Key Points
In this nationwide cohort study, apixaban, dabigatran, and rivaroxaban had similar rates of any stroke or systemic embolism.
Rivaroxaban had higher rates of major bleeding than apixaban and dabigatran but lower rates of myocardial infarction than dabigatran.
Abstract
In the pivotal randomized controlled trials (RCTs) for patients with atrial fibrillation, direct oral anticoagulants (DOACs) had similar or even superior efficacy and safety compared with warfarin. However, RCTs comparing different DOACs are nonexistent and previous observational studies have yielded conflicting results. In this nationwide cohort study, rates of any stroke or systemic embolism (stroke/SE) and major bleeding were compared among new users of apixaban, dabigatran, and rivaroxaban with atrial fibrillation from 2014 to 2019. Inverse probability weighting was used to yield balanced study groups, and outcomes were compared using Cox regression. Stroke/SE rates were similar in patients receiving apixaban, dabigatran, and rivaroxaban. Dabigatran was associated with twofold higher rates of myocardial infarction (MI) than rivaroxaban (1.4 events/100 person-years (py) vs 0.7 events/100-py, hazard ratio [HR] 2.21, 95% confidence interval [CI], 1.00-4.90) and apixaban (1.4 events/100-py vs 0.7 events/100-py, HR 2.26, 95% CI, 0.90-5.67), although the second comparison included the possibility of a null effect. Rivaroxaban was associated with higher major bleeding rates compared with apixaban (2.9 events/100-py vs 1.8 events/100-py, HR 1.64, 95% CI, 1.13-2.37) and dabigatran (2.9 events/100-py vs 1.4 events/100-py, HR 2.18, 95% CI, 1.21-3.93). Specifically, rivaroxaban had higher rates of major gastrointestinal bleeding and other major bleeding than apixaban. In conclusion, although stroke/SE rates were similar for DOACs, rivaroxaban was associated with higher rates of major bleeding than other DOACs and lower rates of MI than dabigatran. These results may help guide oral anticoagulant selection, especially in patients at high risk of bleeding or MI.
Introduction
Pivotal randomized controlled clinical trials (RCTs) demonstrated that direct oral anticoagulants (DOACs) had similar or even superior efficacy compared with warfarin.1-4 In addition, these trials reported higher rates of major bleeding with warfarin than with apixaban, edoxaban, and low-dose dabigatran, whereas major bleeding rates were similar between warfarin and rivaroxaban and high-dose dabigatran.1,4 Whether thromboembolic and major bleeding events differ between individual DOACs remains unclear because no RCT has compared DOACs head-to-head. Assessments of thromboembolic event rates have yielded conflicting results in previous observational studies5-10 whereas rivaroxaban has generally been associated with higher major bleeding rates than other DOACs.5-10
In the initial RCTs, major bleeding was defined according to the International Society of Thrombosis and Hemostasis (ISTH) as a bleeding event leading to hemoglobin drop of ≥20 g/L, transfusion of ≥2 units of red blood cells, bleeding into a critical area (such as the cranium or retroperitoneum), or death.1-4 To the best of our knowledge, no large observational study has used this definition for major bleeding, although a modified version of the ISTH definition was used in a nationwide Norwegian registry study, omitting hemoglobin drop as 1 of the criteria.11 Otherwise, most studies have defined major bleeding as a bleeding event requiring hospitalization, using relevant hospital discharge codes only and without manual verification of events. How closely this resembles the ISTH criteria for major bleeding is unknown. A previous study from our group demonstrated that only 77% of hospitalized patients with gastrointestinal bleeding (GIB) fulfilled the ISTH criteria of major bleeding.12 The proportion is likely lower for other bleeding events such as hematuria, epistaxis, postmenopausal bleeding, or unspecified anemia, which have been included as major bleeding in previous studies.7,8,10,13-15 Thus, population-based real-world studies with well-characterized patients with adequate follow-up are important to elucidate potential differences in the effectiveness and safety of DOACs.
The aim of this study was to compare the rates of any stroke or systemic embolism (stroke/SE) and major bleeding associated with apixaban, dabigatran, and rivaroxaban for patients with atrial fibrillation in a nationwide propensity score–weighted cohort.
Methods
Data source
The creation of the Icelandic oral anticoagulation database has been previously described with emphasis on GIB.16 The database includes data on all patients in Iceland receiving oral anticoagulants (OACs) from 1 March 2014 to 28 February 2019. Using the unique national identification number of each patient, assigned to all Icelanders at birth or upon immigration, data were collected from the Icelandic Medicine Registry, the 5 major hospitals in Iceland, and the Icelandic death registry. This study was approved by the National Bioethics Committee of Iceland (VSN-16-057-V4) and was conducted according to the Declaration of Helsinki.
Patient selection and follow-up
Patients included were those with atrial fibrillation who started treatment with apixaban, dabigatran, and rivaroxaban from 1 March 2014 to 28 February 2019. To establish an OAC naïve cohort, patients were excluded if they had filled a prescription for any OAC in the preceding 12 months before their eligibility into the study. In addition, patients were excluded if their permanent residence was outside Iceland, if they had end-stage renal disease, mitral stenosis, or a mechanical heart valve.
Patients were followed from the date of first drug dispensation to 28 February 2019, or earlier if either the treatment was stopped, primary outcome was achieved, death occurred, they moved out of the country, or were switched to a different OAC.
Exposure and outcomes
The exposure of interest was treatment with apixaban, dabigatran, or rivaroxaban. The primary effectiveness outcome was any stroke/SE. The primary safety outcome was major bleeding. Secondary outcomes were myocardial infarction (MI), venous thromboembolism, arterial thromboembolism, transient ischemic attack (TIA), intracranial hemorrhage, major GIB, other major bleeding, all-cause mortality, vascular mortality, ischemic stroke, and hemorrhagic stroke. Stroke was defined as a focal neurological deficit in an area consistent with the findings of diagnostic imaging or autopsy. TIA was defined as a focal neurological deficit in an area corresponding to a major cerebral artery that lasted for <24 hours and, if applicable, no evidence of infarction or hemorrhage on diagnostic imaging. MI was defined as acute chest pain with 1 of 1) new-onset ST-segment elevation on EKG, 2) >threefold increase in Troponin T from baseline, or 3) MI as cause of death on autopsy. Major bleeding was defined according to the ISTH criteria as bleeding leading to a hemoglobin drop of ≥20 g/L, transfusion of ≥2 units of packed red blood cells, symptomatic bleeding into a closed compartment (such as the cranium or retroperitoneum), or death.17
Identification of events
Data on study outcomes were gathered by 4 separate pathways. First, by use of a thorough International Classification of Diseases, 10th revision (ICD-10) code search (from hospital discharges and emergency department visits) (supplemental Table 1). Second, by querying the Icelandic death registry. Third, by examining endoscopic procedures for GIB. Fourth, by examining computed tomographies of the head and pulmonary arteries to identify missed diagnoses of stroke, intracranial hemorrhage, or pulmonary embolism. Each diagnosis was reviewed and confirmed by manual chart review. Events were excluded if they occurred before the start of the patient's follow-up, the patient had not been receiving OACs in the preceding 2 days, or a bleeding event did not fulfil the ISTH criteria.
For comparison, we identified events by using only previously verified ICD-10 codes and without manual chart review, as reported by other investigators.14,18,19 The sensitivity, specificity, positive predictive value (PPV), and negative predictive value for that method were calculated in comparison to our more robust searching algorithm.
Data on baseline characteristics (ie, treatment indication, comorbidities, concomitant drug use, and area of residence) were collected from the start of the patient's follow-up or earlier (Figure 1; supplemental Tables 1 and 2). The data acquisition for these variables has been previously described in detail.16
Cohort creation diagram. ∗The exclusion criteria were residence outside of Iceland at the index date, prior use of oral anticoagulants within 12 months of the index date, prescription of 2.5 mg of rivaroxaban at the index date, and diagnosis of end-stage renal disease, a mechanical heart valve, or mitral stenosis from the index date or earlier.
Cohort creation diagram. ∗The exclusion criteria were residence outside of Iceland at the index date, prior use of oral anticoagulants within 12 months of the index date, prescription of 2.5 mg of rivaroxaban at the index date, and diagnosis of end-stage renal disease, a mechanical heart valve, or mitral stenosis from the index date or earlier.
Statistical analysis
To account for potential indication bias, inverse probability weighting (IPW) was used to yield balanced study groups. IPW assigns weights to patients based on propensity scores calculated from potential confounders, thereby creating a balanced pseudopopulation that includes the whole study population. The model accounted for age, sex, DOAC dosing (standard or low dose), all variables in the Charlson comorbidity index (except for AIDS, which was too sporadic), bleeding or coagulation disorders, hypertension, history of venous thromboembolism or GIB, concomitant drug use (antihistamines, antiplatelets, corticosteroids, nonsteroidal anti-inflammatory drugs, proton pump inhibitors, selective serotonin reuptake inhibitors, and statins), and area of residence. Study outcomes were compared using propensity score-weighted Cox regression, and the data were visualized using propensity score-weighted Kaplan–Meier survival curves. Finally, the E-value was calculated to estimate the effect of any residual confounding factors.20 All statistical tests were 2-tailed and all CIs were 95%. Statistical analysis was performed in R, version 4.1.2 (R Foundation for Statistical Computing), using RStudio, version 2021.09.2.
Results
Study population
In total, 8892 patients filled an OAC prescription during the study period. Of those, 2869 patients were excluded because they had received an OAC in the preceding 12 months, 1256 patients were excluded because they had treatment indication other than atrial fibrillation, and 97 patients were excluded for other reasons, leaving 4670 patients in the final study cohort (Figure 2). Of those, 1787 patients received apixaban, 420 received dabigatran, and 2463 patients received rivaroxaban. IPW yielded balanced study groups with an average weighted follow-up period of 1.3 years for patients receiving apixaban, 1.9 years for patients receiving dabigatran, and 1.8 years for patients receiving rivaroxaban (Table 1).
Flowchart for study selection. AF, atrial fibrillation; DOACs, direct oral anticoagulants; OAC, oral anticoagulant.
Flowchart for study selection. AF, atrial fibrillation; DOACs, direct oral anticoagulants; OAC, oral anticoagulant.
A total of 44 stroke/SE events were identified during the follow-up period. In total, 38 (86%) events were identified using ICD-10 codes and 4 (9%) from the death registry. In addition, 2 (5%) events were identified during chart review of another diagnosis.
Overall, 191 major bleeding events were identified during the follow-up period. Of those, 137 (72%) originated from the gastrointestinal tract, 28 (15%) had an intracranial location, and 26 (14%) originated from other locations. Of the 191 major bleeding episodes, 159 (83%) were identified using ICD-10 codes, 25 (13%) from endoscopic procedure codes, and 4 (2%) from the death registry. In addition, 3 (2%) events were identified during chart review of another diagnosis.
When only previously validated primary discharge ICD-10 codes were used to identify events and without confirmation by manual chart review, 46 stroke/SE and 73 major bleeding events were identified. Compared with our robust searching algorithm, this method had a sensitivity of 52.3% for identifying any stroke/SE, and sensitivity of 30.4% for any major bleeding (Table 2). The specificity was 99.5% and 99.7% for stroke/SE and major bleeding events, respectively. The PPV for stroke/SE was 50.0%. The PPV was 79.5% for any major bleeding. Specifically, the PPV was 100% for intracranial hemorrhage, 90.2% for GIB, and 39.1% for bleeding from other locations. For other outcomes, the PPV ranged from 14.3% for venous thromboembolism to 91.7% for MI. The negative predictive value was 99.5% for stroke/SE and 97.1% for major bleeding (Table 2).
Comparison of thromboembolic and mortality rates
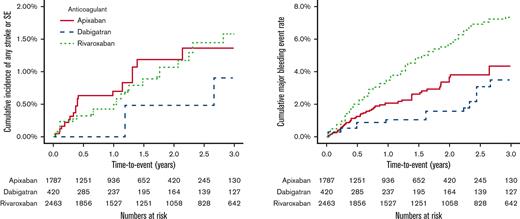
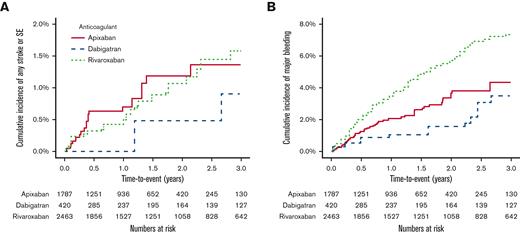
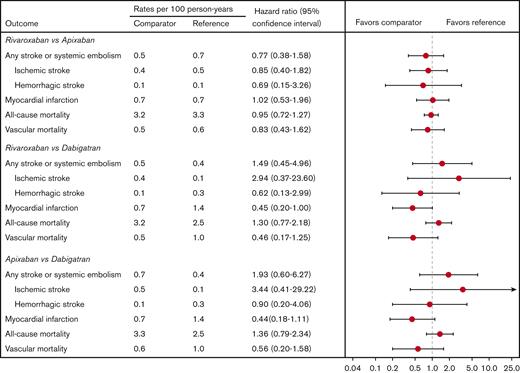
Rates of any stroke/SE were similar among the 3 drugs (Figures 3A and 4). Specifically, the rates of ischemic stroke or hemorrhagic stroke were not markedly different between groups, although the study was likely underpowered for this analysis. Interestingly, dabigatran was associated with >twofold higher rates of MI compared with both apixaban (1.4 events/100 person-years (py) vs 0.7 events/100-py, hazard ratio [HR] 2.26, 95% CI, 0.90-5.67) and rivaroxaban (1.4 events/100-py vs 0.7 events/100-py, HR 2.21, 95% CI 1.00-4.90), although the former comparison included the possibility of a null effect (Figure 4). All-cause mortality was similar among the drugs (Figure 4). Dabigatran was associated with twofold higher vascular mortality compared with apixaban and rivaroxaban, although the CIs had a wide range for these comparisons and included the possibility of a null effect in both instances (Figure 4). The number of venous thromboembolic and TIA events was too low to make any meaningful comparisons among the groups (supplemental Table 3).
Kaplan-Meier cumulative event plots comparing the primary study outcomes. (A) compares rates of any stroke or systemic embolism (SE) for patients receiving apixaban, dabigatran, and rivaroxaban. (B) compares rates of any major bleeding between patients receiving apixaban, dabigatran, and rivaroxaban.
Kaplan-Meier cumulative event plots comparing the primary study outcomes. (A) compares rates of any stroke or systemic embolism (SE) for patients receiving apixaban, dabigatran, and rivaroxaban. (B) compares rates of any major bleeding between patients receiving apixaban, dabigatran, and rivaroxaban.
Propensity score-weighted incidence rates and hazard ratios of thromboembolic and mortality rates. Comparison between patients receiving apixaban, dabigatran, and rivaroxaban.
Propensity score-weighted incidence rates and hazard ratios of thromboembolic and mortality rates. Comparison between patients receiving apixaban, dabigatran, and rivaroxaban.
Comparison of major bleeding rates
Rivaroxaban was associated with higher rates of major bleeding compared with apixaban (2.9 events/100-py vs 1.8 events/100-py, HR 1.64, 95% CI, 1.13-2.37) and dabigatran (2.9 events/100-py vs 1.4 events/100-py, HR 2.18, 95% CI, 1.21-3.93) (Figures 3B and 5). Rivaroxaban users had higher rates of both major GIB and other major bleeding events compared with apixaban. No differences were noted in intracranial hemorrhage rates among the 3 drugs (Figure 5).
Propensity score-weighted incidence rates and hazard ratios of major bleeding rates. Comparison between patients receiving apixaban, dabigatran, and rivaroxaban.
Propensity score-weighted incidence rates and hazard ratios of major bleeding rates. Comparison between patients receiving apixaban, dabigatran, and rivaroxaban.
Estimation of the effect of potential confounders
To estimate the effect of potential confounders, the E-value was calculated. The E-value for the comparison of major bleeding rates between rivaroxaban and apixaban users was 2.66 for the point estimate and 1.51 for the lower limit of the 95% CI. This suggests that an unmeasured confounder, unrelated to the covariates included in the IPW model, would have to be 166% more common in the rivaroxaban group and increase the risk for major bleeding by 166% to explain away the observed difference, or be 51% more common in the rivaroxaban group and increase the risk for major bleeding by 51%, for the CI to include the possibility of a null effect. Similarly, for the comparison between dabigatran and rivaroxaban users, the E-value for the point estimate was 3.79 and 1.72 for the lower limit of the CI.
For the comparison of MI rates between rivaroxaban and dabigatran, the E-value was 3.85 for the point estimate and 1.02 for the lower limit of the CI.
Discussion
In this nationwide propensity score-weighted cohort study, rivaroxaban was associated with higher rates of major bleeding compared with apixaban and dabigatran. Additionally, dabigatran users had twofold higher rates of MI compared with users of apixaban and rivaroxaban, although the former comparison included the possibility of a null effect. Importantly, the rates of stroke/SE were similar among the 3 drugs.
The similar rates of stroke/SE observed in this study are consistent with previous population-based studies from Denmark, the United Kingdom, and Chinese Taipei.7,8,21 However, it contrasts with a study from the USA which found apixaban to be associated with lower rates of ischemic stroke or SE compared with those of rivaroxaban.6 That study was based on the Optum database, which mostly includes privately insured patients, and may therefore be susceptible to selection bias.6 Given the comparable effectiveness of the 3 drugs, oral anticoagulant selection may be guided by the different bleeding and MI risks of the 3 drugs.
The twofold higher rates of MI compared with apixaban and rivaroxaban in the current study contrasts with previous registry studies from the USA, France, and Chinese Taipei that failed to demonstrate significantly different MI rates between dabigatran and rivaroxaban.21-23 The discrepancy between previous studies and this one might, at least partly, be explained by differences in study design. The study from the US was based on patients aged ≥65 years receiving health insurance through the Medicare insurance coverage and only assessed comorbid conditions using relevant ICD-9 codes within 6 months of cohort entry.22 This is likely to lead to missed diagnoses of important baseline covariates. The study from France was limited to the first year after marketing, which, owing to the increased usage of DOACs in recent years, is unlikely to be representative of today's DOAC population.23 Finally, the study from Chinese Taipei was based on a population that was likely at reduced risk of MI compared with that of this study. In that study, only 3% of rivaroxaban and dabigatran users had prior history of MI and 41% to 45% were receiving concomitant antiplatelet treatment. For comparison, 7.6% to 8.1% of rivaroxaban and dabigatran users had history of previous MI in this study and 22% to 27% of patients were receiving concomitant antiplatelet treatment. Therefore, MI rates were, perhaps unsurprisingly, twice as common in this study compared with the one from Chinese Taipei. However, these differences in incidence rates are unlikely to be only because of differences in the study populations. Given that our searching algorithm identified 69% more MI events compared with using ICD-10 codes alone (61 events vs 36 events), previous observational studies have likely suffered from low sensitivity.
A similar discrepancy has been observed between RCTs and observational studies examining MI rates between dabigatran and warfarin. Although a meta-analysis of 14 RCTs demonstrated that dabigatran was associated with higher MI rates compared with warfarin,24 a second meta-analysis limited to observational studies failed to observe any difference in MI rates between the 2 drugs.25 The increased MI risk of dabigatran has been hypothesized to be because of platelet activation. It has been shown that dabigatran increases thromboxane excretion, a marker of platelet activation whereas warfarin usage does not affect thromboxane excretion.26 Similarly, 3 studies have demonstrated increased platelet aggregation in patients after dabigatran initiation but not in rivaroxaban or warfarin users.27-29 These results were replicated using human atherosclerotic plaque homogenates and a mouse arterial injury model using intravital microscopy.29
The higher rates of major bleeding observed for rivaroxaban compared with other DOACs in this study is generally consistent with previous observational studies comparing rates of bleeding events requiring hospitalization.5-10 Although a registry study from Denmark found no difference in bleeding rates among the 3 drugs,8 rivaroxaban has been associated with higher bleeding rates compared with apixaban and/or dabigatran in previous studies from the USA and United Kingdom.5-7,10 The higher bleeding rates of rivaroxaban has been suggested to be because of the different pharmacokinetics of the drugs,6,16 but rivaroxaban is administered once daily whereas apixaban and dabigatran are both administered twice daily. This may cause a higher peak plasma concentration making these patients more susceptible to bleeding. Indeed, previous studies have demonstrated that rivaroxaban has higher peak plasma concentration, higher maximal anti-Xa activity, and higher 24-hour area under the curve for anti-Xa activity compared with apixaban.30,31
This study has several strengths. A centralized national drug prescription database was used that allowed for identification of all DOAC users in the country. Furthermore, study outcomes were gathered from all the major hospitals in Iceland, using a robust searching algorithm that, in addition to the traditional method of using ICD-10 codes, included reviewing diagnostic imaging studies and endoscopic procedures undertaken during the study period, and searching the Icelandic death registry. This study also used the ISTH definition for major bleeding similar to the pivotal RCTs but opposed to most observational studies that have defined major bleeding as a bleeding that leads to hospitalization. In addition, all outcomes were manually verified, greatly increasing the accuracy of the data. In comparison, previous studies have identified outcome events using only a few specific ICD-10 primary discharge codes and without manual verification of events.14,18,19 Although this method is highly specific, the sensitivity was only 52.3% for identification of stroke/SE and 30.4% for identification of major bleeding compared with our searching algorithm. In addition, the PPV was suboptimal for this method, that is, 50.0% for stroke/SE and 79.5% for major bleeding. The low PPV for major bleeding was mostly explained by the fact that a considerable proportion of bleeding events that required hospitalization did not fulfil the ISTH criteria for major bleeding. Meanwhile, the low PPV for stroke/SE was mostly explained by the fact that patients were often issued ICD-10 codes for stroke/SE events that had occurred before the start of the patient's follow-up, ie, a stroke event that led to the patient starting DOAC treatment. This underlines the importance of manual verification of study outcomes and suggests that the results of previous observational studies may be unreliable.
The study also has several limitations. First, although a robust IPW model was used to account for indication bias, it cannot be excluded that some unmeasured confounding exists. For example, this study did not account for socioeconomic or lifestyle variables, such as smoking, alcohol consumption, or obesity. However, because all DOACs in Iceland have equivalent prices and are reimbursed through a universal health insurance, the risk of bias owing to socioeconomic status is unlikely. Second, the study did not have information on baseline laboratory values such as hemoglobin or creatinine values. It did, however, account for prior history of bleeding and diagnosis of chronic kidney disease. Third, data on over-the-counter medication usage were not available. This is important because over-the-counter usage of both nonsteroidal anti-inflammatory drugs and proton pump inhibitors is relatively common in Iceland. However, there was no difference between the groups in concomitant drug prescriptions of nonsteroidal anti-inflammatory drugs or of proton pump inhibitors before or after IPW, making potential differences in over-the-counter consumption less likely. Lastly, the dabigatran group was relatively small compared with previous registry studies.
In conclusion, rates of stroke/SE were similar among DOACs. Rivaroxaban was associated with higher rates of major bleeding compared with other DOACs, but lower rates of MI compared with dabigatran. These results may help guide oral anticoagulant selection, especially in patients at high risk of bleeding or MI.
Acknowledgments
This study was funded by the Landspitali University Hospital Research Fund (A-2018-012 to E.S.B.) and by the Icelandic Centre for Research (207113-051 to A.B.I.). The funding source had no role in the design, conduct, or reporting of the study.
Authorship
Contributions: A.B.I., E.S.B., J.P.H., and P.T.O. designed the study; A.B.I. and E.S.B. were involved in obtaining funding for the study; A.B.I., A.S.A., E.R., D.A.P., I.E.R., and B.R.G. performed the data acquisition; A.B.I. performed the statistical analysis and visualized the data with help from J.P.H. and S.H.L.; A.B.I. wrote the first draft of the manuscript; All other coauthors critically reviewed the manuscript, had access to the primary data, and approved the final manuscript before submission.
Conflicts of interest: P.T.O. and B.R.G. together with the Landspitali University Hospital and the University of Iceland hold a patent for the FIIX-prothrombin measurement. All other authors declare no competing interests.
Correspondence: Einar S. Bjornsson, MD, PhD, Professor of Gastroenterology and Hepatology, University of Iceland, Faculty of Medicine, Chief Physician, Landspitali University Hospital, Department of Gastroenterology and Hepatology; e-mail: einarsb@landspitali.is.
References
Author notes
Study protocol and statistical code are available from E.S.B. through email requests (einarsb@landspitali.is). Data set is available by license through the National Bioethics Committee of Iceland (vsn@vsn.is).
The full-text version of this article contains a data supplement.