Key Points
Patients who underwent core needle biopsy are more likely to have poor-risk disease features and inadequate tissue for molecular analyses.
Increasing tissue requirements of biomarker-driven trials may exclude patients with high-risk DLBCL who need novel agents.
Abstract
An enhanced understanding of the molecular heterogeneity of diffuse large B-cell lymphoma (DLBCL) has opened the door to clinical trials evaluating novel agents with subtype-specific activity. It is an emerging question whether core needle biopsies (CNB) can adequately meet the increasing tissue requirements of these clinical trials. This can potentially lead to selective enrollment of patients who can undergo excisional biopsy (EB). It is also important to know whether patients who can undergo extensive diagnostic work up differ in their disease characteristics and outcomes from those who cannot. In this observational study, we describe the characteristics, outcomes, and adequacy of diagnostic tissue in patients with newly diagnosed DLBCL and primary mediastinal large B-cell lymphoma who underwent EB vs CNB. Of the 1061 patients, 532 (49.8%) underwent EB and 529 (50.1%) underwent CNB. A significantly higher proportion of patients with CNB had advanced stage disease, an international prognostic index of ≥3, and inadequate tissue for molecular analyses. Patients with CNB had significantly worse 5-year event-free survival (67.6% vs 56.9%; hazard ratio [HR], 0.76; confidence interval [CI]95, 0.6-0.9, P < .001) and 5-year overall survival (76.4% vs 69.2%; HR, 0.8; CI95, 0.6-0.9, P < .001). Thus, patients who underwent CNB have poor-risk features and inferior outcomes on frontline chemoimmunotherapy, are more likely to have inadequate tissue for molecular analyses, and might not meet the tissue requirements of biomarker-driven clinical trials. Thus, the increasing tissue requirements of biomarker-driven clinical trials may result in the exclusion of patients with high-risk DLBCL who need novel agents.
Introduction
Advances in molecular profiling have remarkably improved our understanding of diffuse large B-cell lymphoma (DLBCL) biology.1-6 Multiplatform analyses of structural genetic abnormalities and gene expression have identified distinct genetic signatures of DLBCL with differential prognosis with frontline chemoimmunotherapy.2,4,5 Such an enhanced understanding has opened the door to clinical trials evaluating novel therapeutics with activities against specific molecular pathways. Ibrutinib, bortezomib, and lenalidomide have shown activity in relapsed non-GCB DLBCL, but attempts to combine these agents with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP) have not improved the survival of newly diagnosed patients in randomized controlled trials.7-10 Nonetheless, attempts to evaluate biomarker-based targeted novel therapeutics are increasing as our understanding of DLBCL biology expands.
In ECOG1412 trial of RCHOP with lenalidomide in newly diagnosed DLBCL, 53 out of 427 (12%) patients failed expert pathology review due to inadequate tissue for confirmation of diagnosis.11 In ROBUST trial, 8% of patients at screening and 9% of patients after enrollment were found ineligible for the trial due to lack of sufficient tissue.7,11 In REMoDL-B study, about 15% patients were ineligible for randomization due to insufficient tissue.9 Thus, adequacy of diagnostic tissue material is an emerging question for clinical trials evaluating novel therapeutics, and it is likely to be a challenging one in the era of targeted novel therapeutics.
Excisional biopsy (EB) is a standard recommendation for the diagnosis of lymphoma; however, core needle biopsy (CNB) is frequently performed because of the inaccessibility of the tumor, urgency to begin treatment, and instability of the patient. In retrospective studies before 2015, image-guided CNB had obtained tissue diagnosis in 76% to 97% of cases.12-15 Recently, a single-center retrospective review showed that CNB was significantly less likely to yield diagnosis compared with EB; 56.8% of patients were able to be diagnosed with CNB alone in this study because of lack of adequate tissue.16 French lymphopath survey of more than 32 000 patients with lymphoma showed that CNB remained less efficacious in diagnosis.17 In ECOG1412, patients who were ineligible because of inadequate tissue were more likely to have CNB compared with patients who had an ineligible diagnosis (71.6% vs 24.1%, P < .05).11 Thus, the amount of tissue obtained with CNB is limited and might not meet the tissue requirements of biomarker-driven clinical trials can be adequately met by this diagnostic technique.
If the tissue needs of novel clinical trials are not met by CNB, patients who can undergo EB will be selectively enrolled. Therefore, it is important to know whether patients who can undergo extensive EB differ in their clinical characteristics from those who undergo CNB. Here, we describe the characteristics, outcomes, and adequacy of diagnostic tissue in patients with newly diagnosed DLBCL and primary mediastinal large B-cell lymphoma (PMBL) who underwent EB vs CNB in a prospective observational Molecular Epidemiology Resource (MER) cohort of the Mayo Clinic/University of Iowa Lymphoma Specialized Program of Research Excellence.
Methods
Patients and procedures
The study cohort comprised adult patients with newly diagnosed DLBCL and PMBL enrolled in the Mayo portion of the prospective observational MER cohort of Mayo Clinic and University of Iowa.18 Details of this cohort have been described before.18 Briefly, patients with lymphoma who were within 9 months from their initial diagnosis at presentation were enrolled into the MER from 1 September 2002 to 30 June 2015. All participants provided written informed consent, and the cohort protocol was approved by the institutional review boards of the Mayo Clinic and University of Iowa. All participants were treated according to the treating physician’s choice and were systematically contacted every 6 months (±4 weeks) from the date of the initial diagnosis for the first 3 years and annually thereafter for follow-up. Follow-up data included disease recurrence or progression after frontline treatment and vital status. All events were verified through a review of medical records. For this study, we queried the MER database in September 2020 and all consecutive cases of newly diagnosed DLBCL and PMBL were eligible. Demographic (age, sex) and clinical (stage, extranodal involvement, international prognostic index (IPI), and performance status) characteristics were recorded at baseline. Diagnosis to treatment interval (DTI), defined as the time from the date of diagnostic biopsy to commencement of treatment in days, was calculated for study participants. The tissue biopsy method, either EB or CNB, used to obtain the diagnosis was recorded in the electronic health records. For this study, CNB included both fine needle aspiration (FNA) and CNB. Patients who underwent endoscopic, laparoscopic, punch, or open/incisional biopsies were included in the EB group. Patients who required EB for diagnosis after a nondiagnostic CNB/FNA were included in the EB group. All biopsies were reviewed by Mayo Clinic hematopathologists and tissue diagnosis was confirmed. After the initial tissue diagnosis, a Mayo Clinic pathologist (RLK) reviewed the proportion of tissue samples for adequacy of subsequent DNA extraction for whole exome sequencing (WES) and RNA sequencing (RNAseq).
Outcomes
The primary outcome was event-free survival (EFS) according to the tissue biopsy method, defined as the time from diagnosis to progression, relapse, unplanned re-treatment of lymphoma due to lack of efficacy, or death from any cause. The secondary outcome was overall survival (OS) according to the tissue biopsy method. OS was defined as the time from diagnosis to death due to any cause.
Statistical analyses
Categorical variables were described as numbers and proportions. Continuous variables were described using median and range. Chi square test and Fisher exact test were used to assess the differences between categorical variables. Analysis of variance was used to detect the differences between continuous variables. The Kaplan-Meier method was used to calculate the time to event end points. The univariate Cox proportional hazard model was used to calculate the hazard ratios (HRs) and confidence intervals (CIs). All time to event end points were described from the date of diagnostic biopsy. The log-rank test was used to evaluate the differences in the Kaplan-Meier curves between the EB and CNB groups.
Results
Baseline characteristics
A total of 1061 eligible patients with DLBCL and PMBL were identified. Table 1 lists the baseline characteristics of the study population. 1018 were DLBCL (96%) and 43 (4%) were PMBL. The median age of the study population was 63 years (range 18-93) and 610 (58%) were male. In total, 532 (50%) patients underwent EB, 515 (48.4%) underwent CNB, and 14 underwent FNA without CNB. Fourteen patients who underwent FNA were included in the CNB group. Furthermore, 214 (20%) patients had extranodal involvement, 622 (59%) had advanced stage, and 357 (33%) had IPI ≥ 3. Patients who underwent CNB had a higher likelihood of advanced stage (odds ratio [OR], 1.7; 95% confidence interval [CI95], 1.3-2.1, P < .0001), elevated LDH (OR, 2.3; CI95, 1.8-2.9, P < .0001), IPI ≥ 3 (OR, 1.8; CI95, 1.4-2.3, P < .0001) and subdiaphragmatic representation of disease (OR, 1.6; CI95, 1.2-2.0, P < .001). Notably, patients who underwent CNB had a significantly shorter DTI, with a median of 14 days (range: 18-93) compared with 19 days (range: 19-92, P < .0001) for patients who underwent EB (Table 1).
Of the 1061 patients, 871 were reviewed for subsequent WES and RNAseq. Of the 449 patients who underwent EB, 371 (83%) had adequate tissue for WES and RNAseq. Of the 422 patients who underwent CNB, 271 (64%) had adequate tissue for subsequent RNAseq and WES. Patients who underwent CNB were significantly more likely to have inadequate tissue for subsequent molecular analysis (OR, 2.6; CI95, 1.9-3.5, P < .000001).
The majority of patients in the EB and CNB groups received RCHOP. The 2 groups did not differ significantly with respect to frontline regimens (Table 1).
Outcomes
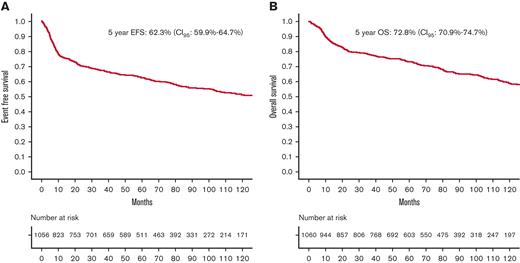
The median follow-up period was 95 months (range: 0.1-240). A total of 1056 patients had EFS and OS data available. Figure 1A-B show the EFS and OS of the entire population. Five-year EFS and OS of the entire study cohort were 62.3% (CI95, 59.9%-64.7%) and 72.8% (CI95, 70.9%-74.7%), respectively.
Survival of entire population. (A) EFS of entire population. (B) OS of entire population.
Survival of entire population. (A) EFS of entire population. (B) OS of entire population.
Figure 2A-B show the EFS and OS according to the type of tissue biopsy. Compared with patients who underwent CNB for the diagnosis of lymphoma, those who underwent EB had significantly higher EFS (HR, 0.76; CI95, 0.6-0.9, P < .001; 5-year EFS 67.6% vs 56.9%, respectively) and OS (HR, 0.8; CI95, 0.6-0.9, P < .001; 76.4% vs 69.2%).
Tissue biopsy method and survival. (A) EFS by type of tissue biopsy. (B) OS by type of tissue biopsy.
Tissue biopsy method and survival. (A) EFS by type of tissue biopsy. (B) OS by type of tissue biopsy.
In univariate analysis, advanced age, male gender, advanced stage, IPI ≥ 3, subdiaphragmatic disease, extranodal disease, DTI, hemoglobin of less than 12, elevated LDH, and receipt of CNB were significantly associated with lower EFS (supplemental Table 1). When adjusted for other adverse disease features, CNB was no longer associated with lower EFS (Table 2).
Similarly, advanced age, advanced stage, IPI ≥ 3, subdiaphragmatic disease, elevated LDH, extranodal disease, and receipt of CNB were significantly associated with lower OS (supplemental Table 1). When adjusted for other adverse disease features, CNB was no longer associated with lower OS (Table 3).
Discussion
In this prospective observational study, we observed that patients diagnosed with CNB were more likely to have high-risk disease features, require prompt care (as suggested by the shorter DTI), have inadequate tissue for molecular analyses, and have lower survival rates. Increasing tissue requirements in biomarker-driven clinical trials may lead to the selective enrollment of patients who can undergo EB and the exclusion of patients with poor-risk features who may require urgent treatment and might not be able to wait for extensive tissue biopsy.
In our study, we observed that patients who underwent CNB were more likely to have a higher IPI, elevated LDH, advanced stage, and shorter DTI. All of these variables have been associated with inferior EFS and OS in the literature.19-21 When adjusted for these adverse-risk features, CNB is no longer associated with differences in survival. Thus, patients who underwent CNB are more likely to have a poor risk of disease and consequently have inferior EFS and OS after frontline chemoimmunotherapy. Moreover, patients who underwent CNB had adequate tissue for diagnosis but were more likely to have inadequate tissue for subsequent molecular analyses. Thus, CNB may not meet the increasing tissue requirements of biomarker-driven clinical trials, necessitating the use of EB. This, in turn, will lead to (1) unintentionally biased exclusion of poor-risk patients who require treatment sooner, cannot wait for EB procedures, have inferior outcomes with standard-of-care treatment, and need novel agents, and (2) increased enrollment of patients who can undergo EB, have more favorable disease characteristics, and better projected outcomes with standard-of-care regimens, making it difficult to see the benefits of novel agents. Patients who are too sick to wait for extensive tissue biopsies before the commencement of treatment are likely to be excluded from these biomarker-driven clinical trials.
Methods to compensate for tissue requirements need to be explored to expand clinical trial access to patients with poor-risk features who cannot wait for EB. Standardization of CNB techniques, such as obtaining multiple cores and using a large gauge needle to obtain extra material for subsequent molecular analysis, are some of the ways to improve the efficiency of CNB. Another way to achieve this goal is to set a time limit for DTI as one of the eligibility criteria; this can facilitate expedition of diagnostic procedures before clinical trial enrollment and accommodate patients who require treatment sooner rather than later.22 Caution may need to be exercised with this strategy, as limiting DTI might impair trial accrual. Increasing the trial sample size by expanding eligibility criteria to include all patients with DLBCL regardless of biological subtype and performing biomarker-based efficacy evaluation as an exploratory analysis is another way to prevent tissue accessibility-related bias in clinical trial enrollment. This approach was adapted in the ECOG-1412 trial as well as in the REMoDL-B study.8,9 Techniques that increase assay capability can be explored so that detailed molecular analyses can be performed using less tissue. Finally, liquid biopsy or peripheral blood circulating tumor DNA (ctDNA) can potentially be utilized to expedite some of the biological analyses.23-28 Recently, ctDNA has been efficiently utilized for molecular characterization of DLBCL; this strategy can compensate some of the tissue needs of clinical trials.29 ctDNA can potentially be used as a surrogate for tumor burden and prognosis in lymphoma. Recently, high pretreatment ctDNA was associated with shorter DTI, inferior EFS, and OS in newly diagnosed DLBCL.26 Decrease of ctDNA tumor burden during therapy has been associated with outcomes in DLBCL.23
The large sample size and prospective follow-up were the 2 major strengths of this study. The lack of a validation cohort is a significant limitation. In our single-center cohort, a very high number of patients who underwent CNB had inadequate materials for subsequent molecular analyses. Although this finding aligns with the evidence reported in the literature, validation in large multicenter cohort is necessary. We were also unable to include patients who were diagnosed and treated after 2015, as data on those patients were not available at the time of publication. Considering advancements in techniques of obtaining CNB over the recent years, it might be interesting to re-assess the efficiency of CNB in providing adequate samples in more modern era.
In conclusion, patients undergoing CNB are more likely to have elevated LDH levels, advanced stage, high-risk IPI, shorter DTI, and lower EFS and OS in response to frontline chemoimmunotherapy. The findings of this study need to be validated in an independent cohort, but they generate an interesting hypothesis. Increasing tissue requirements of biomarker-driven clinical trials may result in the exclusion of patients with high-risk DLBCL who have inferior outcomes with standard treatment and need novel agents and may result in over performance of the control arm. The need for tissues should be carefully balanced against resulting selection bias.
Authorship
Contribution: G.S.N. and S.H.D. were responsible for study design and concept; S.H.D., E.M., U.F., R.L.K., J.R.C., A.L.F., T.M.H., T.E.W., Y.W., and C.A.T. were responsible for data collection; S.H.D., R.M., and M.J.M. were responsible for data analysis; and all authors prepared and reviewed the manuscript.
Conflict-of-interest disclosure: R.L.K. received research funding from BMS/Celgene. M.J.M. received research funding from Genentech, BMS, and MorphoSys, and has served on the advisory board for Pfizer, Kite Pharma, Genmab, and Adaptive Biotechnology. Y.W. received research funding (to institution) from Incyte, Innocare, LOXO Oncology, Novartis, Genentech, and MorphoSys, and reports serving on advisory boards (compensation to institution) for Eli Lilly, TG Therapeutics, LOXO Oncology, Incyte, InnoCare, and Kite. J.C. has received research funding from Kite Pharma. T.E.W. reports consultancy with Celgene, MorphoSys, AbbVie, Incyte, and Spectrum, and research funding from Celgene, Acerta, Karyopharm Therapeutics, and Immune Design. T.M.H. has served on the data monitoring committees for Seagen, and Tess Therapeutics and scientific advisory boards with Eli Lilly & Co, MorphoSys, Incyte, Biegene, and Loco Oncology; G.S.N. reports consultancy with Celgene/BMS, MorphoSys, Ryvu, Kite, Kymera, Curis, and Seattle Genetics, and research funding from Celgene/BMS, MorphoSys, and Nanostring. The remaining authors declare no competing financial interests.
Correspondence: Sanjal H. Desai, Division of Hematology, Oncology and Transplantation, University of Minnesota, 420 Delaware St SE, MMC 480, Minneapolis, MN 55455; e-mail: desai171@umn.edu.
References
Author notes
This is an observational study and individual participant data may not be shared.
The full-text version of this article contains a data supplement.