Key Points
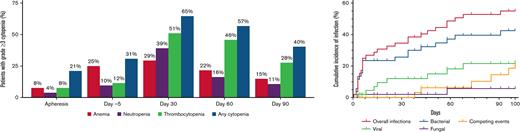
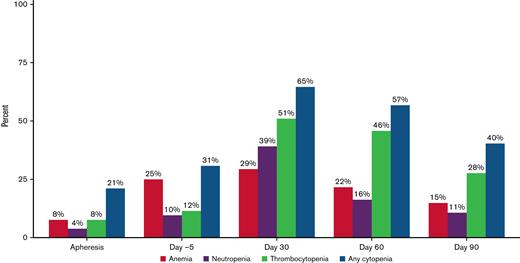
Grade ≥ 3 cytopenias were at their worst at day 7 after ide-cel (94% of patients) but persisted in 40% at day 90, particularly thrombocytopenia.
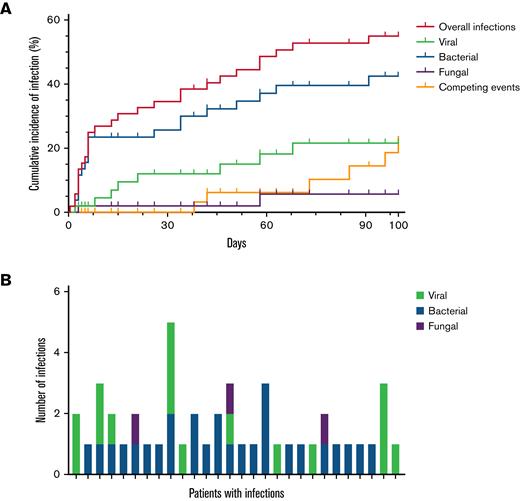
Infections within 30 days of ide-cel were typically bacterial (68%) and severe (50%); moderate viral infections were more prevalent later.
Abstract
Idecabtagene vicleucel (ide-cel) was FDA-approved in March 2021 for the treatment of relapsed/refractory multiple myeloma after 4 lines of therapy. On the KarMMa trial, grade ≥ 3 cytopenias and infections were common. We sought to characterize cytopenias and infections within 100 days after ide-cel in the standard-of-care (SOC) setting. This multi-center retrospective study included 52 patients who received SOC ide-cel; 47 reached day-90 follow-up. Data were censored at day 100. Grade ≥ 3 cytopenia was present among 65% of patients at day 30 and 40% of patients at day 90. Granulocyte colony stimulating factor (G-CSF) was administered to 88%, packed red blood cell transfusions to 63%, platelet transfusions to 42%, thrombopoietin (TPO) agonists to 21%, intravenous immunoglobulin to 13%, and CD34+ stem cell boosts to 8%. At day 100, 19% and 13% of patients had ongoing use of TPO agonists and G-CSF, respectively. Infections occurred in 54% of patients and were grade ≥ 3 in 23%. Earlier infections in the first 30 days were typically bacterial (68%) and severe (50%). Later infections between days 31 and 100 were 50% bacterial and 42% viral; only 13% were grade ≥ 3. On univariate analysis, high pre-CAR-T marrow myeloma burden (≥ 50%), circulating plasma cells at pre-lymphodepletion (LD), and grade ≥ 3 anemia at pre-LD were associated with grade ≥ 3 cytopenia at both days 30 and 90. Longer time from last bridging treatment to LD was the only significant risk factor for infection.
Introduction
Idecabtagene vicleucel (ide-cel) is an autologous anti–B-cell maturation antigen CAR T-cell for adults with relapsed or refractory multiple myeloma (RRMM) that was approved by the US Food and Drug Administration (FDA) in March 2021.1,2 This marked the availability of a promising new therapy for patients with multipally refractory disease,3 leading to an overall response rate of 73% with complete response (CR) or better in 33%, median progression free survival of 8.8 months, and median overall survival of 19.4 months.4 As with FDA-approved autologous anti-CD19 CAR T-cell therapies, ide-cel has been shown to cause cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), in 84% and 18% of patients, respectively.4-9
Cytopenias were common on the pivotal phase 2 KarMMa trial (NCT03361748), including grade 3 or higher neutropenia (89%), anemia (60%), and thrombocytopenia (52%), attributed to fludarabine and cyclophosphamide (Flu/Cy) LD given prior to ide-cel infusion.4,10 Grade 3 or higher cytopenias occurred most commonly within the first 8 weeks following infusion. In patients with persistent grade ≥ 3 neutropenia or thrombocytopenia at 1 month post ide-cel, median time to recovery to grade 2 or lower was 1.9 and 2.1 months, respectively. For prolonged cytopenias, 88% received granulocyte colony stimulating factor (G-CSF), 6% received erythrocyte stimulating agents (ESAs), and 3% received thrombopoietin (TPO) agonists.4 Two patients underwent stem cell boost for hematopoietic reconstitution, and 1 of the 2 recovered.2
Hypogammaglobulinemia and infections occurred more commonly within 8 weeks to 6 months after ide-cel infusion, later than the other toxicities on the KarMMa trial. Sixty-two percent of trial patients received intravenous immunoglobulin (IVIG) due to hypogammaglobulinemia. Infections occurred in 69%, were grade 3 or higher in 22%, and resulted in death in 2% of the patients. Ninety-five percent of patients received antibiotics and antivirals; 62% received antimycotics.4
This multicenter retrospective study aimed to characterize early cellular and humoral reconstitution, infections, and need for supportive therapies due to treatment-related cytopenias in patients with RRMM who received standard-of-care (SOC) ide-cel in the commercial setting. We examined cytopenias, lymphocyte reconstitution, infections, and antibody titers up to 100 days after ide-cel infusion to report on early immune effects after treatment in the “real world.” We aimed to better understand the tolerability and benefit of this therapy in patients who may have been ineligible for the trial due to comorbidities or preexisting cytopenias.
Methods
Patients and data collection
We performed a retrospective analysis of adults with RRMM who underwent treatment with ide-cel as SOC treatment between 1 May 2021 and 30 December 2021. This was a multi-center study from Moffitt Cancer Center, Stanford University, and Medical University of South Carolina. Each center obtained independent institutional review board approval. The study was conducted according to the Declaration of Helsinki. Data extracted from patient records included patient demographics, baseline disease status, prior treatments, complete blood counts, myeloma labs, infection data, pathology reports, and any pertinent medication administration. Data were censored at time of death, 7 days after progressive disease, or at day 100. Patients underwent apheresis based on institutional SOC procedures for ide-cel between 1 April 2021 and 30 October 2021. Flu/Cy conditioning followed by ide-cel infusion were performed as in KarMMa.4,10 Antibiotic prophylaxis was adapted from autologous transplant procedures, as previously described,11 and was consistent among the 3 institutions (supplemental Table 1). The Revised International Staging System12 was used to stage patients at time of CAR T-cell infusion. Adverse events were graded based on the Common Terminology Criteria for Adverse Events (CTCAE) v5.0.13 CRS and ICANS were scored based on the American Society for Transplantation and Cellular Therapy Consensus Grading.5 Responses at day 30 and day 90 were assessed based on International Myeloma Working Group criteria.14
Definition of neutrophil recovery
Neutropenia was defined as per CTCAE v5.0, with grade 1 signifying absolute neutrophil count (ANC) less than the lower limit of normal, or <1,800 cells/μL based on the clinical laboratory. Neutropenia was designated at grade ≥ 3 if ANC was <1000 cells/μL, as per CTCAE. Neutrophil recovery was defined as the first day of ANC > 500 cells/μL sustained on 2 consecutive days without G-CSF use in the past 7 days.
Definition of infection
An infection was defined as any instance of viral, bacterial, or fungal disease identified by microbiological data, radiographic findings, or clinical symptoms per retrospective chart review, censored at day 100. Severe infection was defined as an infection requiring intravenous (IV) antibiotics and/or hospitalization, which by CTCAE criteria would be grade 3 or higher. Infections diagnosed outside of the institution were classified based on patient report and available medical records. Onset of fever following CAR T-cell infusion without microscopic, radiographic, or symptomatic evidence of infection was attributed to CRS and not counted as infection. Fungal infections were classified based on European Organization for Research and Treatment of Cancer (EORTC) definitions.15
Definitions of viral and pneumococcal immunities
All definitions of immunity were based on the clinical laboratory definitions. For herpes simplex virus (HSV) 1/2 immunoglobulin G (IgG), an antibody titer ≤ 0.89 was considered negative and titer ≥ 1.10 was considered positive (immune); titer ≥ 0.90 and ≤ 1.09 was indeterminate. For varicella zoster virus (VZV) IgG antibodies, results were reported as positive (immune), negative, or equivocal. Cytomegalovirus (CMV) IgG antibodies were reported as positive (immune) or negative. Pneumococcal testing was comprised of 14 separate IgG antibody titers, and an antibody concentration > 1.0 μg/mL was considered long-term protection (immunity).
Statistical analysis
Distribution of patient characteristics and outcomes was examined by grade ≥ 3 cytopenia at day 30 and day 90 using Kruskal–Wallis rank sum and χ-square or Fisher’s exact tests. Time-series plots were used to visualize cellular and humoral immune reconstitution at apheresis, day −5, day 0, day 7, day 14, day 21, day 30, day 60, and day 90. Box-and-whisker plots were used to illustrate inflammatory markers at apheresis, day −5, day 0, day 7, day 14, day 21, and day 30. Kruskal–Wallis rank sum tests were used to assess the differences in cellular and humoral reconstitution and inflammatory markers over time. Time-to-event analysis was used to model the cumulative incidence of first infection within 100 days from ide-cel infusion overall and by type of infection (viral, bacterial, or fungal). Progression of disease and death were considered competing events. Evolution of viral titers and pneumococcal titers at apheresis and day 90 were illustrated using alluvial plots16,17 and a heatmap,18 respectively. Statistical tests were 2-sided and a P < .05 was considered statistically significant. All statistical analyses were conducted using R v4.1.2. and GraphPad Prism v8.0.2.
Although underpowered, we performed an exploratory multivariable logistic regression analysis of risk factors associated with grade ≥ 3 cytopenia at day 30 and day 90. The a priori risk factors assessed included high marrow burden (≥50%; yes, no), grade ≥ 3 cytopenia at day −5 (yes, no), CRS grade (no CRS or grade < 2 CRS, grade ≥ 2 CRS), steroid use (yes, no), and number of prior lines of therapy (continuous, per 1 line of therapy). A forest plot was used to depict the odds ratios (ORs) and 95% confidence intervals (CIs) from the multivariable analysis.
Results
Patient and manufacturing characteristics
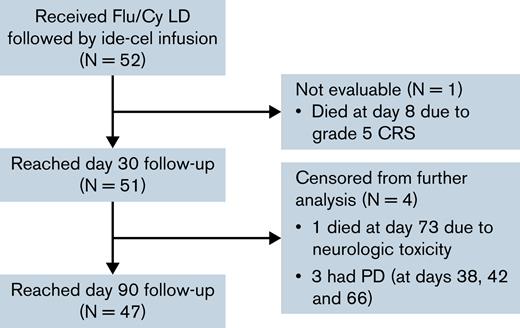
A total of 52 patients received Flu/Cy LD followed by infusion of ide-cel in the SOC setting (Figure 1). Patient baseline characteristics are described in Table 1. All patients received at least 4 prior lines of therapy, and most (87%) were refractory to an immunomodulatory agent, proteasome inhibitor (PI), and daratumumab. Eighty-one percent had previously received autologous stem cell transplant; none had received prior allogeneic stem cell transplant. Only 25% of the patients would have met eligibility criteria for the pivotal phase 2 KarMMa trial at time of apheresis. Reasons for trial ineligibility included cytopenias (29%), comorbidities (42%), myeloma disease features (13%), and prior treatments (25%), and are delineated in supplemental Table 2.
CONSORT diagram description of the patient cohort. Patients were censored at time of death or 7 days after progressive disease (PD). CRS, cytokine release syndrome; Flu/Cy, fludarabine and cyclophosphamide; ide-cel, idecabtagene vicleucel; LD, lymphodepletion chemotherapy.
CONSORT diagram description of the patient cohort. Patients were censored at time of death or 7 days after progressive disease (PD). CRS, cytokine release syndrome; Flu/Cy, fludarabine and cyclophosphamide; ide-cel, idecabtagene vicleucel; LD, lymphodepletion chemotherapy.
Apheresis and product characteristics are detailed in supplemental Table 3. Fifty-two percent of the patients collected via a non-tunneled internal jugular central venous catheter, while 42% collected successfully via peripheral IV line. The median time required for collection was 227 minutes. The median time from apheresis to ide-cel infusion was 46 days (range 36–75 days). The infused product contained a median of 415.8 million CAR T-cells (range 306.8–456.4), meeting the target dose range from KarMMa.2,4
Safety and response to therapy
Eighty-five percent of the patients experienced any grade CRS, and 85% received tocilizumab (Table 2). Sixty-seven percent of all patients experienced grade 1 CRS; median time to onset and maximum severity of CRS for all patients was 1 day. Trends in lactate dehydrogenase, ferritin, and C-reactive protein are shown in supplemental Figure 1. Though grade ≥ 3 CRS only occurred in 3 patients (6%), 1 patient died of grade 5 CRS on day 8 after ide-cel infusion. Nineteen percent of the patients experienced any grade ICANS, and in 8% of all patients ICANS was grade ≥ 3. In total, 35% of patients received steroids and 1 (2%) received anakinra for management of CAR T-cell–related toxicities. No patient has died of ICANS, but 1 patient died of late-onset progressive ascending weakness without evidence of myeloma at day 73.
Ninety-two percent of the patients achieved a partial response or better as their best response in the first 90 days, including 48% with CR or stringent CR (sCR) and 81% of these negative for minimal residual disease by clonoSEQ® testing to the level of 10−6 nucleated cells, based on International Myeloma Working Group criteria.
Cytopenias
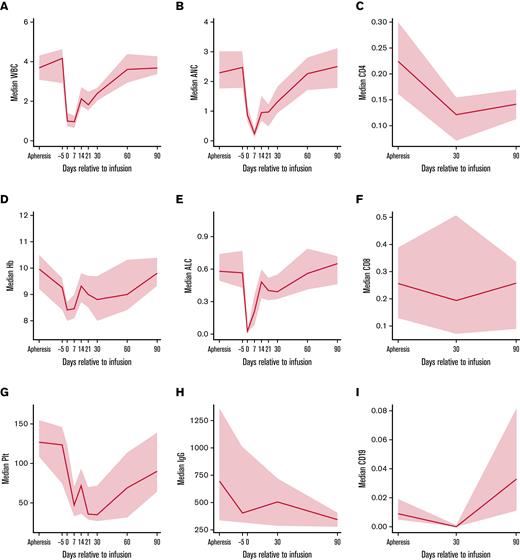
Cellular and humoral immune reconstitution after ide-cel is demonstrated in Figure 2. Though cytopenias were common at baseline with 92% of the patients having any grade cytopenia at both apheresis and day −5, grade ≥ 3 cytopenias were more commonly seen after LD, with 94% of patients experiencing any grade ≥ 3 cytopenia at day 7 (Table 3). Hematologic parameters generally improved with time, but any grade ≥ 3 cytopenia was still present among 65% of the patients at day 30 and 40% of the patients at day 90. Prevalence of grade ≥ 3 cytopenias and recovery over time is shown graphically in Figure 3. At day 30, 51% of patients had grade ≥ 3 thrombocytopenia and 39% had grade ≥ 3 neutropenia. At day 90, 28% of patients still had grade ≥ 3 thrombocytopenia and 11% grade ≥ 3 neutropenia.
Cellular and humoral immune reconstitution after idecabtagene vicleucel (ide-cel). Time-series plots for cellular and humoral immune reconstitution. Solid blue lines reflect median values and gray areas reflect 95% confidence intervals. (A) Median white blood cell (WBC) trend (k/μL) from apheresis to day 90. (B) Median absolute neutrophil count (ANC) trend (k/μL) from apheresis to day 90. (C) Median CD4 T-cell count (k/μL) from apheresis to day 90, based on flow cytometry. (D) Median hemoglobin (Hb) trend (g/dL) from apheresis to day 90. (E) Median absolute lymphocyte count (ALC) trend (k/μL) from apheresis to day 90, based on complete blood count. (F) Median CD8 T-cell count (k/μL) from apheresis to day 90, based on flow cytometry. (G) Median platelet (Plt) trend (k/μL) from apheresis to day 90. (H) Median immunoglobulin G (IgG) trend (mg/dL) from apheresis to day 90. (I) Median CD19 B-cell count (k/μL) from apheresis to day 90, based on flow cytometry.
Cellular and humoral immune reconstitution after idecabtagene vicleucel (ide-cel). Time-series plots for cellular and humoral immune reconstitution. Solid blue lines reflect median values and gray areas reflect 95% confidence intervals. (A) Median white blood cell (WBC) trend (k/μL) from apheresis to day 90. (B) Median absolute neutrophil count (ANC) trend (k/μL) from apheresis to day 90. (C) Median CD4 T-cell count (k/μL) from apheresis to day 90, based on flow cytometry. (D) Median hemoglobin (Hb) trend (g/dL) from apheresis to day 90. (E) Median absolute lymphocyte count (ALC) trend (k/μL) from apheresis to day 90, based on complete blood count. (F) Median CD8 T-cell count (k/μL) from apheresis to day 90, based on flow cytometry. (G) Median platelet (Plt) trend (k/μL) from apheresis to day 90. (H) Median immunoglobulin G (IgG) trend (mg/dL) from apheresis to day 90. (I) Median CD19 B-cell count (k/μL) from apheresis to day 90, based on flow cytometry.
Prevalence of grade ≥ 3 cytopenias before and after idecabtagene vicleucel (ide-cel) CAR-T therapy. Prevalence of grade ≥ 3 cytopenias at apheresis, day −5, day 30, day 60, and day 90. Grade 3 cytopenias: Anemia with hemoglobin < 8 g/dL; Neutropenia with absolute neutrophil count < 1000/μL; Thrombocytopenia with platelet trend < 50 000/μL.
Prevalence of grade ≥ 3 cytopenias before and after idecabtagene vicleucel (ide-cel) CAR-T therapy. Prevalence of grade ≥ 3 cytopenias at apheresis, day −5, day 30, day 60, and day 90. Grade 3 cytopenias: Anemia with hemoglobin < 8 g/dL; Neutropenia with absolute neutrophil count < 1000/μL; Thrombocytopenia with platelet trend < 50 000/μL.
Bone marrow biopsies were done at baseline, day 30 and day 90, with findings shown in Table 4. Baseline marrows were collected at median of 19 days prior to ide-cel infusion (range 4–64 days) and were typically normo- to hypercellular with median of 11.2% CD138+ plasma cells (range 0% to 100%). At day 30 and day 90, comparatively, hypocellular marrows were most common, in 40% and 49% of patients, respectively. The median CD138+ plasma cells in the bone marrow declined to 0% (range 0% to 80%) at both time points. Marrow fibrosis and dysplasia were uncommon.
We conducted univariate analyses to examine risk factors for grade ≥ 3 cytopenias. Any grade ≥ 3 cytopenia at day 30 after ide-cel was associated with female sex, high baseline marrow plasma cell burden (≥50% CD138+ plasma cells on pre–ide-cel bone marrow core biopsy), presence of circulating plasma cells at pre-LD, prior B-cell maturation antigen (BCMA)-directed therapy, more time from last bridging treatment to LD (median 29 days versus [vs] 15 days), any grade anemia at pre-LD, and lower day-30 bone marrow biopsy cellularity (median 14% vs 37.5%; supplemental Table 4). In comparison, any grade ≥ 3 cytopenia at day 90 was associated with extramedullary disease, high baseline marrow tumor burden, circulating plasma cells at pre-LD, any reason for KarMMa trial ineligibility at apheresis, and any grade ≥ 3 cytopenia at pre-LD (supplemental Table 5). On univariate analysis, treatment-related outcomes after ide-cel that were predictive of any grade ≥ 3 cytopenia at day 30 included occurrence of any grade CRS, longer duration of CRS (median 3 days vs 1.5 days), steroid use, and tocilizumab use (supplemental Table 6).
The multivariable analysis showed no association of the selected risk factors and grade ≥ 3 cytopenia at day 30. At day 90, patients with a high baseline marrow plasma burden (≥50%) had a higher risk of grade ≥ 3 cytopenia (OR = 6.40, 95% CI = 1.40, 33.60; supplemental Table 7; supplemental Figure 2). There was a positive but not statistically significant association for grade ≥ 3 cytopenia at day −5 and prolonged grade ≥ 3 cytopenia at day 90 (OR = 5.11, 95% CI = 0.91, 34.90).
Supportive therapies
Therapies including transfusions, G-CSF, TPO agonists, and IVIG were given at the discretion of the treating physician to support patients through their cytopenias and hypogammaglobulinemia (Table 5). In total, 88% of patients received G-CSF, starting at a median of 9 days (range 1–93 days) and continued through median of 29 days (range 6–100 days) postinfusion. At day 100 when data were censored, 6/47 patients (13%) had ongoing G-CSF use. Due to persistent neutropenia despite G-CSF, 4 patients (8%) received CD34+ hematopoietic stem cell boosts, administered at a median of 54 days with a median cell dose of 3.12 million cells/kg. For the 41 patients in whom ANC fell below 500 cells/μL after ide-cel and recovered prior to day 100 in the absence of death or progression, median time to sustained neutrophil recovery without G-CSF support was 30 days (range 7–94 days). Seventy-five percent of patients (3/4) who received stem cell boosts recovered counts prior to day 100, with median time to neutrophil recovery 10 days after CD34+ stem cell infusion (range 10–14 days).
In total, 65% of the patients required transfusion within the first 100 days after ide-cel: 52% were transfused packed red blood cells (pRBCs) and 29% received platelets within 7 days of CAR T-cell infusion; 46% pRBCs and 42% platelets beyond 7 days. No patient received ESAs. Twenty-one percent of patients (11/52) received TPO agonist support, starting at a median of 35 days (range 23–63 days). Of these, 82% (9/11), or 19% of all patients (9/47), remained thrombocytopenic and continued on TPO agonists at day 100. Thirteen percent of patients (7/52) received IVIG, starting at a median of 39 days (range 25–99 days) and ongoing for 11% of all patients (5/47) at day-100 postinfusion.
Infections and immunity
Within 100 days after ide-cel infusion, 46 documented infections occurred in 28/52 patients (54%). Fourteen infections were severe and occurred in 12/52 patients (23%). No patient died of infection in the first 100 days after ide-cel. Cumulative incidence of first infection and infection prevalence by type (viral, bacterial, or fungal) per patient are shown in Figure 4. Details of infections can be found in supplemental Table 8. Earlier infections in the first 30 days after ide-cel were typically bacterial (68%), and 50% were considered severe. Of these earlier bacterial infections, unspecified bacterial pneumonia, Clostridioides difficile colitis, and skin or soft tissue infections were most common. Later infections between days 31 and 100 were 50% bacterial, most commonly sinusitis, and 42% viral, most commonly coronavirus disease 2019 (COVID-19, SARS-CoV2). Infections were overall evenly distributed between the 2 time periods with 22 infections in the first 30 days and 24 infections between days 31 and 100. Only 13% of the later infections were severe. Univariate analyses examining risk of any infection by treatment-related factors identified that longer time from last bridging treatment to LD (median 29 days vs 15 days) had the only statistically significant association (supplemental Table 9A). We were unable to identify any statistically significant association between treatment-related factors and severe infection (supplemental Table 9B). Importantly, there was no observed difference in risk of infection or severe infection with use of tocilizumab, steroids, or anakinra. There were no significant associations on univariate analysis between infection and cell counts or IVIGs (supplemental Table 10).
Cumulative incidence of infection and infection density. Cumulative incidence of infection and infection prevalence within 100 days following idecabtagene vicleucel (ide-cel) infusion. (A) Cumulative incidence of first infection by type of infection (viral, bacterial, and fungal) among the total cohort (N = 52) over 100 days post ide-cel infusion. Patients were censored at the time of last follow-up (maximum of 100 days). Competing events were defined as disease relapse or progression and death. Two patients had concurrent bacterial and fungal infections. (B) Number of infections among the 28 patients with any infection annotated by type of infection (viral, bacterial, and fungal).
Cumulative incidence of infection and infection density. Cumulative incidence of infection and infection prevalence within 100 days following idecabtagene vicleucel (ide-cel) infusion. (A) Cumulative incidence of first infection by type of infection (viral, bacterial, and fungal) among the total cohort (N = 52) over 100 days post ide-cel infusion. Patients were censored at the time of last follow-up (maximum of 100 days). Competing events were defined as disease relapse or progression and death. Two patients had concurrent bacterial and fungal infections. (B) Number of infections among the 28 patients with any infection annotated by type of infection (viral, bacterial, and fungal).
Finally, we looked at the evolution of viral (supplemental Figure 3) and pneumococcal (supplemental Figure 4) immunity after ide-cel by comparing changes in antibody titers between apheresis and day 90. In total, 27 patients had paired HSV 1/2 IgG antibodies. Of these, 14/27 (52%) maintained HSV-immune status, 5/27 (19%) lost HSV immunity after ide-cel, and 2/27 (7%) gained HSV immunity; the remaining 6 patients were never immune to HSV (supplemental Figure 3A). The same 27 patients had paired VZV IgG antibody results, but 8 of these patients were excluded due to equivocal results at apheresis and/or day 90. Of these, 1/19 (5%) maintained VZV-immune status, 3/19 (16%) lost VZV immunity, and 2/19 (11%) gained VZV immunity; 13 patients were never immune to VZV (supplemental Figure 3B). The 27 patients also had paired CMV IgG antibodies. Of these, 15/27 (56%) maintained CMV-immune status and 2/27 (7%) lost CMV immunity; no patient gained CMV immunity and 10 were never immune to CMV (supplemental Figure 3C). For these 27 patients who had paired viral antibody titers, median CD4 count at apheresis was 221 cells/μL (range 53–663); at day 90 25/27 had CD4 counts available with median 145 cells/μL (range 49–572). Nine patients in total lost immunity to at least 1 of the 3 viruses, and for these the median CD4 count at day 90 was 90 cells/μL (range 49–572).
Pneumococcal testing was comprised of 14 separate IgG antibody titers, for which 31 patients had paired results. Per the clinical laboratory definition, an antibody concentration > 1.0 μg/mL was considered long-term protection (immunity). No patient maintained universal pneumococcal immunity over time, but 4/31 (13%) maintained partial pneumococcal immunity while acquiring new pneumococcal antibodies (supplemental Figure 4). An additional 3/31 (10%) started without any pneumococcal immunity and acquired new pneumococcal antibodies after ide-cel. In total, 10/31 (32%) lost immunity in one or more pneumococcal antibodies. Of note, 1 patient who had paired viral titers as well as paired pneumococcal titers received IVIG post-CAR T (started at day 84), and 1 different patient who had paired viral titers and paired pneumococcal titers received IVIG within 30 days prior to apheresis. The other 29 patients with paired titers did not receive IVIG post-CAR T or within 30 days of apheresis.
Discussion
We present here a detailed analysis of cytopenias and infections in patients who received SOC ide-cel infusion. Though all patients met the FDA label criteria of 4 prior lines of therapy and exposure to an immunomodulatory agent, PI, and anti-CD38 monoclonal antibody, most patients (75%) in this study would not have met eligibility criteria to receive ide-cel within the KarMMa trial. For these 52 patients, all underwent apheresis/cell manufacturing successfully and proceeded with ide-cel infusion at median of 46 days after apheresis.
Clinical outcomes including incidence of any grade and grade ≥ 3 CRS, time to CRS onset, incidence of any grade ICANS, time to ICANS onset, and minimal residual disease negative responses were similar to those on KarMMa. Notable differences in outcomes between this patient cohort and the KarMMa trial patients included greater use of tocilizumab per institutional protocols and physician discretion (85% vs 52% on trial), greater steroid use (35% vs 15% on trial), shorter duration of CRS (median 3 days with range 0–10 days vs median 5 days with range 1–63 days on trial), shorter median ICANS duration (1.5 days vs 3 days on trial), and slightly higher rate of severe ICANS (6% grade 3 and 2% grade 4 vs 3% grade 3 and no grade 4 on trial), albeit limited by small number of patients experiencing ICANS in the SOC setting. Although responses in our analysis including best overall response rate and best CR/sCR rates by day 90 appear higher than on the KarMMa trial (92% vs 73% and 48% vs 33%, respectively), a larger number of patients, longer follow-up, and multivariable analysis are needed to better quantify duration of response, survival, and predictors of efficacy and toxicity.
As on the KarMMa trial, severe cytopenias were common. At day 7 when any grade ≥ 3 cytopenia was most pervasive (94%), 81% of patients had grade ≥ 3 neutropenia and 52% had grade ≥ 3 thrombocytopenia. Grade ≥ 3 anemia was less common, affecting 35% of patients on day 0 when incidence was highest, compared with 52% incidence on trial. This may at least partially explain why 6% of patients on trial received ESAs while no SOC patients did. As cited previously, these early cytopenias are likely due to Flu/Cy LD chemotherapy,4,10 with the temporal relation and global decrease in bone marrow cellularity supporting this underlying etiology.
Persistent severe cytopenias remain a challenge in the care of patients treated with CAR T-cell therapy and may require a team approach for management between the CAR-T center and local hematologists. At day 30, we observed a high rate of grade ≥ 3 cytopenia (65%), including 51% grade ≥ 3 thrombocytopenia, 39% grade ≥ 3 neutropenia, and 29% grade ≥ 3 anemia. This was similar to that seen in the KarMMa trial. We identified risk factors for grade ≥ 3 cytopenia at day 30 by univariable analysis, including female sex, high baseline marrow tumor burden, presence of circulating plasma cells at pre-LD, prior BCMA-directed therapy, more time from last bridging treatment to LD, any grade anemia at pre-LD, lower day-30 bone marrow biopsy cellularity, any grade CRS, longer duration of CRS, steroid use, and tocilizumab use. Notably, neither use of any bridging therapy nor use of alkylating bridging therapy was associated with grade ≥ 3 cytopenia at day 30. We were unable to identify risk factors for cytopenias at day 30 by multivariable analysis; however, power to detect an association was limited due to our relatively small sample size.
A total of 88% of patients in our analysis as on KarMMa trial received G-CSF due to persistent neutropenia. Though incidence of grade ≥ 3 neutropenia had decreased to 11% by day 90 for the SOC patients, 13% of patients had ongoing G-CSF use at day 100. This highlights the variability of neutrophil recovery and is why the definition of ANC > 500 cells/μL sustained on 2 consecutive days without G-CSF use in the prior 7 days was used. Higher incidence of prolonged grade ≥ 3 cytopenia at day 90 was associated with extramedullary disease, high baseline marrow burden, circulating plasma cells at pre-LD, any reason for KarMMa trial ineligibility at apheresis, and any grade ≥ 3 cytopenia at pre-LD by univariate analysis. Although limited due to small patient numbers, high baseline marrow burden was a significant risk factor for grade ≥ 3 cytopenia at day 90 in multivariable analysis.
Prolonged grade ≥ 3 thrombocytopenia after ide-cel was present in 28% of patients at day 90, and 17% of patients had ongoing TPO agonist use at day 100. Despite this, no patient died of a bleeding complication. Compared with cytopenias after anti-CD19 CAR T-cell therapy,11,19-21 this persistent grade ≥ 3 thrombocytopenia seems to be more common with anti-BCMA CAR T-cell therapy and has been reported after treatment with ciltacabtagene autoleucel (cilta-cel) as well.22,23 This cannot be explained by on-target off-tumor toxicity, as BCMA is preferentially expressed on mature B-cells.24 Immune thrombocytopenia is also an unlikely explanation, because these patients respond to platelet transfusions. Potential etiologies could be inflammation related to CAR T-cell therapy as well as the marrow involvement from myeloma. Of the risk factors we identified on univariate analysis, only high baseline marrow burden, presence of circulating plasma cells at pre-LD, and grade ≥ 3 anemia at pre-LD were associated with grade ≥ 3 cytopenia at both day 30 and day 90, and all of these are notable effects of advanced multiple myeloma.
Kambhampati et al recently published on infectious complications within 1 year after anti-BCMA CAR T-cell therapy.25 Their retrospective single-center 55-patient study exclusively included patients on clinical trials: 42% bb2121 (ide-cel), 13% bb21217 (ide-cel with PI3K inhibitor incubation), 15% JNJ-4528 (cilta-cel), and 31% JCARH125 (orvacabtagene autoleucel, now abandoned). Authors identified 47 infections in 29 patients (53%) with 53% of infections in the first 100 days, 40% bacterial, 53% viral, 92% mild to moderate, and 68% involving the respiratory tract. Similar to our study, authors defined severe infection by the need for IV antibiotics; however, they specified that bacteremia from skin contaminants or fever without systemic symptoms would be categorized as moderate infection. While their study did not find any statistically significant risk factors for infection, our study did identify longer time from last bridging treatment to LD as a risk factor for infection. This difference could be attributed to a large number of our patients (75%) not meeting trial eligibility criteria (supplemental Table 2) and shorter follow-up time post-CAR T (100 days vs 1 year). The risk for infection early after ide-cel may be distinct compared with longer-term infections that may be influenced by other factors beyond CAR T-cell therapy.
Comparing our SOC infection data with the data from Kambhampati et al,25 we had a similar proportion of patients affected by infection (54% vs 53%) but a higher number of infections within 100 days of CAR T-cell infusion (46 vs 25) and more patients with severe infection (23% vs 6%) despite antibiotic prophylaxis. Institutional guidelines were similar across the 3 centers in our study with all utilizing antiviral, antibiotic, antifungal, and Pneumocystis pneumonia prophylaxis (supplemental Table 1). Notably, none of the severe infections within our patients would have been re-classified as moderate based on the definition used by Kambhampati et al.25 Further, we showed that earlier infections in the first 30 days after ide-cel were typically bacterial (68%) and severe in nature (50%), while later infections between days 31 and 100 were more likely to be moderate by definition and were more evenly distributed between bacterial (50%) and viral (42%) causes. Compared with KarMMa, fewer of our patients experienced an infection (54% vs 69% on trial) but a similar proportion had severe infection (23% vs 22% on trial).
One question regarding post-CAR T-cell therapy management that remains largely unanswered in the literature is the need for revaccination, and many institutions have simply adopted post-autologous stem cell transplant revaccination practices. Our comparison of paired IgG antibody titers for HSV 1/2, VZV, CMV, and pneumococcus found evidence that immunity is lost after ide-cel in a proportion of patients, but not universally (in 19%, 16%, 7%, and 32%, respectively). Patients will likely benefit from revaccination, especially for pneumococcus. Future studies and longer follow-up are needed to evaluate antibody titer data for all commonly used vaccines.
Limitations of this study include its small sample size and retrospective design. Longer follow-up and a larger number of SOC patients are needed to fully appreciate the risk factors and longer-term patterns of hematologic and infectious toxicities after ide-cel in the “real world.” Prospective studies will be important to provide insights into more standardized use of supportive care measures, including common strategies for antimicrobial prophylaxis as well as use of G-CSF and stem cell boosts.
In summary, cytopenias and infections are common after SOC ide-cel, but no patient on this study died due to complications of cytopenia or infection by day 100; rather, progression of myeloma remains the most pressing problem.
Acknowledgments
This work was supported in part by the Moffitt Cancer Center National Cancer Center Institute Core Grant (P30-CA076292) and a generous donation from the Hyer family. This work was also supported by the Pentecost Family Myeloma Research Center. F.L.L. is supported by a Scholar in Clinical Research award from The Leukemia and Lymphoma Society. S.S. is supported by Stanford Clinical and Translational Science KL2 Career Development Award program, award #KL2 TR003143.
Authorship
Contribution: J.M.L., L.C.P., H. Hashmi, S.S., and D.K.H. designed the research; J.M.L., H. Hashmi, A.M.S., H. Hosoya, R.M.G., C.C., K.H.K., V.H., B.S., S.P., S.S., and D.K.H. collected data; J.M.L., L.C.P., and C.M.C.-L. analyzed results and made the figures; J.M.L., L.C.P., H. Hashmi, S.S., and D.K.H. wrote the paper; R.M.G., A.L., M.D.J., A.B., O.V.K., N.B., R.G.F., H.E., F.K., M.L.D., A.M., B.J.B., A.F.G.-C., O.A.C.P., H.D.L., T.N., C.L.F., J.B.B., K.H.S., R.C.B., F.L.L., and M.A. contributed vital analytical skills.
Conflict-of-interest disclosure: H. Hashmi serves on the advisory boards for Janssen, Sanofi, and Bristol Myers Squibb (BMS). M.D.J. reports consultancy/advisory roles with Kite/Gilead, BMS, Novartis; and research funding from Incyte and Kite. N.B. served as a consultant or had an advisory role with Medexus Pharmaceuticals, Magenta Therapeutics, CareDx Pharma, Sanofi, and CTI BioPharma. R.G.F. reports research funding from Kite/Gilead and Novartis. H.E. reports research funding from BMS. B.J.B. reports a role with the speakers’ bureau for Sanofi pharmaceuticals. A.F.G.-C. serves on the advisory boards for Janssen and Sanofi; and reports a role with the speakers’ bureau for Sanofi. H.D.L. reports a role with the speakers’ bureau for Sanofi. C.L.F. reports honoraria/consulting roles for BMS, Seattle Genetics, Celgene, Abbvie, Sanofi, Incyte, Amgen, and Janssen; and research funding from Teva, Janssen, and Roche/Genentech. K.H.S. has consultancy, advisor, and/or speaker roles with Adaptive Biotech, Janssen, BMS, Takeda, Sanofi, and Glaxo Smith Kline; research funding with Karyopharm and Abbvie, and funds from BMS, Amgen, and Janssen-funded clinical trials. F.L.L. reports a scientific advisory role for Allogene, Amgen, Bluebird Bio, BMS/Celgene, Calibr, Cellular Biomedicine Group, GammaDelta Therapeutics, Iovance, Kite Pharma, Janssen, Legend Biotech, Novartis, Sana, Takeda, Wugen, and Umoja; research funding from Kite Pharma (Institutional), Allogene (Institutional), Novartis (Institutional), Blue-Bird Bio (Institutional), CERo Therapeutics (Institutional), and BMS (Institutional); patents, royalties, and other intellectual property including several patents held by the institution in his name (unlicensed) in the field of cellular immunotherapy; consulting roles for Cowen, EcoR1, Emerging Therapy Solutions, and Gerson Lehrman Group; and education or editorial activity for Aptitude Health, ASH, BioPharma Communications CARE Education, Clinical Care Options Oncology, Imedex, and Society of Immunotherapy of Cancer. D.K.H. reports OncLive honoraria and research funding from BMS. J.M.L, L.C.P., C.M.C.-L., A.M.S., H. Hosoya, R.M.G., C.C., K.H.K., V.H., B.S., S.P., A.L., A.B., O.V.K., F.K., M.L.D., A.M., O.A.C.P., T.N., J.B.B., R.C.B., M.A., and S.S declare no competing financial interests.
Correspondence: Doris K. Hansen, Department of Blood and Marrow Transplant and Cellular Immunotherapy, H. Lee Moffitt Cancer Center and Research Institute, 12902 USF Magnolia Dr, CSB Room 7165, Tampa, FL 33612; e-mail: doris.hansen@moffitt.org; and Surbhi Sidana, Division of Blood and Marrow Transplantation and Cellular Therapy, Department of Medicine, Stanford University School of Medicine, 300 Pasteur Dr, Room H0101, Stanford, CA 94305; e-mail: surbhi.sidana@stanford.edu.
References
Author notes
∗S.S. and D.K.H. are joint senior authors.
The full-text version of this article contains a data supplement.
For original de-identified data that underlie the reported results, please contact Doris.Hansen@moffitt.org.