Key Points
Patients without detectable lymphoma at the time of CD19 CAR T-cell therapy had excellent outcomes.
Administering CD19 CAR T cells to patients without detectable disease is safe, feasible, and warrants further exploration.
Abstract
CD19 chimeric antigen receptor (CAR) T-cell therapy represents a breakthrough for patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL), inducing sustained remissions in these patients. However, CAR T cells can result in significant toxicities. Preinfusion disease burden is associated with toxicities and outcomes after CAR T-cell therapy. We identified 33 patients with R/R DLBCL treated at 8 academic centers who had no detectable disease at the time of CAR T-cell therapy. The median time from leukapheresis to CAR T-cell infusion was 48 (19-193) days. Nine patients received axicabtagene ciloleucel, and 24 received tisagenlecleucel. There was no severe (grade ≥3) cytokine release syndrome, and only 1 patient developed severe neurotoxicity (grade 4). After a median follow-up of 16 months, 13 patients relapsed (39.4%) and 6 died (18.1%). One-year event-free survival and overall survival were 59.6% and 81.3%, respectively. Our findings suggest that, in patients with R/R DLBCL who have an indication for CAR T-cell therapy, treating patients in complete remission at the time of infusion is feasible, safe, and associated with favorable disease control. Further exploration in a larger clinical trial setting is warranted.
Introduction
CD19 chimeric antigen receptor (CAR) T-cell therapy has revolutionized the treatment of relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL). Data from pivotal trials and real-world practice demonstrate excellent efficacy in these difficult-to-treat patients.1-4 However, CAR T cells can have unique toxicities, including cytokine release syndrome (CRS), immune effector–cell associated neurotoxicity syndrome (ICANS), and prolonged cytopenia. Data suggest that pretreatment disease burden is associated with toxicities and outcomes after CAR T-cell therapy.5-7 However, because CAR T-cell kinetics are partly influenced by the engagement between CAR and its target antigen, pretreatment disease burden may be a prerequisite for CAR T-cell expansion, which could influence outcomes.8 Recently, Bishop et al9 reported outcomes of 7 patients with no residual disease at the time of tisagenlecleucel (tisa-cel) infusion. Five patients (71%) remained in remission at 1 year after treatment. The peak CAR T-cell expansion and toxicities in these patients were comparable to the original JULIET cohort. However, the study was limited by a small number of patients and restricted to the use of tisa-cel. Here, we report real-world data focusing on 33 patients with R/R DLBCL with no residual diseases at the time of CAR T-cell infusion to provide additional insights in this setting.
Methods
This retrospective multicenter analysis includes adults aged ≥18 years with DLBCL undergoing apheresis for commercial CAR T-cell therapy at 8 US academic centers that offer either axicabtagene ciloleucel (axi-cel) or tisa-cel.10 Data collection occurred from 1 May 2018 through 30 June 2021. Baseline data, details on CAR T-cell treatment, toxicity, response, and outcomes were recorded in a centralized research electronic data capture (REDCap) database. The study was approved by the individual member institutional review boards and ethics committee. The study was performed in accordance with the Declaration of Helsinki.
Preinfusion disease status from positron emission tomography-computed tomography (PET-CT) was interpreted using the 5-point scale Deauville score according to the 2014 revised International Working Group response criteria for malignant lymphoma.11 Complete remission (CR) without detectable disease was defined by the resolution of all FDG-avid lesions consistent with a complete metabolic response on the PET-CT scan (Deauville score 1-3) without evidence of lymphomatous involvement by other clinically indicated studies, including brain magnetic resonance imaging study, bone marrow pathology, or cerebrospinal fluid if available. CAR T cells were administered in accordance with the standard package insert of each product. CRS and ICANS were graded using the American Society of Transplantation and Cellular Therapy consensus criteria.12 Event-free survival (EFS) was defined as the time from CAR T-cell infusion to relapse/progression, the initiation of new antilymphoma treatment, death, or last follow-up. Overall survival (OS) was defined as time from infusion until last follow-up or death. Relapse incidence was the time from CAR T-cell infusion to lymphoma relapse, with nonlymphoma–related death being a competing event. Baseline demographic and clinical variables were compared across patients with and without CR pre-CAR T-cell therapy using Wilcoxon rank sum tests for continuous variables and Fisher exact tests for categorical variables. OS and EFS were analyzed using Kaplan-Meier methodology. Differences in survival based on disease status pre-CAR T-cell therapy were assessed using log-rank tests. All statistical analyses were performed using R statistical software version 4.1.1.
Results
Of the 363 patients receiving CD19 CAR T cells during the study period, 33 patients received CD19 CAR T-cell therapy when their disease status was in CR without evidence of residual disease (9 axi-cel and 24 tisa-cel). Baseline characteristics and details of 33 patients with no detectable disease at CAR T-cell therapy are described in Table 1. The median age was 63.8 years (interquartile range [IQR], 58-70 years). Indications for CAR T-cell therapy included 23 with R/R de novo DLBCL, 5 with high-grade B-cell lymphoma, and 6 with transformed DLBCL.
Of 33 patients, 26 (78.8%) had measurable disease at leukapheresis and achieved a CR after bridging therapy (systemic therapy; n = 20, radiotherapy; n = 4 and corticosteroids; n = 2). The remaining 7 patients who were in CR before leukapheresis were deemed to have high-risk disease by their treating physician (including 5 heavily treated patients, 1 post-ASCT relapse, and 1 ASCT ineligible). The details of bridging therapy are described in the supplemental Material. The median time from leukapheresis to CAR T-cell infusion was 48 days (IQR, 37-56 days). The median time from preinfusion PET-CT to CAR T-cell infusion was 14 days (IQR, 10-29 days). Lymphodepletion chemotherapy was given according to the specific CAR T product’s package insert. Of the 24 patients receiving tisa-cel, bendamustine was given to 15 patients, whereas the other 9 tisa-cel recipients, and all axi-cel–treated patients received fludarabine and cyclophosphamide. Compared with patients with detectable disease, patients with no residual disease at the time of infusion had a lower proportion of primary refractory disease and had a lower median pretreatment lactate dehydrogenase (Table 1).
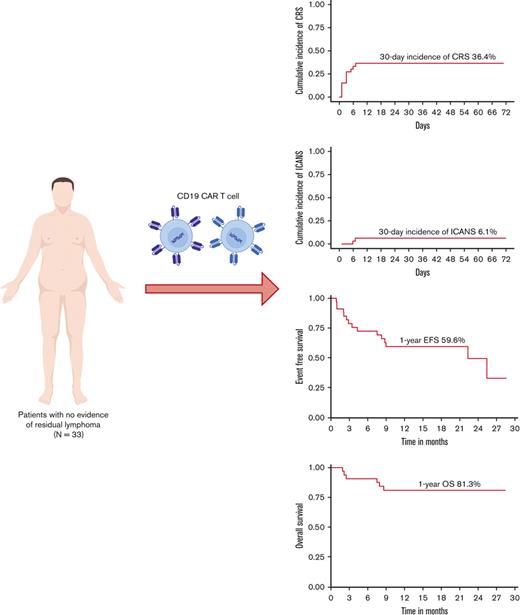
CRS was observed in 12 patients (36.3%) (5 of 9 axi-cel, 7 of 24 tisa-cel) with a median onset of 3 days (range, 0-7 days) from the day of infusion (supplemental Material). There were no grade ≥3 CRS events. Four patients received tocilizumab, and 2 received standard-dose corticosteroids (≤40 mg/d dexamethasone or equivalent) for the treatment of CRS. Two patients (6.1%) developed ICANS (grade 1; n = 1 and grade 4; n = 1, both of whom received axi-cel) requiring corticosteroids. The occurrence of CRS and ICANS was significantly lower in patients with no residual lymphoma at the time of CAR T-cell therapy (Table 1). It should be noted that the use of tisa-cel was significantly higher in patients with no residual disease compared with patients with disease (72.7% vs 40.2%). This may in part explain the observed differences in CRS and ICANS, as tisa-cel is associated with lower rates of CAR T-cell therapy toxicity than axi-cel, which was more commonly used in the cohort of patients with residual disease.10
At day 100, 26 patients (78.8%) remained in remission (3 relapsed within the first 30 days). There was no treatment-related mortality within the first 100 days. The incidence of prolonged cytopenias (>28 days) grade 3 or higher was low (grade ≥3 neutropenia in 3 patients and grade ≥3 thrombocytopenia in 4 patients), with only 1 patient (3%) experiencing grade 4 neutropenia and 2 patients (6%) having grade 4 thrombocytopenia (requiring a thrombopoietin receptor agonist). Details on bridging treatment, inflammatory markers, and hematological parameters are provided in the supplemental Material.
At the time of data cutoff, with the median follow-up of 16 months, 13 patients (39.3%) relapsed; the corresponding 1-year relapse incidence was 37.3% (95% confidence interval [CI], 20.1-54.3) (4 DLBCL, 1 high-grade B-cell lymphoma). Of the 13 patients who relapsed after CAR T-cell therapy, CD19 status at the time of relapse was available in 8 patients, including 6 with a CD19+ relapse. Six patients (18.1%) died as of last follow-up; causes of death included progressive disease in 5 patients and therapy-related myelodysplastic syndrome in 1 patient. The 1-year EFS and OS of 33 patients without residual disease at the time of infusion were 59.6% (95% CI, 44.7-79.3) and 81.3% (95% CI, 68.8-96.0), which were significantly higher than those with detectable disease at CAR T-cell infusion (Figure 1).
Survival outcomes after CD19 CAR T-cell therapy in patients with and without evidence of disease at the time of infusion. (A) EFS after CAR T-cell therapy. (B) OS after CAR T-cell therapy.
Survival outcomes after CD19 CAR T-cell therapy in patients with and without evidence of disease at the time of infusion. (A) EFS after CAR T-cell therapy. (B) OS after CAR T-cell therapy.
Discussion
Our study highlights the excellent safety and outcomes using CAR T cells in patients without detectable disease at the time of infusion and supports the role of disease burden on CAR T-cell–related toxicities and outcomes. The survival in these 33 patients appeared superior to that in patients with persistent disease at the time of CAR T-cell infusion (Figure 1). However, our results also suggest that having a significant disease burden at infusion is not a prerequisite for CAR T-cell function. Similar to the cohort of 7 patients without measurable disease from the JULIET study, the incidences of CRS and ICANS in our study were low. Although not a matched controlled comparison, our study demonstrated better safety and outcomes in patients with no detectable disease compared with those with residual disease at the time of CD19 CAR T-cell infusion.
Although the number of patients in our report is relatively small, this is the largest study to date and represents real-world practice. Furthermore, our study is important to the cell therapy community as it includes both tisa-cel and axi-cel. CRS and ICANS were uniformly graded using the American Society of Transplantation and Cellular Therapy consensus criteria, allowing comparisons across products. A major limitation of the study is the retrospective design. Other limitations include the lack of a central radiology review and the absence of data on postinfusion CAR T-cell kinetics and persistence. A potential concern about treating patients in CR is the absence of antigenic stimulus to drive CAR T-cell expansion. However, the fact that we did observe CRS and ICANS suggests that there was an expansion of CAR T cells. This could be driven in part by the presence of residual normal B cells. Furthermore, although preclinical data shows that optimal expansion of T cells in the lymphopenic state is driven by recognition of specific antigens, T cells also expand in part through homeostatic proliferation.13 The results of our study are also not applicable to other hematological malignancies or CAR T-cell products. Finally, data are lacking on outcomes in patients who achieved a CR and did not receive CAR T cells.
The excellent safety of CAR T-cell therapy in our cohort bolsters the feasibility of CAR T-cell therapy in the ambulatory setting,14,15 particularly in patients with low disease burden. Moreover, 1-year EFS and OS from our cohort appear promising, with survival rates comparing favorably to the results of the pivotal studies.1,2,16,17 The 3 patients who relapsed early following CAR T-cell infusion may be explained by intrinsic CAR T-cell deficits or other host-related factors and deserve further exploration. Along with other reports, our study highlights that pretreatment disease burden can significantly influence the safety and efficacy profile of CAR T cells. Recently, a post hoc analysis of the BELINDA study showed that patients who achieved a CR or PR before tisa-cel had a better response rate and a superior EFS.18 These results, however, also need to be seen in the context of an overall negative trial, likely due in part to an extended vein-to-vein time.19
Although pivotal trials included very specific recommendations on bridging therapy, real-world data show that clinicians are increasingly using bridging therapy in patients, typically chemoimmunotherapy, including in recipients of axi-cel. In addition, some of the patients in our cohort received nonchemotherapy–based bridging therapy. Novel agents, including small molecule inhibitors or antibody drug conjugates, may provide additional bridging options in the real-world setting compared with pivotal clinical trials. A potential benefit of bridging is a reduction in tumor burden, which may translate into higher efficacy and lower toxicity. However, patients may also develop complications related to bridging therapy, which can result in treatment delays or even the inability to proceed to CAR T-cell therapy, or may progress on bridging therapy with resultant increased tumor burden. The interpretation of data on bridging therapy may also be subject to selection biases. Patients without residual disease at the time of infusion may represent a biologically distinct group of patients with a high probability of doing well after treatment compared with patients with a higher disease burden. The lower baseline disease burden may also offer an ideal effector to target ratio compared with patients with higher disease burden. In contrast, patients who require bridging may have a highly proliferative disease that is less likely to respond to CAR T cells. In sum, the exact role and/or benefit of bridging therapy, as well as the optimal bridging therapy, remain open questions.
Although we report a relatively small cohort, our results with CAR T therapy in patients in CR compare favorably to outcomes in patients with R/R lymphoma who underwent ASCT after achieving a CR with salvage therapy.20,21 CAR T cells may be able to eradicate chemorefractory subclones at the minimal residual level that might not be eradicated by high-dose therapy given for ASCT.
Finally, our study supports further exploration of CAR T-cell therapy in patients with no measurable disease in a prospective trial to better understand the impact of CAR T cells as a consolidation therapy.
Acknowledgments
The authors thank the data managers at each Cell Therapy Consortium member institution who collected patient data and maintained the database.
This research was supported in part by a grant from Novartis Pharmaceuticals, Inc. (D.L.P.), National Institutes of Health, National Cancer Institute grant P30 CA008748 (M.-A.P.), and the National Center for Advancing Translational Sciences of the National Institutes of Health Award UL1-TR002494 (V.B.).
Authorship
Contribution: K.W. and M.-A.P. proposed the study concept, performed the analysis, and drafted the manuscript; A.A.T. and J.B. created the data collection form, abstracted, and recorded data; A.A.T., V.B., L.J.N., J.P.M., R.T.M., O.O.O., S.J.S., D.L.P., and M.R.B. provided extensive input and helped edit the manuscript; P.A.R. supervised the data collection, data abstraction, provided extensive input, and edited the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: V.B. reports research funding from Incyte, Bristol Myers Squibb (BMS), Gamide Cell, Incyte, Citius Pharmaceuticals, and FATE Therapeutics; and consultancy at Incyte, Karyopharma, ADC, BMS, Gamida Cell, FATE Therapeutics, Kite; and DSMB membership at Miltenyi Biotec. L.J.N. reports honoraria from ADC Therapeutics, BMS, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, Janssen, MorphoSys, Novartis, and Takeda, and research support from BMS, Caribou Biosciences, Epizyme, Genentech, Genmab, Gilead/Kite, Janssen, IGM Biosciences, Novartis, and Takeda. J.P.M. serves on the advisory boards and receives funding from Novartis, Kite/Gilead, BMS, AlloVir, and Caribou. R.T.M. is an adviser or consultant for AlloVir, CRISPR Therapeutics, Incyte, and Novartis; reports honoraria from Incyte; research support from AlloVir and Novartis; participation in a data and safety monitoring board for Athersys, Vor Pharma, and Novartis; reports a patent with Athersys Inc.; and serves on scientific advisory board of Artiva and Lyrik Therapeutics. O.O.O. reports consultancy and advisory board for Pfizer, Kite, Gilead, AbbVie, Janssen, TGR Therapeutics, Novartis, curio science, and Nekktar; received institution funding from Kite, Pfizer, Daichi Sankyo, and AlloGene; and receives honoraria from Pfizer and Gilead. S.J.S. is an adviser or consultant for Acerta, AlloGene, AstraZeneca, BeiGene, Celgene (Juno), Genentech (Roche), LoxoOncology, Novartis, and Tessa Therapeutics; reports honoraria from Acerta, AlloGene, AstraZeneca, BeiGene, Celgene, Genentech (Roche), LoxoOncology, Novartis, Nordic Nanovector, Pfizer, and Tessa Therapeutics; reports steering committee participation for AbbVie, Celgene, Novartis, Juno, Nordic Nanovector, and Pfizer; reports research support from AbbVie, Acerta, Celgene (Juno), DTRM Bio, Genentech, Incyte, Merck, Novartis, Portola, and TG Therapeutics; and reports a patent with Novartis. D.L.P. reports honorarium for consulting or advisory board participation from Novartis, Kite/Gilead, Incyte, Gerson Lehrman Group, Janssen (Johnson & Johnson), Jazz, Adepcet Bio, DeCART, BMS, Bluebird Bio, and Kadmon, Angiocrine, and Mirror Biologics; research support from Novartis; patents and royalties related to CAR T-cell therapy for malignancies licensed to Novartis and Tmunity; and stock/equity in Genentech/Roche (spouse former employment). M.R.B. reports membership on an advisory board or consultancy for Kite/Gilead, Novartis, CRISPR Therapeutics, Autolus Therapeutics, BMS, Incyte, Sana Biotechnology, and Iovance Biotherapeutics, and has served on a speaker’s bureau for BMS, Kite/Gilead, Agios, and Incyte. P.A.R. has served as a consultant and/or advisory board member for AbbVie, Bayer, BeiGene, BMS, Janssen, Karyopharm, Kite/Gilead, Novartis, Nurix, Sana Biotechnology, and Takeda; served as a speaker for Kite Pharma; received honoraria from Novartis; and received research support from BMS, Calibr, CRISPR Therapeutics, FATE Therapeutics, Kite Pharma, MorphoSys, Novartis, Tessa Therapeutics, and Xencor. M.-A.P. reports honoraria from AbbVie, AlloVir, Astellas, BMS, Celgene, Equilium, Exevir, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Novartis, Nektar Therapeutics, Omeros, OrcaBio, Takeda, VectivBio AG, and Vor Biopharma; serves on DSMBs for Cidara Therapeutics, Medigene, Sellas Life Sciences, and Servier, and the scientific advisory board of NexImmune; has ownership interests in NexImmune and Omeros; and received institutional research support for clinical trials from Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. The remaining authors declare no competing financial interests.
A complete list of the members of the Cell Therapy Consortium Study Group appears in “Appendix.”
Correspondence: Miguel-Angel Perales, Department of Medicine, Adult Bone Marrow Transplant Service, Memorial Sloan Kettering Cancer Center, 530 East 74th St, Box 59, New York, NY 10021; e-mail: peralesm@mskcc.org.
Appendix
Cell Therapy Consortium Study Sites
| Study Site . | Principal Investigator . |
|---|---|
| Memorial Sloan Kettering Cancer Center (coordinating center) | Miguel-Angel Perales |
| Oregon Health Science University | Richard T. Maziarz |
| University of Chicago | Peter A. Riedell |
| University of Kansas | Joseph P. McGuirk |
| University of Minnesota | Veronika Bachanova |
| University of Pennsylvania∗ | David L. Porter |
| University of Texas MD Anderson Cancer Center | Loretta J. Nastoupil |
| Vanderbilt University | Olalekan O. Oluwole |
| Study Site . | Principal Investigator . |
|---|---|
| Memorial Sloan Kettering Cancer Center (coordinating center) | Miguel-Angel Perales |
| Oregon Health Science University | Richard T. Maziarz |
| University of Chicago | Peter A. Riedell |
| University of Kansas | Joseph P. McGuirk |
| University of Minnesota | Veronika Bachanova |
| University of Pennsylvania∗ | David L. Porter |
| University of Texas MD Anderson Cancer Center | Loretta J. Nastoupil |
| Vanderbilt University | Olalekan O. Oluwole |
Coordinating site.
References
Author notes
∗P.A.R. and M.-A.P. are joint senior authors.
This study was presented in part at the 64th annual meeting of the American Society of Hematology, December 2021.
Data are available on request from the corresponding author, Miguel-Angel Perales (peralesm@mskcc.org).
The full-text version of this article contains a data supplement.