytokines (Figure 2A-D). The data suggest that production of cytokines, degranulation, and short-term target cell–killing were mostly comparable for conventional CAR-T and CAR-Tm. CAR-Tm were also compared with CD3+ allogeneic healthy donor-derived CAR T cells in capacity to lyse Jeko-1 red target cells (Figure 2A-B). But this was not the case when cytotoxicity of CAR-Tm and CAR-T were assessed in the prolonged cytotoxicity experiment. The sequential killing assay tests the ability of the CAR T cells to repeatedly eliminate tumor cells in multiple rounds of coincubation. In that stress test, we demonstrated that on average, CAR-Tm loses cytotoxicity much faster than CD45RA+ CAR-T (Figure 2E). The individual sequential killing curves obviously demonstrate that CAR-Tm have shorter functional lifespans and cytotoxicity than CAR-T (Figure 2E-F; supplemental figure 6). The sequential killing assay was performed on CAR-T and CAR-Tm with “adjusted-to-1” CD4:CD8 ratios to demonstrate that reduced cytotoxicity CAR-Tm doesn’t depend on CD4:CD8 ratio (supplemental Figure 7A-B). To clarify the reason for drastic differences in long-term cytolytic activity, we performed a transcriptomic analysis of CAR-Tm and standard CAR-T products using NanoString RNA protocol (Figure 2G; supplemental Figures 4 and 5). Not surprising, CD19 CAR-Tm cells significantly differed from standard CD19 CAR T cells in chemokine’s expression profile and peripheral T-cell differentiation markers (LEF1, TCF7, CD86, CD38, IRF8) (Figure 2G; supplemental Figure 4). Interestingly, TCF7 transcription factor was recently suggested as an important marker of high functionality of the CAR T-cell products.16,18 A profound increase in expression of IL-2 superfamily interleukins (IL-4, IL-15, IL-21) and T-cell exhaustion markers (CTLA-4, TIGIT, KLRB1) was detected in CAR19-Tm cells in contrast to conventional CAR19-T (for summary, see Figure 2G and supplemental Figures 3-5).
Functional comparison of memory-derived CD19 CAR T cells (CAR-Tm) and CD19 CAR-T. (A) CAR-Tm and CAR-T were coincubated with Jeko-1 at a 3:1 ratio and analyzed by Incucyte (the curves represent number of live Jeko-1 cells in time). Average cytotoxicity of CAR-Tm (n = 4) and CAR T cells (n = 6) (left). Individual measurements of CD19 CAR-T and CD19 CAR-Tm cytotoxicity; CAR-T products were manufactured using T cells isolated from patients #1 and #2 (right). (B) Area under curve (AUC) and cytotoxity from panel A. CD19 CAR-Tm compared with CD19 CAR-T (n = 4 and n = 6, respectively; each dot is the mean value of individual donor). (C) Secretion of interferonγ and tumor necrosis factor-α by CD4+ and CD8+ fractions of CD19 CAT-Tm (n = 2) in comparison with respective subsets of CD19 CAR-T (n = 10). (D) Degranulation of CD19 CAR-Tm (n = 3) and CD19 CAR T (n = 21) cells in CD4+ and CD8+ populations after coincubation with Jeko-1. (E) Sequential killing of Jeko-1 by CD19− CAR T cells (1:5 ratio). Lines represent the mean values for CAR-Tm (n = 4) and CAR-T (n = 6). Control indicates target-cell proliferation in the absence of CAR-T. The number of target cells was analyzed every 3 days. The remaining CAR T cells were mixed with a fresh portion of target cells at the same ratio and incubated for another 3 days. The procedure was repeated several times until day 18. Plots show the number of survived target cells. (F) Sequential killing assay of CD19 CAR T-cells of individual patients from panel E. (G) Transcriptomic analysis of CD19 CAR-Tm (n = 4) and CD19 CAR T (n = 6) cell products. Heatmap represents genes with highest (green) and lowest (red) expression (P < .01, fold change >2). Color key indicates the intensity associated with normalized expression values. Numbers indicate patients. For panels B-D, statistical analysis was performed using paired t tests. For all panels, ∗P < .05, ∗∗P < .01, ∗∗∗P < .005. For panels A and E, statistical analysis was performed using a 2-way analysis of variance with Turkey multiple comparisons test. For all panels, ∗P < .05, ∗∗P < .01, ∗∗∗P < .005.
Functional comparison of memory-derived CD19 CAR T cells (CAR-Tm) and CD19 CAR-T. (A) CAR-Tm and CAR-T were coincubated with Jeko-1 at a 3:1 ratio and analyzed by Incucyte (the curves represent number of live Jeko-1 cells in time). Average cytotoxicity of CAR-Tm (n = 4) and CAR T cells (n = 6) (left). Individual measurements of CD19 CAR-T and CD19 CAR-Tm cytotoxicity; CAR-T products were manufactured using T cells isolated from patients #1 and #2 (right). (B) Area under curve (AUC) and cytotoxity from panel A. CD19 CAR-Tm compared with CD19 CAR-T (n = 4 and n = 6, respectively; each dot is the mean value of individual donor). (C) Secretion of interferonγ and tumor necrosis factor-α by CD4+ and CD8+ fractions of CD19 CAT-Tm (n = 2) in comparison with respective subsets of CD19 CAR-T (n = 10). (D) Degranulation of CD19 CAR-Tm (n = 3) and CD19 CAR T (n = 21) cells in CD4+ and CD8+ populations after coincubation with Jeko-1. (E) Sequential killing of Jeko-1 by CD19− CAR T cells (1:5 ratio). Lines represent the mean values for CAR-Tm (n = 4) and CAR-T (n = 6). Control indicates target-cell proliferation in the absence of CAR-T. The number of target cells was analyzed every 3 days. The remaining CAR T cells were mixed with a fresh portion of target cells at the same ratio and incubated for another 3 days. The procedure was repeated several times until day 18. Plots show the number of survived target cells. (F) Sequential killing assay of CD19 CAR T-cells of individual patients from panel E. (G) Transcriptomic analysis of CD19 CAR-Tm (n = 4) and CD19 CAR T (n = 6) cell products. Heatmap represents genes with highest (green) and lowest (red) expression (P < .01, fold change >2). Color key indicates the intensity associated with normalized expression values. Numbers indicate patients. For panels B-D, statistical analysis was performed using paired t tests. For all panels, ∗P < .05, ∗∗P < .01, ∗∗∗P < .005. For panels A and E, statistical analysis was performed using a 2-way analysis of variance with Turkey multiple comparisons test. For all panels, ∗P < .05, ∗∗P < .01, ∗∗∗P < .005.
The use of allogeneic donor-derived CAR T cells is increasingly reported as a candidate approach to the treatment of B-ALL relapse after HSCT.19-22 GVHD is one of the potential serious adverse effects in the setting of HLA-mismatched HSCT.5 Despite the dominant central memory phenotype of the CAR-T produced with currently used manufacturing platform, the T-cell receptor repertoire of the final T-cell population could still be diverse enough to initiate GVHD reactions, especially in the setting of HLA-mismatched HSCT. Therefore, the dose of transfused CAR T cells should be relatively low to avoid the development of GVHD. Based on the approach proposed by Chang et al, we have demonstrated that allogeneic CD45RA-depleted T cells could be used to manufacture CAR19-Tm cells in a good manufacturing practice-compliant settings as recently suggested.7,8 We observed high transduction efficiency with a lower ex vivo expansion of CAR19-Tm and a phenotype with a predominance of effector memory T-cell fraction, contrary to the conventional approach, which generates a product with mostly central memory phenotype. We reason that ex vivo proliferation of memory T cells could skew the final population phenotype toward short-lived effector memory, whereas CD45RA depletion leads also to depletion of stem cell memory T cells, a population with high proliferative potential that might contribute significantly to high functionality of conventionally manufactured CAR-T.23 In vivo application of the CD19 CAR-Tm product was safe as it neither induced CAR-T–specific toxicity exceeding mild cytokine release syndrome/Immune effector cell-associated neurotoxicity syndrome nor, importantly, did it result in significant GVHD or graft dysfunction.
Our data suggest that, indeed, allogeneic memory CAR19-T can be manufactured on a clinical scale and further tested in the prospective clinical study. Correlative research and in vivo data on CAR19-Tm persistence demonstrate that the phenotype and functional capacity of the therapeutic cell product diverges with properties of the conventional CAR19-T product. CD45RA depletion prior to CAR19-Tm manufacturing resulted in a significant change of the CD4/CD8 ratio toward an increase in CD4 fraction and a predominantly effector memory phenotype. Functional activity of CAR19-Tm in vitro was comparable to conventional CAR19-T in short-term killing assays, cytokine production, and degranulation tests. However, in long-term experiments, CAR19-Tm showed faster decline in cytotoxicity that correlates with an increase in expression of T-cell exhaustion markers as well as elevated level of transcripts characteristic for T-cell chronic activation and exhaustion. Limited persistence in vivo of memory T-cell–derived CAR-T product has clear implications on the potential clinical development of the like products because for the B-lineage–associated targets, long-term persistence of the CAR T-cells was clearly shown to be associated with long-term control of the tumor. Thus, dose escalation and repeated application should be further assessed as a way to achieve relevant clinical goals of allogeneic CAR19-Tm therapy.
Acknowledgments: This work was supported by Science for Kids charitable foundation via the Cellular Immunotherapy in Pediatric Oncology program and the Russian Scientific Foundation project No. 17-74-30019.
Contribution: M.M., L.S., and D.P. designed the clinical application and immunomonitoring approach; Y.R. and A.S. designed the in vitro study; V.U., O.M., L.S., D.P., E.K., D.V., A.R., V.B., and D.O. performed and analyzed the experiments; Y.M. and A.K. manufactured CAR T cells; O.M., L.S., D.P., A.R., and M.M. contributed to patient clinical care and data collection; V.U., Y.R., A.S., and M.M. wrote the paper; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: M.M. received a speaker’s fee from Miltenyi Biotec. The remaining authors declare no competing financial interests.
Correspondence: Michael Maschan, Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Samory Mashela 1, Moscow 117997, Russia; e-mail: mmaschan@yandex.ru; and Alexey Stepanov, The Scripps Research Institute, 10550 N Torrey Pines, La Jolla, CA 92037; e-mail: stepanov.aleksei.v@gmail.com.
References
Author notes
For original data, please contact stepanov@scripps.edu.
The full-text version of this article contains a data supplement.