TO THE EDITOR:
In 2014, the first targeted therapy for chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), ibrutinib, was approved by the Food and Drug Administration. Targeted therapies, such as inhibitors of both Bruton’s tyrosine kinase and BCL2, have shown superior efficacy when compared with chemoimmunotherapy in the treatment-naive setting.1-4 However, when treatments are implemented in the real world, inequalities and increased comorbidities in real-world patients can hinder improvements in survival observed in clinical trials.5 Specifically, a barrier to the use of these treatments is cost, with monthly costs estimated to be >$10 000.6,7 This high expense may disproportionately affect patients who are in minority and have a lower neighborhood socioeconomic status (nSES).8,9 Here, we performed an analysis of 2 separate Surveillance, Epidemiology, and End Results Program (SEER) data sets to assess the prognostic impact of race and nSES for patients with CLL/SLL.
CLL/SLL cases reported to 17 SEER Program registries from 2006 to 2019 were identified through SEER∗Stat case listing sessions. Two separate but overlapping data sets were analyzed. These 2 data sets could not be linked and thus were analyzed separately. Cohort 1 includes a SEER data set from 2009 to 2019. Patient characteristics such as age at diagnosis, sex, year of diagnosis, race, ethnicity, and prior malignancy to CLL/SLL diagnosis were examined. Information on ethnicity, as represented by Hispanic vs not Hispanic, was collected independently of race. Cohort 2 includes a SEER data set from 2006 to 2016. In addition to variables in cohort 1 (except prior malignancy), nSES was included in cohort 2 as represented by the Yost index (a composite measure of 7 variables assessing different aspects of the socioeconomic status of a census tract),10 and rural/urban residence as represented by the rural-urban commuting area. Patient characteristics were presented with descriptive statistics. Overall survival (OS) was calculated from the date of CLL/SLL diagnosis to the date of death; patients who were alive were censored at the date of last follow-up. OS was estimated using the Kaplan-Meier method. Cox proportional hazards models estimated the association between OS and patient characteristics, wherein univariable models were first fit for each variable, then a full multivariable model was fit including all variables in univariable analyses (UVAs). Complete case analysis was used in the modeling analysis without imputing missing data. SAS software version 9.4 was used for statistical analysis. All tests were two-sided, and statistical significance was declared to be α=0.05.
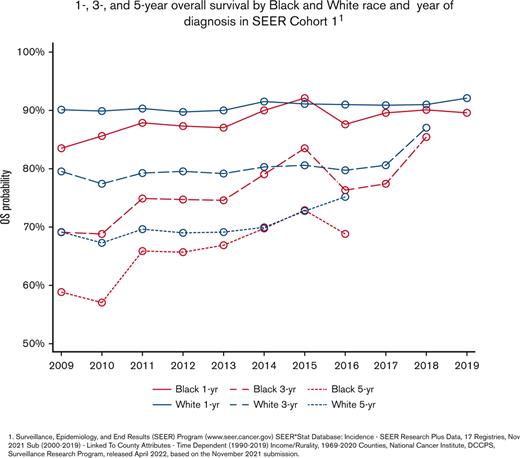
In cohort 1, 57 783 cases of CLL/SLL from 2009 to 2019 were identified. The median age was 70 years (range, 18 to ≥100), and 60.3% of patients were men. A median of 5379 patients was diagnosed with CLL/SLL per year (range, 4100-5620). In addition, 89.8% of the patients were White, 7.3% were Black, 2.6% were Asian/Pacific Islander, and 0.4% were American Indian/Alaska Native; 5.8% of patients identified as Hispanic; and 21.2% of patients had malignancy before receiving a diagnosis of CLL/SLL. Of 57 212 patients with follow-up data, after a median follow-up of 4.2 years (range, 0-10.9 years), the median OS was 9.8 years (95% CI, 9.5-10). The 1-, 3-, and 5-year OS was 90.6%, 79.8%, and 70.0%, respectively (Table 1). In cohort 1, multivariable analysis (MVA) showed that increased age, male sex, diagnosis before 2014, Black race, and malignancy before CLL/SLL were independently associated with a shorter OS. Hispanic ethnicity was not significant in UVA but was associated with inferior OS after accounting for other factors (Table 2). Over time, Black race was consistently associated with poorer OS than White race, and the disparity was maintained when evaluating patients diagnosed after 2014 (Figure 1). The median age of diagnosis for Black patients was significantly younger than White patients, with 67 years (range, 22-100) and 70 years (range, 18-100) (P<.0001), respectively.
Overall survival of patients with CLL/SLL by race. (A) 1-, 3-, and 5-year OS by Black and White race and year of diagnosis in SEER cohort 1. (B) 5-year OS by Black and White race diagnosed before 2014. (C) 5-year OS by Black and White race diagnosed after 2014. 1. SEER Program (www.seer.cancer.gov) SEER∗Stat Database: Incidence-SEER Research Plus Data, 17 Registries, Nov 2021 Sub (2000-2019)-Linked To County Attributes-Time Dependent (1990-2019) Income/Rurality, 1969-2020 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2022, based on the November 2021 submission.
Overall survival of patients with CLL/SLL by race. (A) 1-, 3-, and 5-year OS by Black and White race and year of diagnosis in SEER cohort 1. (B) 5-year OS by Black and White race diagnosed before 2014. (C) 5-year OS by Black and White race diagnosed after 2014. 1. SEER Program (www.seer.cancer.gov) SEER∗Stat Database: Incidence-SEER Research Plus Data, 17 Registries, Nov 2021 Sub (2000-2019)-Linked To County Attributes-Time Dependent (1990-2019) Income/Rurality, 1969-2020 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2022, based on the November 2021 submission.
In cohort 2, we identified 47 867 cases of CLL/SLL from 2006 to 2016. Median age and OS, as well as sex, race, and ethnicity distributions were similar to cohort 1. In addition, 24.8%, 33.8%, and 41.4% of patients were categorized as Yost tertile 1, 2, and 3, respectively, where higher Yost tertile was associated with higher nSES. Furthermore, 8.9% of patients were categorized as rural vs 91.1% as urban per rural-urban commuting area (Table 1). Of 47 867 patients, 47 277 patients with follow-up data were analyzed. In cohort 2, MVA showed that increased age, male sex, diagnosis of CLL before 2014, Black race, and lower nSES were independently associated with a shorter OS. Here, Hispanic ethnicity and American Indian/Alaska Native race became significantly associated with inferior OS after accounting for other factors. Rural categorization was significant in UVA but not MVA (Table 2).
In this analysis, we show that Black race is an independent prognostic variable for poor OS for patients with CLL/SLL. In a prior analysis of SEER data that extended through 2009, non-White race and lower nSES (based on a county-level measurement of percent living below poverty) were associated with worse OS in patients with CLL/SLL.11 This finding had been recapitulated in prognostic modeling of a longitudinal study that enrolled patients between 2010 and 2014, in which risk of death increased in non-White patients.12 In this study, data up to 2019 were included, representing treatment of CLL/SLL at a time when modern therapies were readily available. We included the variable “diagnosis after 2014” to account for a time period that included modern therapies, and found this variable is associated with improved OS despite increased age; however, Black race and Hispanic ethnicity continued to be independently associated with worse OS. This analysis showed that racial disparities continue to be prevalent in CLL/SLL even when targeted agents were approved.
One unanswered question is whether Black patients develop a more aggressive CLL/SLL biology, which would explain the observed disparities. In line with this hypothesis, Black patients are diagnosed with CLL/SLL at an earlier age13 and may have poor prognostic features at presentation.14 However, in a single-center study performed before ibrutinib was approved, when patients were treated similarly, equal outcomes in White vs non-White patients were observed.15 Our study continues to show that Black patients are diagnosed younger than White patients reaffirming that Black patients may have different disease biology than their White counterparts.
The prevalence of these disparities before the approval of ibrutinib along with the high cost of new therapies put patients in minority and with lower socioeconomic status at high risk for continuing disparities.8,9 It is unclear if patients from different races are benefiting equally from the progress seen in CLL/SLL treatment. Here, we found that lower nSES, represented by lower Yost index, is an independent prognostic variable for worse OS. In this second analysis, Black race continued to be an independent variable despite accounting for nSES. This cohort only had data up to 2016, which makes conclusions regarding nSES in the era of targeted therapy less reliable.
This study is significant in that it identifies racial disparities in this modern era of CLL/SLL therapy. However, identification is only the first step, and further research is necessary to determine the root cause of these disparities with the goal of developing interventions to eliminate disparities. This will require more granular data than are available within public databases, which limits the ability to control for comorbidities and specific treatments. Further research is needed to determine whether racial disparities in CLL/SLL are due to differences in access to therapy, quality of care, social determinants of health, or disease biology. This research is critical to developing interventions to ensure that progress in CLL/SLL therapy benefits all patients.
Acknowledgments: A.S.K. is a recipient of the Conquer Cancer, the ASCO Foundation, Career Development Award. K.A.R. and J.A.W. are Scholars in Clinical Research of The Leukemia & Lymphoma Society.
Contribution: J.L.F. collected data; A.S.K., Y.H., J.L.F., E.D.P., and J.A.W. interpreted the data and were involved in the conception and design of the study; A.S.K., Y.H., J.L.F., and J.A.W. wrote the manuscript; and all authors analyzed the data, edited and revised the manuscript, provided critical intellectual content, and approved the revised manuscript.
Conflict-of-interest disclosure: A.S.K. receives research funding from AstraZeneca and has consulted for AbbVie, AstraZeneca, BeiGene, and Janssen. S.A.B. receives research funding from AstraZeneca; has consulted for Pharmacyclics, Janssen, BeiGene, and AstraZeneca; has received honorarium from OncLive; and has received travel funding from Arqule. E.D.P. is an MPI on grants to the university from Pfizer, Merck Foundation, and Genentech, outside the submitted work. K.A.R. receives research funding from AbbVie, Genentech, Novartis, and Janssen; has consulted for Genentech, AbbVie, Pharmacyclics, Innate Pharma, AstraZeneca, and BeiGene; and has received travel funding from AstraZeneca. J.C.B. has received honoraria from Janssen; consulted for AstraZeneca, Pharmacyclics/AbbVie, BeiGene, MEI, and BMS; and has received research funding from Oncternal, AstraZeneca, and Velosbio/Merck. J.A.W. receives research funding from AbbVie, Janssen, Pharmacyclics, Karyopharm, Loxo, Morphosys, and Schrodinger and has consulted for AbbVie, AstraZeneca, BeiGene, Genentech, Janssen, Loxo, Pharmacyclics, and Newave. The remaining authors declare no competing financial interests.
Correspondence: Adam S. Kittai, 1140D Lincoln Tower 1800 Cannon Dr, Columbus, OH 43210; e-mail: adam.kittai@osumc.edu.
References
Author notes
∗J.L.F. and J.A.W. contributed equally to this work.
Data are available on request from the corresponding author, Adam S. Kittai (adam.kittai@osumc.edu).