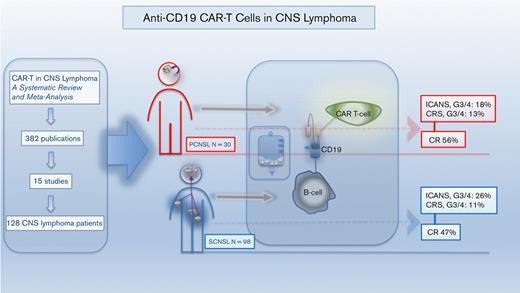
Abstract
Relapsed/refractory primary central nervous system lymphoma (PCNSL) and secondary central nervous system lymphoma (SCNSL) are associated with short survival and represent an unmet need, requiring novel effective strategies. Anti-CD19 chimeric antigen receptor (CAR) T cells, effective in systemic large B-cell lymphoma (LBCL), have shown responses in PCNSL and SCNSL in early reports, but with limited sample size. We, therefore, performed a comprehensive systematic review and meta-analysis of all published data describing CAR T-cell use in PCNSL and SCNSL. This identified 128 patients with PCNSL (30) and SCNSL (98). Our primary objectives were to evaluate CAR T-cell specific toxicity (immune effector cell-associated neurotoxicity syndrome [ICANS] and cytokine release syndrome [CRS]) as well as response rates in these 2 populations. Seventy percent of patients with PCNSL had CRS of any grade (13% grade 3-4) and 53% had ICANS of any grade (18% grade 3-4). Comparatively, 72% of the SCNSL cohort experienced CRS of any grade (11% grade 3-4) and 48% had ICANS of any grade (26% grade 3-4). Of the patients with PCNSL, 56% achieved a complete remission (CR) with 37% remaining in remission at 6 months. Similarly, 47% of patients with SCNSL had a CR, with 37% in remission at 6 months. In a large meta-analysis of central nervous system (CNS) lymphomas, toxicity of anti-CD19–CAR T-cell therapy was similar to that of registrational studies in systemic LBCL with no increased signal of neurotoxicity observed. Encouraging efficacy was demonstrated in patients with CNS lymphoma with no discernible differences between PCNSL and SCNSL.
Introduction
The treatment landscape of relapsed and refractory (R/R) systemic large B-cell lymphoma (LBCL) has recently been altered by the development and high efficacy of anti-CD19 chimeric antigen receptor (CAR) T-cell therapy. Currently, axicabtagene ciloleucel (axi-cel) (ZUMA-1),1 tisagenlecleucel (tisa-cel) (JULIET)2 and lisocabtagene maraleucel (liso-cel) (TRANSCEND)3 are all U.S. Food and Drug Administration approved for R/R systemic LBCL. Improving outcomes in LBCL involving the central nervous system, a subset with a particularly poor outcome,4-6 represents an unmet clinical need. This is challenging for many reasons, including the need for systemic therapies to penetrate the blood-brain barrier,7 which has led to distinct management paradigms for primary central nervous system lymphoma (PCNSL) and secondary central nervous system lymphoma (SCNSL) with the exclusion of these entities from many novel agent clinical trials. This was the case with recent registrational CAR T-cell studies. There was particular concern that patients with CNS involvement may be more susceptible to immune effector cell-associated neurotoxicity syndrome (ICANS) owing to unpredictable effects of anti-CD19 CAR T cells in the CNS. Few studies have elucidated this theoretical concern; 1 identified a brain mural pericyte population that expresses CD19 and represents a potential off-tumor target for CAR T-cell therapies.8 In addition, it was unclear if CAR T cells undergo peripheral expansion without the antigenic stimulation of systemic lymphoma or if they can sufficiently traffic to the CNS. For these and other reasons, PCNSL was excluded from the above 3 trials, and only a small proportion of SCNSL was included in the TRANSCEND study.
Despite the above mentioned historical concerns, CAR T cells have since been shown to successfully traffic to the CNS. In a case series of 2 patients with R/R acute lymphoblastic leukemia who were successfully treated with anti-CD19 CAR-modified cells, the investigators were able to show the presence of CAR T cells in the patients’ cerebrospinal fluid (CSF) samples. Furthermore, these cells persisted in the CSF at high levels for at least 6 months.9 Similarly, T cells that are modified to express CAR targeting on a variety of primary brain tumor antigens have been shown to migrate from the blood to the tumor sites,10 and the efficiency of this migration is modifiable.11 In CNS lymphoma, early studies sought to understand the kinetics of CAR T-cell expansion in vivo using different detection techniques to quantify the transgenic cells. Data suggest that not only can these CAR T cells traffic to the CNS but they expand and persist in the absence of measurable disease at the time of infusion.12-14 Early clinical proof of concept was highlighted by describing a durable complete remission (CR) after CAR T-cell infusion for a heavily pretreated patient with refractory diffuse large B-cell lymphoma (DLBCL) with a right temporal lobe lesion,15 and reproduced (CR in 5 of 9 patients with R/R PCNSL) in a small retrospective case series.16 Prospective study of anti-CD19 CAR T cells in PCNSL is ongoing, with one published study noting manageable rates of serious cytokine release syndrome (CRS; G3 or higher 0%) and ICANS (G3 or higher 8%), with 50% of patients achieving CR.13
Although prospective trials are now active and accruing to investigate the use of CAR T-cell therapy in both PCNSL and SCNSL, there is a paucity of published safety and outcome data, making any broad-reaching conclusions challenging. Therefore, our objective was to perform a comprehensive systematic review and meta-analysis of all published data describing CAR T-cell use in primary and secondary CNS lymphoma. Our goal was to combine and analyze a sizable data set to evaluate the safety and efficacy of CAR T cells in these rare and difficult-to-treat cases.
Methods
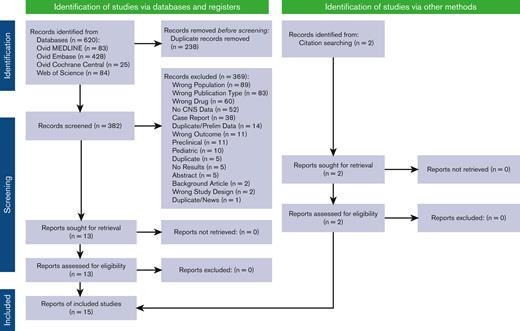
We performed a comprehensive search of the literature for the use of CAR T-cell therapy in adults with PCNSL and SCNSL. The inclusion criteria for this study were prospective or retrospective studies that reported the safety and/or efficacy of CAR T-cell therapy in adult patients (≥18 years of age) with either PCNSL or SCNSL. Preclinical/in vitro studies, reviews/editorials, and single-patient case reports were excluded. Refer to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) diagram as well as the supplement/appendix for full reproducible search strategies. We searched MEDLINE, Embase, and Cochrane CENTRAL via Ovid and Web of Science on April 22, 2022, spanning the years from 2017 through the date of search. This study followed the PRISMA reporting guideline and the PRISMA extension statement.17 Duplicate citations were removed using EndNote X9 (Clarivate Analytics, Philadelphia, PA) and uploaded to Rayyan for screening.18
The literature search, abstract/manuscript review for inclusion/exclusion, and data collection were performed independently by both the first and the last author of this analysis and then cross reviewed for accurate data collection. Our primary outcome was CAR T-cell-specific safety signals, namely CRS and ICANS. Secondary end points were CR rate, overall response rate (ORR), duration of response, time to the achievement of CR, and ongoing responses at the data cutoff date of the corresponding published studies. Information regarding CRS, ICANS, CR rate, time to CR, and ongoing responses at the datacut off date were pulled directly from published literature. We defined the median time of response by the duration of time between CAR T-cell infusion and the progression of disease or data cutoff date for patients who experienced a partial or complete response to therapy. We collected the duration of response if it was reported in each individual trial, or if it was calculated by both the investigators of the study if the appropriate data points were available, and the study did not specifically report this end point. When available, the following information was collected from each study: study name, first author, year of publication, study design/phase, patient age, cell-of-origin, prior lines of therapy, type of CAR T-cell product, and bridging/conditioning therapies.
Statistical analysis
Selected characteristics of the included studies were summarized as means, medians, ranges or frequencies, and percentages. Meta-analysis was conducted separately for the selected outcomes of the study. Heterogeneity of proportions/risks across studies was tested using Cochran’s Q statistic. The I2 statistic was also used as an indicator of the percentage of variation among the studies due to true heterogeneity rather than chance, with 25% indicating low heterogeneity, 50% moderate heterogeneity, and 75% high heterogeneity.19 Both the fixed effect or random effects approach were followed using the inverse-variance weighting method depending on whether the study heterogeneity hypothesis was significant, and a high value of the I2-statistic was attained. For the random effects approach, heterogeneity variance was estimated using the DerSimonian and Laird approach.20 Studies were synthesized using the metaprop function in the package META in R for windows.21
Results
Overall, the literature search identified 382 abstracts and publications (PRISMA flow diagram, Figure 1), which resulted in the inclusion of 15 studies (8 prospective and 7 retrospective), identifying 30 patients with PCNSL and 98 patients with SCNSL who were treated with CAR T-cell therapy.3,13,16,22-33 Patients with PCNSL were nearly all nongerminal center B-cell type (93.75%) with parenchymal disease (86.2%), were heavily pretreated (median, 3.75), and required bridging therapy (80%) before CAR T-cell infusion. Most patients with PCNSL (63.33%) received tisa-cel. Patients with PCNSL and SCNSL did not differ significantly in age (median ∼56 vs 50 years; Wilcoxon rank-sum test, P = .46). The SCNSL cohort primarily had DLBCL (where cell-of-origin was available, 66.7% had nongerminal center B-cell type DLBCL), equally heavily pretreated (4 prior lines) and ∼51% were treated with axi-cel. In the SCNSL cohort, 58.1% of SCNSL lesions were parenchymal, 38.7% nonparenchymal (CSF, leptomeningeal, ocular, cranial/spinal nerve, dural/ventricular disease), and 3.2% had both. In the SCNSL cohort, 51.8% had the concurrent non-CNS systemic disease at the time of CAR T-cell infusion. The majority of both cohorts received fludarabine/cyclophosphamide as their conditioning regimen. One prospective protocol gave a dual targeting (CD19, CD22) CAR as consolidation for autologous stem cell transplant (4 PCNSL, 9 SCNSL).23 Of the total patients, 96.7% of patients with PCNSL and 74.1% of patients with SCNSL had documented evidence of disease at the time of CAR T-cell infusion. In the SCNSL cohort, 51.8% were documented to have systemic and CNS disease before therapy. Full demographic and pretreatment data are shown in Table 1.
PRISMA flow diagram.17 For more information, visit: https://www.prisma-statement.org/.
PRISMA flow diagram.17 For more information, visit: https://www.prisma-statement.org/.
Overall, statistical analysis for the proportion of CRS, grade 3 to 4 CRS, ICANS, grade 3 to 4 ICANS, CR, CR at 3 months, CR at 6 months, PR, ORR, and ongoing responses at the time of data collection did not identify any significant heterogeneity in the studies for PCNSL, SCNSL or the entire study sample (supplementary appendix). Therefore, for these outcomes, the fixed effect model was used to estimate the overall proportion of these primary and secondary outcomes. The only outcome that showed significant heterogeneity across publications was CR at 28 days, thus the random effects model was employed for this analysis.
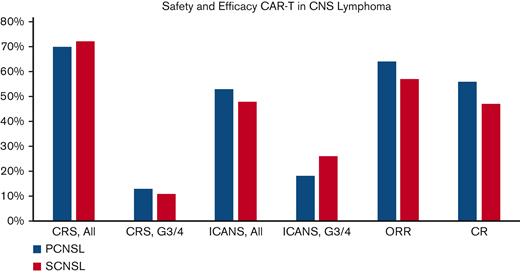
Median follow-up was 12.2 months (8.5-14.2) for the PCNSL publications and 10.1 months (5.1-18.8) for the SCNSL studies. Primary outcome data (Table 2; Figure 2-4) suggested that 70% of patients with PCNSL had CRS of any grade, with 13% of patients developing grade 3 to 4 CRS. Comparatively, 72% of the SCNSL cohort experienced CRS of any grade, and 11% developed grade 3 to 4 CRS. ICANS of any grade occurred in 53% of patients with PCNSL and 48% of patients with SCNSL, respectively. Grade 3 to 4 ICANS occurred in 18% and 26% of patients with PCNSL and SCNSL, respectively.
Primary and major secondary outcomes:graphical representation of CRS, ICANS, ORR, and CR rates for primary CNS lymphoma (PCNSL, blue bars) and secondary CNS lymphoma (SCNSL, red bars).
Primary and major secondary outcomes:graphical representation of CRS, ICANS, ORR, and CR rates for primary CNS lymphoma (PCNSL, blue bars) and secondary CNS lymphoma (SCNSL, red bars).
Proportion of CRS, all grades. Left panel, PCNSL. Right panel, SCNSL.
Proportion of ICANS, all grades. Left panel, PCNSL. Right panel, SCNSL.
Evaluation of secondary efficacy end points revealed that 64% of patients with PCNSL achieved a response to therapy (ORR), with 56% of this cohort achieving a CR (Table 2; Figure 2 and 5). Thirty-one percent of patients were noted to be in a CR by day 28 after CAR T-cell infusion, with the CR rate increasing to 40% and 37% on day 90 and 180, respectively. In the PCNSL group, 37% were found to have an ongoing response at the time of the data cutoff date, and the median duration of response was 8.97 months. Similarly, 57% of the SCNSL cohort had an objective response to therapy, with 47% achieving a CR (Table 2; Figure 2 and 5). In the SCNSL cohort, 32% of patients were noted to be in CR by day 28 after CAR T infusion, with the CR rate increasing to 37% on both day 90 and day 180 (Figure 6). Ongoing responses at the data cutoff date were estimated to be present in 46% of patients, and the median duration of response was 4.63 months in the SCNSL group.
Proportion of CR as best response. Left panel, PCNSL. Right panel, SCNSL.
Combining PCNSL and SCNSL cohorts, 95 patients’ CAR T-cell costimulatory domain was explicitly reported, and 57 were treated with a CD28 costimulatory domain, compared with 38 patients with a 4-1BB costimulatory domain. A fraction of these patients’ key primary and secondary outcomes could be correlated with the costimulatory domain. Subgroup safety analysis found the CD28 cohort had a 77.8% incidence of all grade CRS, with 10.4% being grade 3 to 4. ICANS of any grade was seen in 68.9% of this group, with 30% being grade 3 or higher. The 4-1BB subpopulation had a 59.3% rate of any grade CRS, all reported to be grade 1 to 2. Any grade ICANS was reported in 44.4% with 11.1% being grade 3 or higher. Subgroup efficacy analysis reported the CD28 cohort to have an ORR of 54.0%, with 51.5% achieving CR and 38.7% remaining in CR at 6 months. The 4-1BB group had an ORR of 51.9%, a CR rate of 44.4%, with 25% achieving CR at 6 months.
Discussion
To our knowledge, this is the largest systematic review and meta-analysis that combines and analyzes the data published to date on the utility of CAR T-cell therapy in PCNSL and SCNSL. Overall, our data set shows a safety and efficacy profile in accordance with the published literature leading to the regulatory approval of CAR T-cell products in R/R LBCL. Our analysis shows that the majority of patients with PCNSL and SCNSL develop CRS, but only 13% and 11% of those respective cohorts report a G3-4 CRS. Similarly, ICANS occurred in roughly half of each cohort, with 18% and 26% documented G3-4 neurotoxicity. These safety data are comparable to what is reported by ZUMA-11 (G3/4 CRS: 13%, G3/4 ICANS: 28%), JULIET2 (G3/4 CRS 22%, G3/4 ICANS 12%), and TRANSCEND3 (G3/4 CRS 2%, G3/4 ICANS 10%). The slightly higher incidence rate of G3-4 ICANS in the SCNSL cohort may be explained by more patients in this cohort receiving axi-cel, which has been associated with a higher incidence rate and severity of neurotoxicity compared with other products owing to a difference in costimulatory domains, among other factors.34 On the contrary, the PCNSL cohort was mainly treated with tisa-cel. In addition, a lack of the systemic burden of disease in PCNSL can result in less systemic inflammation and lower levels of circulating cytokines. This phenomenon could have also contributed to the difference in ICANS rates. However, one would expect a lower incidence rate of CRS in PCNSL as well, which was not found in our study. Overall, our data show no major differences in toxicity incidence or severity when CNS lymphoma is treated with CAR T-cell products compared with systemic lymphoma without CNS involvement.
The efficacy analysis of our study is equally encouraging. A significant portion of patients responded to CAR T-cell therapy, with 56% of patients with PCNSL and 47% of patients with SCNSL achieving CR as their best response. Importantly, ∼37% of both groups had an ongoing CR at 6 months after CAR T-cell therapy. In the long-term follow-up analyses of pivotal CAR T-cell trials in LBCL, the majority of patients with a CR at 3 months, 6 months, or both developed durable event-free survival.35,36 This suggests that CR at 3 or 6 months could be a surrogate for the durable response. Given the comparable ORR/CR rates as well as the 3-month and 6-month CR rates between our data and the pivotal CAR T-cell trials in LBCL, it is reasonable to hypothesize that approximately one-third of patients with CNS lymphoma could have durable responses when treated with CAR T-cell therapy. We acknowledge the limitations of this hypothesis, especially given the heterogenous treatments (several CAR T-cell products, varying bridging therapies, use of concurrent autologous stem cell transplant) that were included in this analysis. Consequently, this hypothesis will need to be confirmed with appropriately designed prospective studies of patients with PCNSL and SCNSL.
Analyzing the costimulatory domain subgroup suggests that patients with CNS lymphoma treated with CD28 products had higher rates of ICANS and CRS than those treated with 4-1BB CAR T cells. In addition, the CD28 cohort had numerically better outcomes for CR rates. We feel that these data reflect the aforementioned registrational studies 1-3; axi-cel has been documented to show higher response rates, as well as higher rates of ICANS when compared with other CAR products. Although our data do not reflect the rate of grade 3 to 4 CRS within the JULIET study, one could hypothesize that this was related to tisa-cel use in patients with PCNSL, in which the absence of systemic disease may lead to less cytokine release. In addition, the 4-1BB cohort included patients treated with liso-cel, which has been shown to have very low rates of severe CAR T–specific toxicity.
Bridging therapy is vital when considering the global care of these patients. Although the SCNSL data regarding bridging therapy were sparse, almost all patients with PCNSL were documented to have received bridging therapy before CAR T-cell therapy. Bridging therapy varied greatly, from standard blood-brain barrier traversing chemotherapy to targeted therapies (immunomodulators, Bruton’s tyrosine kinase inhibitors), whole brain radiation, and finally, corticosteroids. We did not have a large enough sample size to evaluate each bridging therapy’s impact on safety and efficacy, but it is noteworthy to mention that in studies that employed whole brain radiation, we did not find a noticeable difference in safety or efficacy. In real-world practice, there is a long time between the initial evaluation of the patient to the infusion of the CAR product. Most patients with CNS lymphoma will likely require a bridging therapy while the CAR T cells are being manufactured. Finally, the combination of fludarabine and cyclophosphamide was by far the most common conditioning therapy used and alternative conditioning regimens are not seemingly required to ensure the efficacy of CAR T cells in this disease group.
This study is not without its limitations. First, meta-analysis is prone to the heterogeneity of the data that are included within the analysis. Given the limited literature on this topic, both retrospective and prospective data were included. This will potentially introduce significant heterogeneity regarding patient inclusion, demographics, treatment strategies, and outcomes. Nevertheless, with the use of Cochran’s Q and I2 statistical methods, we were able to show the absence of a significant amount of heterogeneity within the data retrieved. In addition, retrospective studies are prone to publication bias, in which it is less likely for a negative CAR T-cell study in CNS lymphoma to be published. The data used for this analysis are also summarized published data, which are less reliable than analyzing individual patient data from each of these publications. Different bridging therapies were utilized before CAR T-cell infusion by different groups and the effect of that on the outcomes of the patients is uncertain. Notably, one prospective study described the use of high-dose chemotherapy and stem cell rescue before CAR T-cell infusion (Wu et al23; Figure 3-5). Although this approach did not appear to improve the outcome of these patients, no conclusions can be drawn given the small sample size. Finally, important variables such as classification of CNS disease, CAR T-cell construct/costimulatory domain, and CNS/systemic burden of disease were not homogenously reported, hindering our ability to make conclusions regarding their impact on overall outcomes. Future prospective work with a focus on these elements will be vital to understanding the intricacies of CAR T-cell therapy in CNS lymphoma.
In conclusion, to our knowledge, our study is the largest pooled analysis reported to date of anti-CD19 CAR T cells in PCNSL and SCNSL. The findings demonstrate encouraging response rates with manageable ICANS and CRS that are consistent with results in systemic LBCL,1-3 supporting the conclusion that CAR T-cell therapy is safe and effective in patients with PCNSL and SCNSL. Though we did not identify differences in neurotoxicity rates for primary vs secondary CNS cases, distinct pathophysiologic mechanisms of ICANS are likely differentially important in PCNSL vs SCNSL. Future studies should focus on better elucidating these mechanisms and developing prognostic models to identify subsets of patients who will preferentially benefit from prophylactic strategies to prevent ICANS. Our report and other recent studies support the expeditious upfront investigation of anti-CD19 CAR T-cell based approaches in PCNSL and SCNSL, where survival outcomes remain significantly inferior to systemic LBCL.
Authorship
Contribution: M.R.C. and A.A. designed the study concept, reviewed literature, performed the inclusion/exclusion of publications, data collection, manuscript writing and editing, and visual abstract/figure creation; S.C.D. contributed to the literature review, Rayyan review creator, PRISMA figure design, and editing of the manuscript; K.M. and Y.L. contributed to the biostatistical design, analysis, and figure creation and editing of the manuscript; K.D. contributed to the editing of the manuscript and the visual abstract creation; and P.N.M., M.D., S.R., and A.G. contributed to the editing of the manuscript.
Conflict-of-interest disclosure: K.D. served on the advisory board/consulting for AstraZeneca, Beigene, AbbVie, Daiichi Sankyo, ADC Therapeutics, Incyte, Morphosys, Genmab. P.N.M. served on the advisory board for Incyte; consultancy: Incyte; speakers bureau: Incyte, Kite Pharma. A.G. acted in a consulting or advisory role for AstraZeneca, Bristol Myers Squibb, Celgene, Hoffmann-La Roche, Janssen, Kite, Morphosys, Allopex, Gilead, Novartis, Vincerx, Resilience, received research funding (institutional) from Acerta, AstraZeneca, Bristol Myers Squibb, Celgene, Genentech, Hoffmann-La Roche, Infinity, Janssen, Karyopharm, Kite, Morphosys, Pharmacyclics, Seattle Genetics, Verastem. A.S. served as a consultant (Kite/Gilead, Magenta Therapeutics, Incyte Pharmaceuticals, CareDx) and received royalty fees from In8Bio Inc. A.A. served on the advisory board/consulting for Incyte. The remaining authors declare no competing financial interests.
Correspondence: Alaa Ali, Stem Cell Transplant and Cellular Immunotherapy Program, Georgetown University, 3800 Reservoir Road, NW, Washington, DC 20007; e-mail: alaa.ali@gunet.georgetown.edu.
References
Author notes
All analysis generated from this systematic review and meta-analysis is available within the manuscript, or in the supplemental appendix.
The full-text version of this article contains a data supplement.
K.D. and A.A. are joint senior authors.