Key Points
High-resolution typing at HLA-A, -B, -C, and -DRB1 should be standard practice when selecting units for double UCBT.
UCB units with a maximum of 3 allele mismatches should be selected for double UCBT adult candidates with hematologic malignancies.
Abstract
In single unrelated cord blood transplantation (UCBT), an increasing number of HLA allele mismatches (MM) has been associated with inferior overall survival (OS) and attributed to higher transplant-related mortality (TRM). Previous studies on the role of allele-level HLA matching after double UCBT (dUCBT) showed conflicting results. In this study, we report the impact of allele-level HLA matching on the outcomes of a large dUCBT cohort. We included 963 adults with hematologic malignancies, with available allele-level HLA matching at HLA-A, -B, -C, and -DRB1, receiving dUCBT between 2006 to 2019. Assignment of donor-recipient HLA match was performed considering the unit with the highest disparity with the recipient. Three hundred ninety-two patients received dUCBT with 0 to 3 MM and 571 with ≥4 allele MM. For recipients of dUCBT with 0 to 3 MM, day-100 and 4-year TRM were 10% and 23%, respectively, compared with 16% and 36% for those with ≥4 MM. A higher degree of allele MM was also associated with the worse neutrophil recovery and lower incidence of relapse; no significant effect on graft-versus-host disease was observed. Patients receiving units with 0 to 3 MM had a 4-year OS of 54% compared with 43% for those receiving units with ≥4 MM. The inferior OS associated with higher HLA disparity was only partially mitigated by increased total nucleated cell doses. Our results confirm that allele-level HLA typing is a significant factor for OS after dUCBT, and units with ≥4 MM (≤4/8 HLA-matched) should be avoided if possible.
Introduction
Cell dose is a critical determinant of the outcomes after unrelated cord blood transplantation (UCBT), especially in adults.1 Infusion of 2 unrelated cord blood (UCB) units, known as double-unit UCBT (dUCBT), is a way to circumvent the issue of low number of cells delivered by a single cord blood unit (CBU), allowing for an adequate cell dose even for adult patients with a high body weight with malignant and nonmalignant diseases.2 Previous studies report similar overall survival (OS) after single-unit UCBT (sUCBT) with adequate cell doses and dUCBT.3-5 Although human leukocyte antigen (HLA) matching is long known to affect survival after UCBT, most studies are based on antigen-level matching at HLA-A and HLA-B and allele-level matching at HLA-DRB1.6 In sUCBT, increasing numbers of allele-level HLA-mismatches (MM) have been associated with inferior OS which is attributed to higher transplant-related mortality (TRM). In addition, in one of the studies, when comparing the distribution of allele-level HLA-match with HLA matching at a lower resolution, historically used for CBU selection, a considerably higher number of MM was identified.7,8 These findings have prompted current guidelines to endorse high-resolution HLA typing in the selection of CBUs.9,10
Conversely, data on allele-level HLA typing after dUCBT is limited to a few single-center studies reporting conflicting results.11-13 A comprehensive appraisal of the effect of allele-level HLA matching was likely hindered by the relatively small sample sizes in these previous studies. Although only 1 CBU is engrafted in most dUCBT recipients (winning or dominant unit), the total nucleated cell (TNC) dose of the losing unit (or nondominant unit) has been shown to affect the engraftment of the winning unit.14 Similarly, as there may be long-term microchimerism, the allele-level HLA matching of the losing unit might also affect other outcomes, which is suggested by some studies showing a higher incidence of acute graft-versus-host disease (GVHD)3,4,15 and lower rate of relapse12,16,17 in dUCBT, in comparison with similarly HLA-matched sUCBT recipients. This is particularly relevant given the growing evidence in favor of other types of alternative donors18-20 and the emergence of modern but costly techniques of ex vivo expansion of UCB units.21 Therefore, in this study, we propose an analysis of the impact of allele-level HLA matching on the outcomes of a large cohort of dUCBT recipients with the objective of improving CBU selection and further exploring the unique dUCBT biology.
Methods
Data collection and patient selection
Eurocord/EBMT registry collects data on UCBT performed in Europe and other participating countries. Patients are followed longitudinally until death or until they are lost to follow-up. In this study, we included adults (≥18 years) who received a dUCBT as their first allogeneic transplant for any hematologic malignancy at EBMT centers between 2006 to 2019.
Only dUCBT that had either available HLA typing for HLA-A, -B, -C, and -DRB1 at the allele-level for the recipient and both units or had enough data allowing for the imputation of the CBU-recipient pair allele-level matching for the same loci were included. A validated high-resolution imputation tool, HaploStats, was used, as previously reported, to adjudicate allele-level match status for a subset of 430 CBU-recipient pairs lacking complete high-resolution typing.7 A sensitivity analysis, excluding imputed cases, for OS was performed, and HLA disparity was still an independent risk factor for survival (data not shown). The outcomes of a proportion of patients with HLA typing at high resolution included in this analysis have been previously reported by our group (N = 337).6 Patients provided informed consent for data entry into the Eurocord/EBMT database for observational studies. The study was performed in accordance with the Declaration of Helsinki. The institutional review board of Eurocord reviewed and approved this study.
Definitions and endpoints
Donor-recipient HLA matching at the allele-level was classified according to the CBU with the highest degree of HLA mismatch with the patient (eg, if a patient received a CBU with 2 allele MM and another with 4 allele MM, the graft would be considered as having 4 allele MM) and interunit matching was not considered.22 ABO matching was defined by the CBU with the highest ABO disparity (eg, if a patient with blood type O+ received an O+ and an A+ CBU, the graft would be considered as bearing major ABO incompatibility). Myeloablative conditioning regimen (MAC) was defined as a regimen containing either total body irradiation (TBI) with a dose of >6 Gy or a dose of busulfan administered orally at >8 mg/kg or intravenously at >6.4 mg/kg.23 Other conditioning regimens were defined as reduced intensity (RIC). Patients were classified as having low, intermediate, high, and very high-risk disease according to standard classification.24 Winning unit was defined as the cord unit representing >50% of the total marrow hematopoiesis by day 130 after transplant.25
The primary end point was OS. Secondary end points were relapse/progression, TRM, neutrophil engraftment, and acute and chronic GVHD. The cumulative incidence of neutrophil recovery was defined as the first of 3 consecutive days of an absolute neutrophil count (ANC) ≥0.5 × 109/L. The cumulative incidence of platelet recovery was defined as the first of 3 consecutive days of achieving platelets ≥20 × 109/L unsupported by platelet transfusions for at least 7 days. The diagnosis and grading of acute and chronic GVHD were performed according to standard criteria.26,27
Statistical methods
Survival curves were constructed using the Kaplan-Meier method, and the log-rank test was used to assess differences between curves. Pairwise comparisons of the numbers of allele MM showed that receiving units with ≥4 vs 0 to 3 allele HLA MM had the highest negative impact on OS (supplemental Figure 1) and was used for all the main analyses of the transplant outcomes. Survival or incidence estimates were reported with 95% confidence intervals (CI). For time-to-event end points with competing events, cumulative incidence curves were estimated using Kalbfleisch and Prentice method28 and compared using Gray’s test.29 The effect of covariates (age at transplant, gender, weight, disease type, leukemia type, disease risk, recipient-unit ABO match, recipient-unit cytomegalovirus match, recipient-unit gender match, interunit gender match, interunit ABO match, conditioning intensity, TBI dose, antithymocyte globulin (ATG) use, cryopreserved TNC, cryopreserved CD34+ cell dose, and year of transplant) on time-to-event end points were assessed by applying the Cox regression models or Fine-Gray competing-risk models. Covariates with P values <.20 in the univariate regression analyses were included in a multiple Cox regression analysis or multiple Fine-Gray regression model with the backward elimination method (P value <.05 to stay). The competing risk for relapse/progression was death without relapse and for TRM was relapse/progression. For acute and chronic GVHD, death without the event was considered a competing risk. Continuous covariates were dichotomized to find an optimal cutoff value using the method of Contal and O’Quigley,30 except for cryopreserved CD34 and total nucleated cell (TNC) count, whose cutoffs were based on previous findings reported by our group.6 HLA match at allele level was forced in all multiple regression final models. Proportions were compared between independent groups according to the Qui-square test or Fisher exact test with Monte Carlo (MC) simulation (with 10 000 MC replications).31 Non-normally distributed quantitative variables were compared by using Mann-Whitney tests and described as median with interquartile range. Categorical variables were described with counts and proportions. Two exploratory analyses were done, one to compare OS of patients with class I and II MM vs only class I MM in all patients and in those with ≥4 MM and another to compare the sum of allele MM of both CBU to the worst-matched CBU. In addition, the effect of HLA typing at high resolution on OS was tested in a subset of patients with available information on the winning unit (N = 476). Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC). All tests were 2-sided, and P values <.05 were considered significant.
Results
Patients, disease, and graft characteristics
A total of 963 patients met the eligibility criteria, of which 392 received dUCBT with 0 to 3 allele MM and 571 with ≥4 MM. Acute leukemia and myelodysplastic syndrome (MDS) accounted for 70% of the diagnoses. There was a larger proportion of patients receiving UCB units with fewer allele MM after 2012 (57% with up to 3 MM vs 49% with ≥4 MM; P = .01). Other patient-, disease-, and UCB unit–related characteristics are summarized in Table 1. Of those receiving dUCBT ≥4 allele MM, 18% had a maximum HLA disparity of 5 out of 6 at lower HLA-typing resolution, 72% of 4 out of 6, and 10% of ≤3 out of 6 (supplemental Table 1).
Hematologic recovery
Although no significant difference was seen between 0 to 3 MM vs ≥4 MM, day-60 cumulative incidences of neutrophil recovery for dUCBT with maximum HLA disparity of 0 to 2, 3, 4, and ≥5 MM were 95% (95% CI, 90-98), 89% (95% CI, 84-92), 87% (95% CI, 82-90), and 87% (95% CI, 83-91; P = .03), respectively. The percentages of nonengraftment in dUCBT recipients with 0 to 2, 3, 4, and ≥5 allele MM were 3%, 12%, 12%, and 11% (P = .02), respectively. Patients receiving CBUs with 3 or 4 allele MM had a lower likelihood of neutrophil recovery compared with those having 0 to 2 allele MM (Table 2).
TRM and relapse/progression
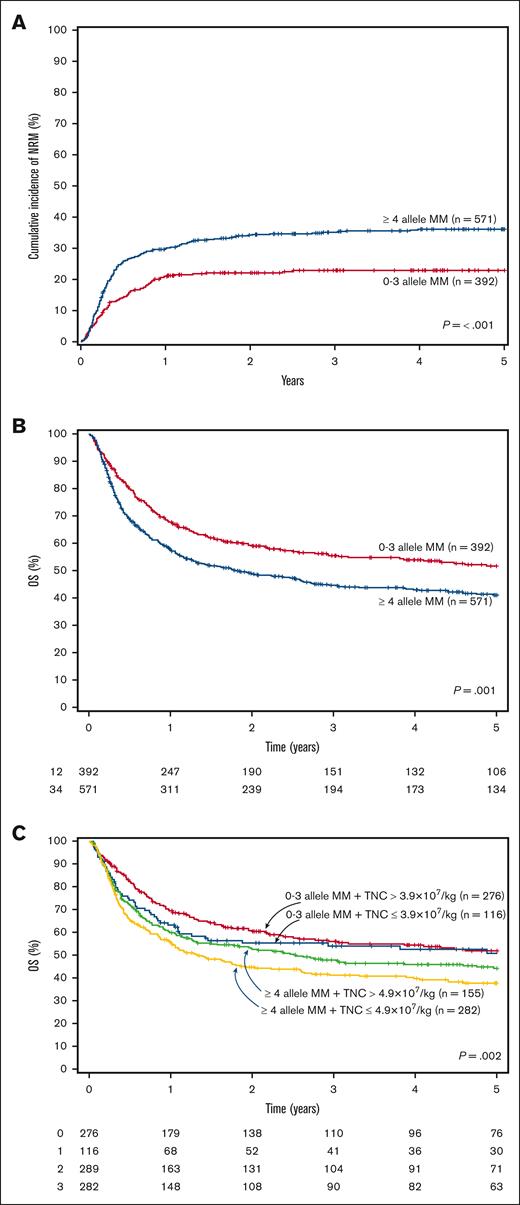
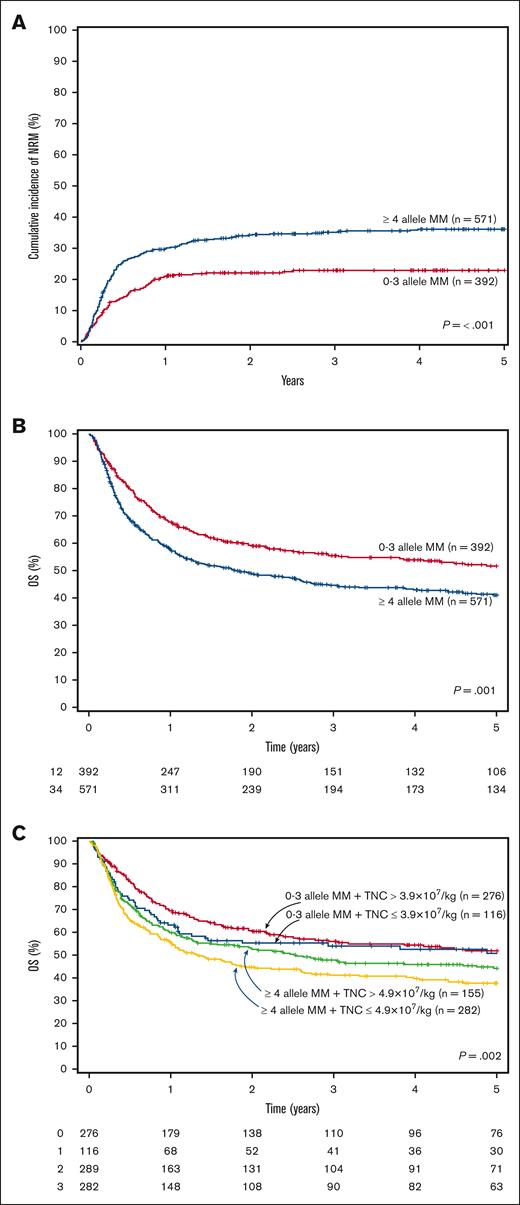
The cumulative incidence of TRM at 100 days was 16% (95% CI, 14-20) and at 4 years was 36% (95% CI, 32-40) for patients receiving dUCBT with ≥4 MM vs 10% (95% CI, 8-14) and 23% (95% CI, 19-28) for those with 0 to 3 MM (P < .001; Figure 1A). In multivariate analysis, having 4 or ≥5 MM was an independent risk factor for an increased 100-day cumulative incidence of TRM compared with those with up to 2 allele MM (Table 2). Double-unit UCBT recipients with ≥4 MM also had a significantly higher early and overall TRM than those with 0 to 3 allele MM (Table 2).
Transplant-related mortality and overall survival curves. (A) Cumulative incidence of TRM according to the number of allele HLA-mismatches, (B) Overall survival according to the number of allele HLA-mismatches, (C) Overall survival according to the number of allele HLA-mismatches and TNC count. TRM, transplant-related mortality; MM, mismatches; OS, overall survival; TNC, total nucleated cells.
Transplant-related mortality and overall survival curves. (A) Cumulative incidence of TRM according to the number of allele HLA-mismatches, (B) Overall survival according to the number of allele HLA-mismatches, (C) Overall survival according to the number of allele HLA-mismatches and TNC count. TRM, transplant-related mortality; MM, mismatches; OS, overall survival; TNC, total nucleated cells.
The 1 and 4-year cumulative incidences of relapse/progression were 21% (95% CI, 18-23) and 28% (95% CI, 25-31) for the whole cohort, respectively. Receiving dUCBT with ≥5 vs 0 to 2 allele MM was associated with a lower risk of relapse/progression (Table 2).
GVHD
The 100-day cumulative incidences of grade 2-4 and 3-4 acute GVHD were 40% (95% CI, 35-45) and 19% (95% CI, 16-23) in recipients of CBUs with ≥4 MM vs 40% (95% CI, 37-45; P = .75) and 14% (95% CI, 11-17; P = .03) in those with up to 3 MM, respectively. The 100-day cumulative incidences of grade 3-4 acute GVHD for dUCBT recipients with maximum HLA disparity of 0 to 2, 3, 4, and ≥5 MM were 7% (95% CI, 4-14), 16% (95% CI, 12-21), 17% (95% CI, 13-22), and 22% (95% CI, 17-27; P = .01). In multivariate analysis, patients receiving CBUs with ≥4 MM did not have a significantly higher risk of grade 2-4 (Table 2) and 3-4 acute GVHD (hazard ratio [HR] 1.27, 95% CI, 0.83-1.90; P = .26 after adjusting for TNC and ATG use) than those with up to 3 MM. There was a trend for higher risk of grade 3-4 acute GVHD in dUCBT recipients with ≥5 MM than those with 0 to 2 MM after adjusting for TNC and the use of ATG (HR 2.34, 95% CI, 1.00-5.49; P = .05).
The 4-year cumulative incidences of overall and extensive chronic GVHD were 35% (95% CI, 32-40) and 15% (95% CI, 13-18) for the whole cohort, respectively. Allele HLA disparity was not an independent risk factor for overall (Table 2) or extensive chronic GVHD (≥4 vs 0-3 allelic MM: HR 0.84, 95% CI, 0.59-1.21; P = .36; after adjusting for recipient-unit sex match).
Overall survival
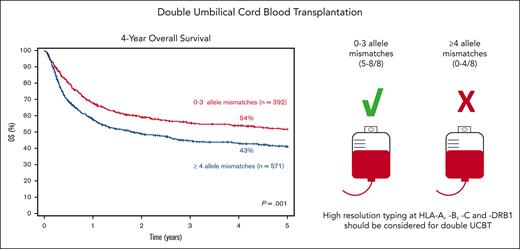
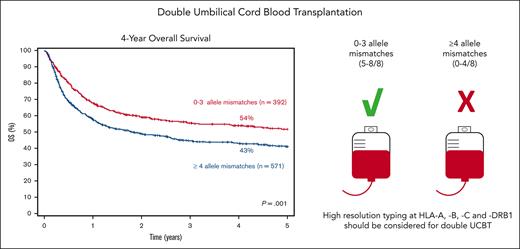
Patients receiving dUCBT with 0 to 3 allele MM had a 4-year survival of 54% (95% CI, 49-59) compared with 43% (95% CI, 39-47) for those with ≥4 allele MM (P = .001; Figure 1B). HLA allele-level matching was an independent risk factor for survival (≥ 4 vs 0-3 allele MM: HR, 1.32; 95% CI, 1.06-1.66; P = .015) TNC (Table 3). Consistent with these findings, in a subset analysis of patients with available data on chimerism (N = 476), patients whose winning CBU had ≥ 4 allele MM had a significantly inferior OS compared with those whose winning UCB unit had up to 3 allele MM (Table 3). Regardless of the number of HLA MM, having HLA class I + II MM vs only class I MM had no significant difference on OS (Table 3).
We also aimed to determine the combined effect of HLA match and TNC dose on OS. Cutoffs of TNC with the highest impact on OS were defined separately for dUCBT recipients with HLA disparity between 0 to 3 MM (TNC dose = 3.9 × 107/kg) and those with ≥4 MM (4.9 × 107/kg). The inferior OS associated with higher allele HLA disparity was only partially mitigated by increasing the TNC count in multivariate analysis (0-3 MM + TNC ≤ 3.9 × 107/kg vs 0-3 MM + TNC > 3.9 × 107/kg [HR, 1.50 (0.97-2.32); P = .07]; ≥4 MM + TNC > 4.9 × 107/kg vs 0 to 3 MM + TNC > 3.9 × 107/kg [HR, 1.42 (1.06-1.89); P = .02]; ≥4 MM + TNC ≤ 4.9 × 107/kg vs 0 to 3 MM + TNC > 3.9 × 107/kg [HR, 1.66 (1.24-2.22); P < .001] after adjusting for age at transplant, weight, disease risk index, and ATG use; Figure 1C).
There were 509 deaths in the cohort for the following causes: 195 (38%) owing to disease relapse/progression, 287 (56%) owing to TRM, 9 (2%) due to secondary malignancies, and 18 (4%) owing to other causes or unknown. Recipients of dUCBT with ≥4 allele MM had a higher proportion of deaths owing to TRM (62%; mainly infectious complications) than those with CBUs with up to 3 allele MM (47%; P = .01). During the first 100 days after the transplant, the main cause of death was TRM regardless of the number of HLA MM (0-3 MM, 85% vs ≥ 4 MM, 90%; P = .41). After D+100, relapse was the most common cause of mortality in the recipients of UCB units with 0 to 3 MM (49%), whereas TRM was the main cause in the group with ≥4 MM (61%; P = .03; supplemental Table 2).
The sum of HLA-mismatches of the 2 UCB units vs the UCB unit with the highest HLA disparity
To determine the most appropriate way to assess the impact of allele-level HLA matching between the recipient and the 2 CBUs, we performed an analysis of OS considering the CBU with the highest HLA disparity (≥4 vs 0-3 allele MM) compared with the sum of allele MM of the 2 UCB units. The optimal cutoff of the sum of MM for OS was 8. The 4-year OS for patients receiving 2 UCB units bearing a total of up to 8 allele MM was 49% (95% CI, 45-53) vs 42% (95% CI, 34-50) for those with a combined CBU sum of ≥9 MM (P = .03; supplemental Figure 2). In a multivariate analysis of OS, only HLA match considering the UCB unit with the highest HLA disparity remained in the final model (data not shown).
Discussion
Our findings corroborate that allele-level HLA typing is a relevant risk factor for OS after dUCBT. This had been demonstrated in sUCBT and other donor types,7,8,32 but not in dUCBT yet. We also showed that the optimal cutoff-point of the number of allele MM having maximum impact on survival was 0 to 3 vs ≥4 MM, which was used in all analyses in this study. In line with the findings in the main cohort, the impact of allele-level HLA match on the OS was confirmed when considering only the winning unit. Across these different analyses, the inferior OS associated with the increasing number of allele-level MM was consistently driven by a higher rate of TRM.
The finding herein that HLA disparity affected TRM and OS differs from a previous report by the Minnesota group which included 342 dUCBT recipients12 and did not find any significant effect of allele-level HLA match on OS and TRM. One could argue that the small number of patients with ≥4 MM or even the grouping of such patients along with recipients of 5 of 8 HLA-matched UCB units in that study may have hampered the detection of the deleterious effect of allele-level HLA disparity. In contrast, similar to our findings, a previous report of 133 dUCBT recipients showed a significantly higher TRM and worse OS in patients aged >32 years old and whose winning unit had ≥4 MM.13
It is noteworthy that we did not find a significant impact of having 3 allele MM on survival in this large cohort of dUCBT recipients. This contrasts a joint report by the Center for International Blood and Marrow Transplant Research and our group in sUCBT recipients with malignant diseases, which showed worse TRM with ≥1 allele MM and worse survival with 3 or more allele MM.7 Studying the effect of HLA match in dUCBT is challenging because the 2 CBUs potentially contribute to outcomes despite only 1 unit becoming dominant (winning unit).25 In dUCBT, the losing unit may attenuate the effect of allele MM on neutrophil engraftment and in consequence reduce early TRM, as previously reported.33 In our cohort, the losing unit may have mitigated the effect of HLA mismatching on graft failure in the recipients of 3 allele MM, not affecting TRM and OS in these patients. This was also consistent with the finding that the recipients of a winning unit with 3 allele MM had similar OS compared with those with 0 to 2 MM. However, we cannot ascertain that the lack of impact of having 3 allele MM on survival was that because the comparison group consisted of patients with a maximum of 2 HLA MM instead of 8 out of 8 HLA-matched recipients, as there was a small number of UCBT recipients with none or only 1 allele-level MM.
The worse TRM in recipients of dUCBT with ≥4 MM found in this study seemed to be associated with a higher proportion of deaths owing to infectious complications. Higher allele–level HLA disparity was associated with worse neutrophil recovery as previously reported,7,8,34,35 and receiving units with 4 and ≥5 MM was an independent risk factor for early TRM in this study. Notably, allele-level HLA class I MM, particularly allele MM at HLA-C, have been implicated in the augmented incidence of viral infections and inferior OS in the setting of UCBT, possibly owing to insufficient cytotoxic T-cell recognition and dendritic cell priming.36 Cytomegalovirus prophylaxis with letermovir has been shown to be highly effective in mitigating cytomegalovirus-related mortality and avoiding toxic anti-cytomegalovirus therapy in UCBT,37 though we did not have information on cytomegalovirus reactivation or disease and cytomegalovirus treatment–associated toxicity in this registry study, and most patients received dUCBT before the approval of letermovir in Europe. Extended prophylaxis with letermovir beyond D + 100 has shown encouraging results38 and might be of benefit to UCBT recipients with GVHD and poor immune reconstitution.39 This drug may also be particularly significant in patients receiving highly HLA-mismatched UCB units. GVHD, an important cause of TRM, was not significantly associated with allele HLA mismatch in our study. A higher risk of acute GVHD has been reported in UCBT with increasing allele-level HLA disparity in some7,8,35 but not all studies.12,13,34,40 Interestingly, as described in UCBT and transplants using other donor types,7,12,35,41 recipients of at least 1 unit with ≥5 MM in this study had a lower risk of relapse, but this advantage was offset by higher TRM.
HLA disparity in dUCBT has been classically dealt with considering the worst-matched unit. The results obtained in this study using this approach were concordant with analyses restricted to the winning unit.6 Considering the worst-matched unit allows us to study the effects of HLA mismatching on graft failure and early TRM while also considering the potential influence of the second unit on these outcomes by considering the two-unit TNC and CD34+-cell dose. However, such an approach has the caveat of overestimating the eventual impact of HLA mismatching if the better matched CBU becomes the winning unit. Moreover, independent of whether the worst or better HLA-matched unit becomes the winning one, it is known that there may be long-term microchimerism of the losing unit.42 A higher incidence of GVHD after dUCBT when compared with similarly HLA-matched single-unit UCBT was reported in different studies including a prospective phase 3 trial,3,4,15 perhaps highlighting the additive effect of HLA disparity of the 2 units. We hypothesized that summing the number of allele HLA MM of the 2 units could be a better approach than considering the worst-matched unit. We performed a subanalysis considering the summed MM of the 2 CBU units comprising the graft and compared it with the results obtained when considering only the worst HLA- matched unit for the HLA match assignment. Nevertheless, the latter proved to be the best independent predictor for survival in this large cohort. Thus, our data corroborate the current approach of considering the UCB unit with the highest HLA disparity in the setting of dUCBT.
In this study, TNC count was independently associated with OS, neutrophil recovery, and early TRM. The main advantage of double-unit in relation to single-unit UCBT is the possibility of attaining higher TNC and CD34+ doses and reducing early TRM owing to graft failure or delayed neutrophil recovery. However, similar to a previous report by our group that considered HLA matching at a lower resolution in dUCBT,6 the negative effect of HLA disparity on OS seemed to persist despite higher TNC doses. We recently showed that a cryopreserved TNC dose of 3.5 × 107/kg was the optimal cutoff for OS and ∼90% of dUCBT recipients had a TNC count at this level or above.6 Our present results suggest that selecting better HLA-matched CBUs at the allele-level should be prioritized in dUCBT even at the cost of somewhat lower TNC count as long as they are kept above the aforementioned optimal cutoff. The Memorial Sloan Kettering group retrospectively reviewed the selection of CBUs for dUCBT based on HLA typing at a lower resolution and showed that the combination of CBU pairs would change in one-third of the patients if HLA matching at allele-level were considered but with minimal effect on cell dose.43 Approximately 40% of dUCBT recipients received a graft with maximum HLA disparity of 5 out of 8 in our cohort. Although one could argue that a large proportion of transplant candidates lacking HLA-matched conventional donors would not have available UCB units with up to 3 allele MM, we think that current evidence supports the selection of alternative donor types for such patients, for example, haploidentical related donors or 7 out of 8 HLA-matched unrelated donors with new types of GVHD prophylaxes.18,20 Moreover, a recent phase 3 trial demonstrated that omidubicel, an ex vivo expanded hematopoietic progenitor cell product derived from a single UCB unit, was associated with a faster hematopoietic recovery and lower early transplant-related complications.21 This innovative approach has recently been given priority review by the Food and Drug Administration and may allow the selection of better allele-level HLA-matched UCB units for patients who would otherwise require dUCBT of grafts bearing 4 or more MM.
Apart from the aforementioned limitations, this study is limited by the fact it is a retrospective analysis in which the selection of UCB units was physician- and center-dependent and possibly affected by unmeasured factors. Our analysis of the effect of HLA disparity considering the winning unit was also limited by the fact we did not have data on the chimerism of a significant proportion of patients. Despite that, we could still show overall concordant findings between the winning unit and the main analysis. Another drawback was the small number of patients receiving UCB units with single locus MM, preventing us from analyzing the individual effect of mismatching at HLA-A, -B, -C, and -DRB1 on transplant outcomes. Although found to be significant factors for transplant outcomes in sUCBT,44,45 there were only a few unit-recipient pairs with complete typing at HLA-DQB1 and -DPB1 available (44% and 6% in our database, respectively), precluding the inclusion of these factors in our analyses. Alloreactive HLA class II MM–specific CD4+ T cells from the winning unit toward the loser CBU have been implicated in unit dominance and graft-versus-leukemia in dUCBT,46,47 but investigating this in our study was not possible owing to the small number of CBU-recipient pairs with information on the winning CBU (including lymphocyte subset chimerism) and extended class II HLA typing. Lastly, this study included transplants performed from 2006 to 2019, a period during which CBU selection and supportive treatment changed significantly (eg, use of reduced toxicity conditioning regimens, letermovir–based cytomegalovirus prophylaxis, and other drugs), yet the year of transplants was carefully accounted for in the different multivariate analyses. This may explain why the overall outcomes observed in this registry study seem to be inferior compared with more contemporary reports on UCBT from experienced single centers,48,49 suggesting the relevance of historical changes in clinical practice in addition to a center effect in this transplant modality.
In conclusion, as demonstrated for sUCBT, the use of high-resolution matching at HLA-A, -B, -C, and -DRB1 should be standard practice when selecting units for dUCBT. Our findings support the selection of UCB units with up to 3 allele MM and adequate cell doses for transplant candidates with hematologic malignancies lacking conventional HLA-matched donors for whom dUCBT is contemplated. In patients for whom such UCB units are not available, the risks and benefits of performing dUCBT with more HLA-mismatched units should be balanced against disease risk and the availability of other alternative donors.
Acknowledgment
This work was submitted on behalf of Eurocord and the Cellular Therapy & Immunonbiology Working Party of the European Society for Blood and Marrow Transplantation.
Authorship
Contribution: G.F. and V.R. designed the study; F.V. retrieved and prepared the data; L.M. classified HLA matching at high resolution; F.M. performed the statistical analyses; G.F, L.M., F.V., H.R., M.M.R.-F., C.K., G.M.S., B.C., A.R., E.G., and V.R. analyzed the data; G.F. wrote the manuscript; J.C., S.F., E.D., A.S., and R.P.d.L. provided cases for the study; and all authors edited and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Giancarlo Fatobene, Hospital das Clínicas and LIM31, Faculty of Medicine University of São Paulo, Av. Dr. Enéas Carvalho de Aguiar, 155 – 1º andar 01246-000, São Paulo, Brazil; e-mail: giancarlo.fatobene@hc.fm.usp.br.
References
Author notes
Data are available on request from the corresponding author, Giancarlo Fatobene (giancarlo.fatobene@hc.fm.usp.br).
The full-text version of this article contains a data supplement.