Key Points
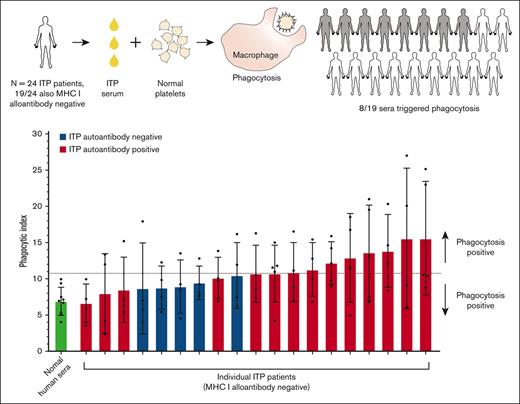
Incubation of ITP serum with normal donor platelets triggered platelet uptake via macrophage phagocytosis for sera from half of the patients with ITP.
Platelet autoantibody presence, lower platelet count, and increased serum pentraxin 3 were associated with increased macrophage phagocytosis.
Abstract
Humoral antiplatelet factors, such as autoantibodies, are thought to primarily clear platelets by triggering macrophage phagocytosis in immune thrombocytopenia (ITP). However, there are few studies characterizing the capacity and mechanisms of humoral factor–triggered macrophage phagocytosis of platelets using specimens from patients with ITP. Here, we assessed sera from a cohort of 24 patients with ITP for the capacity to trigger macrophage phagocytosis of normal donor platelets and characterized the contribution of humoral factors to phagocytosis. Sera that produced a phagocytosis magnitude greater than a normal human serum mean + 2 standard deviations were considered phagocytosis-positive. Overall, 42% (8/19) of MHC I alloantibody-negative ITP sera were phagocytosis-positive. The indirect monoclonal antibody immobilization of platelet antigens assay was used to detect immunoglobulin G (IgG) autoantibodies to glycoproteins (GP)IIb/IIIa, GPIb/IX, and GPIa/IIa. Autoantibody-positive sera triggered a higher mean magnitude of phagocytosis than autoantibody-negative sera. Phagocytosis correlated inversely with platelet counts among autoantibody-positive patients but not among autoantibody-negative patients. Select phagocytosis-positive sera were separated into IgG-purified and -depleted fractions via protein G and reassessed for phagocytosis. Phagocytosis was largely retained in the purified IgG fractions. In addition, we assessed serum concentrations of C-reactive protein, serum amyloid P, and pentraxin 3 as potential phagocytosis modulators. Pentraxin 3 concentrations correlated inversely with platelet counts among patients positive for autoantibodies. Taken together, sera from approximately half of the patients with ITP studied triggered macrophage phagocytosis of platelets beyond a normal level. An important role for antiplatelet autoantibodies in phagocytosis is supported; a role for pentraxins such as pentraxin 3 may be suggested.
Introduction
The accelerated clearance of platelets is a central feature of immune thrombocytopenia (ITP) pathophysiology. The Harrington-Hollingsworth experiment provided evidence that accelerated platelet clearance may be because of factors present in the circulatory system of patients with ITP. Harrington et al demonstrated that the transfusion of blood or plasma from patients with ITP can produce a precipitous platelet count decrease in recipients without ITP.1 It was hypothesized that circulating “thrombocytopenic factors” were responsible for the platelet count decreases and thus also in ITP.1 Overall, the transfusion of ITP blood or its plasma equivalent produced a >50% recipient platelet count decrease in 16 of 26 (61.5%) of such instances.2 Subsequent work by Shulman et al provided more direct evidence that antiplatelet immunoglobulin G (IgG) autoantibodies are a thrombocytopenic factor in ITP.3 Antiplatelet autoantibodies are thought to opsonize platelets and trigger clearance by macrophage phagocytosis,4,5 particularly in the spleen, which is the dominant site of platelet clearance in ITP.6 It is now appreciated that IgG autoantibodies in ITP target multiple platelet antigens, including glycoprotein (GP)IIb/IIIa, GPIb/IX, GPV, and GPIa/IIa.7-10 More recently, it has been demonstrated that C-reactive protein (CRP) can enhance the phagocytosis of blood cells, such as erythrocytes11 and platelets,12 and may potentially be a thrombocytopenic factor in ITP.
There is a general agreement that thrombocytopenic factors, such as autoantibodies, can trigger platelet clearance via macrophage phagocytosis in ITP. McMillan et al first demonstrated that the incubation of splenic leukocytes of patients with ITP with healthy donor platelets led to platelet uptake or binding.13 Handin et al14 and Tsubakio et al15 demonstrated that incubation of healthy donor platelets with ITP serum promoted platelet uptake or binding by peripheral blood granulocytes14 or peripheral blood leukocytes.15 Hoemberg et al, observed monocyte uptake of normal donor platelets when platelets were incubated with IgG or IgM antiplatelet autoantibody-positive ITP plasma.16
These studies support the finding that platelets from patients with ITP or normal donor platelets incubated with ITP sera or plasma are internalized by phagocytes beyond the level observed for normal controls. However, these studies used peripheral blood leukocyte populations,15 granulocytes,14 or monocytes16 as phagocytes, which are not generally considered the main phagocytes clearing platelets in ITP.17 Furthermore, the mechanisms of phagocytosis triggered by patient specimens remain poorly characterized. Recently, we demonstrated that ITP splenic macrophages phagocytosed normal donor platelets incubated with anti-GPIIb/IIIa autoantibody-positive adult ITP sera by a mechanism using FcγRI and FcγRIII.4 Here, we expand on this work by studying macrophage phagocytosis of platelets using a larger cohort of adult patients with ITP and investigated the role of autoantibodies as well as pentraxins in phagocytosis.
We characterized the sera from 24 adult patients with ITP for their potential to trigger macrophage phagocytosis of platelets. Sera from patients with ITP were incubated with normal donor platelets and assessed for induction of macrophage phagocytosis. As a source of phagocytes, we used a recently developed human monocyte cell line (THP-1-CD16A), which endogenously expresses the major activating FcγRs, including FcγRI, FcγRIIA, and FcγRIIIA,18 and differentiated these monocytes into macrophages. ITP sera were characterized for autoantibodies against GPIIb/IIIa, GPIb/IX, and GPIa/IIa by the indirect monoclonal antibody-specific immobilization of platelet antigens (MAIPA). In addition, we investigated the serum concentration of pentraxins, such as CRP, serum amyloid P (SAP), and pentraxin 3 (PTX3), as possible phagocytosis modulators.
Materials and methods
For full details, refer to supplemental Data, Materials, and Methods.
Patient enrollment
Patient inclusion criteria included adult age (≥18 years old) and a diagnosis of primary ITP. Patients with ITP part of primary Evans syndrome were included if they presented exclusively with ITP at study enrollment and serum collection. Exclusion criteria included administration of intravenous immunoglobulin or anti-D within the last 4 weeks and secondary causes of thrombocytopenia. Twenty-six patients with a diagnosis of ITP were initially recruited. Two patients had alternate explanations for thrombocytopenia (lupus and viral infection) and were subsequently excluded. In the remaining 24 patients, 19 had a sole diagnosis of ITP and 5 other patients were diagnosed with ITP, as part of Evans syndrome (patients ITP1, 4, 19, 20, and 21) but had exclusively active ITP when recruited. The nature of these patients’ Evans syndrome is described in the supplemental Methods. The study was conducted under approved ethics protocols with written consent at Sunnybrook Health Sciences Centre, the University Health Network, and Canadian Blood Services. The study was conducted in accordance with the Declaration of Helsinki.
Detection of antiplatelet antibodies
The indirect MAIPA was used to detect IgG autoantibodies in serum from patients with ITP.19 The MAIPA was done with both homozygous test platelets of a/a or b/b human platelet antigen (HPA) genotypes for each respective antigen for GPIIb/IIIa, GPIb/IX, or GPIa/IIa. Alloantibodies to MHC class I were detected via a Luminex bead system.
Generation of THP-1-CD16A monocyte/macrophages
THP-1 cells stably expressing FcγRIIIA (THP-1-CD16A) were generated by lentiviral transduction, as previously described.18 Transductants were cultured in complete RPMI media (10% fetal bovine serum, supplemental L-glutamine, and antibiotics), plus puromycin for transductant selection.
Flow cytometry
THP-1-CD16A monocytes were differentiated into macrophages by phorbol 12-myristate 13-acetate (PMA). FcγR expression of THP-1-CD16A macrophages was evaluated by flow cytometry using fluorescent antibodies to FcγRI (clone 10.1), FcγRIIA (clone IV.3), FcγRIIA/B/C (clone AT10), and FcγRIIIA/B (clone 3G8).
In vitro macrophage phagocytosis of ITP serum-opsonized platelets
Human macrophage phagocytosis assays were performed as previously described with minor modifications.4,20 In brief, platelets were collected from citrate-anticoagulated healthy donor whole blood, fluorescently labeled with 5-chloromethylfluorescein diacetate, then incubated with ITP sera or control sera. Platelets were then added to PMA-differentiated THP-1-CD16A macrophages for phagocytosis. After stopping phagocytosis, surface-bound, nonphagocytosed platelets were stained with a mouse anti-human GPIX-AlexaFluor 647 antibody. Phagocytosis was quantified from confocal microscopy photomicrographs, as previously described.4,20 Phagocytic index was calculated as (engulfed platelets counted/macrophages counted) × 100.
Serum pentraxin measurement
The serum concentrations of SAP and PTX3 were determined using a Luminex assay platform. Serum concentration of CRP was determined using a sandwich enzyme-linked immunosorbent assay.
Protein G fractionation of ITP sera
Protein G beads were used as per the manufacturer’s instructions. Briefly, ITP serum was diluted with binding buffer and incubated with a protein G bead slurry, then separated by centrifugation. IgG was eluted from the protein G beads and the eluate was pH neutralized. The IgG-depleted supernatant and the eluted IgG were washed and concentrated using a molecular weight cutoff concentrator with phosphate-buffered saline (PBS), then resuspended in PBS.
Results
THP-1-CD16A macrophages phagocytose IgG antibody-opsonized platelets
To study ITP humoral factor–triggered phagocytosis of platelets, THP-1 cells stably expressing FcγRIIIA (THP-1-CD16A) via lentiviral transduction were used as a source of phagocytes.18 Similar to human splenic macrophages,4,21 THP-1-CD16A cells express several major activating FcγRs, including FcγRI, FcγRIIA, and FcγRIII, and use FcγRI and FcγRIII in the phagocytosis of IgG-opsonized red blood cells and platelets.18 An immortalized cell line (THP-1) was selected to avoid potential interdonor variability, which may be associated with the use of primary monocytes or macrophages.
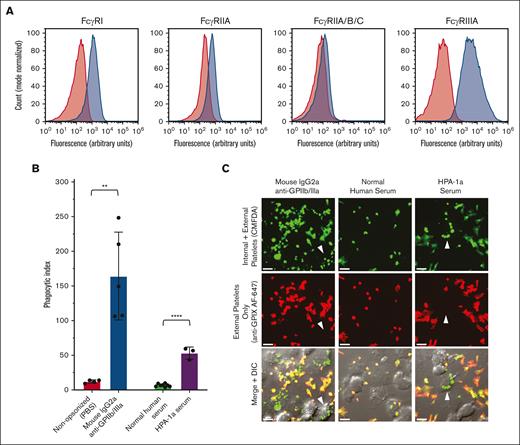
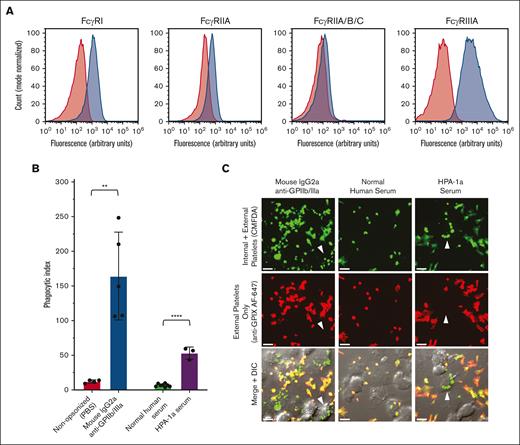
THP-1-CD16A cells were differentiated into adherent macrophage-like cells by PMA. FcγRI, FcγRIIA, and FcγRIII were detected via flow cytometry on the surface of THP-1-CD16A cells after macrophage differentiation via PMA (Figure 1A). We next evaluated the phagocytic capacity of THP-1-CD16A for human platelets opsonized with 1 of 2 control sources of anti-GPIIb/IIIa antibody: a mouse monoclonal IgG2a antibody (clone A2A9/6) or anti-HPA-1a alloantibody-positive human serum (Figure 1B).
THP-1-CD16A macrophages phagocytosed human platelets opsonized with IgG anti-GPIIb/IIIa antibodies. (A) THP-1-CD16A surface expression of FcγRs was evaluated by flow cytometry using fluorescent antibodies to FcγRI (clone 10.1), FcγRIIA (IV.3), all FcγRII isoforms (FcγRIIA/B/C) (AT10), and FcγRIII (3G8). Histograms from a representative experiment are presented. Red shade histogram = unstained control; blue shade histogram = macrophages stained with fluorescent antibody. y-axis, counts (normalized to mode); x-axis, log10 fluorescence intensity. (B) THP-1-CD16A macrophage phagocytosis of platelets opsonized with anti-GPIIb/IIIa mouse IgG2a antibody clone A2A9/6 or human serum positive for HPA-1a alloantibodies (HPA-1a serum). Phagocytosis was quantified from fluorescent photomicrographs as described in the “Methods.” Phagocytic index: number of phagocytosed platelets per 100 counted macrophages. Statistical significance was calculated by performing unpaired, parametric t tests; ∗∗P < .01; ∗∗∗∗P < .001. Data error: mean ± 1 standard deviation (SD). (C) Example of confocal photomicrographs of THP-1-CD16A macrophages after incubation with human platelets opsonized anti-GPIIb/IIIa antibodies (clone A2A9/6, HPA-1a serum) or NHS. White arrows point toward example macrophages with phagocytosed platelets. Platelets were labeled with the cytoplasmic dye 5-chloromethylfluorescein diacetate (CMFDA) (green, top row). External (nonphagocytosed) platelets were identified after phagocytosis using an AlexaFluor 647 (AF647)-conjugated anti-GPIX antibody (red, middle row). Merged fluorescence and differential interference contrast (DIC) images are shown in the bottom row. Areas of red and green colocalization in the merge appear as yellow-orange (nonphagocytosed) vs green (phagocytosed) platelets. Scale bar, 10 μM. Photomicrographs were taken by spinning-disk confocal microscopy under 63× objective oil immersion (numerical aperture 1.47) with DIC and laser fluorescence (488, 647) channels on a Quorum multimodal imaging system equipped with 50 μm pinhole spinning disk and ORCA-Flash 4.0 V2 PLUS sCMOS camera. At least 4 images at distinct locations were taken near the center of each coverslip with Z-stacking every 0.33 μm. Z-stacked images were 3D reconstructed for analysis using Imaris v8.0.2.
THP-1-CD16A macrophages phagocytosed human platelets opsonized with IgG anti-GPIIb/IIIa antibodies. (A) THP-1-CD16A surface expression of FcγRs was evaluated by flow cytometry using fluorescent antibodies to FcγRI (clone 10.1), FcγRIIA (IV.3), all FcγRII isoforms (FcγRIIA/B/C) (AT10), and FcγRIII (3G8). Histograms from a representative experiment are presented. Red shade histogram = unstained control; blue shade histogram = macrophages stained with fluorescent antibody. y-axis, counts (normalized to mode); x-axis, log10 fluorescence intensity. (B) THP-1-CD16A macrophage phagocytosis of platelets opsonized with anti-GPIIb/IIIa mouse IgG2a antibody clone A2A9/6 or human serum positive for HPA-1a alloantibodies (HPA-1a serum). Phagocytosis was quantified from fluorescent photomicrographs as described in the “Methods.” Phagocytic index: number of phagocytosed platelets per 100 counted macrophages. Statistical significance was calculated by performing unpaired, parametric t tests; ∗∗P < .01; ∗∗∗∗P < .001. Data error: mean ± 1 standard deviation (SD). (C) Example of confocal photomicrographs of THP-1-CD16A macrophages after incubation with human platelets opsonized anti-GPIIb/IIIa antibodies (clone A2A9/6, HPA-1a serum) or NHS. White arrows point toward example macrophages with phagocytosed platelets. Platelets were labeled with the cytoplasmic dye 5-chloromethylfluorescein diacetate (CMFDA) (green, top row). External (nonphagocytosed) platelets were identified after phagocytosis using an AlexaFluor 647 (AF647)-conjugated anti-GPIX antibody (red, middle row). Merged fluorescence and differential interference contrast (DIC) images are shown in the bottom row. Areas of red and green colocalization in the merge appear as yellow-orange (nonphagocytosed) vs green (phagocytosed) platelets. Scale bar, 10 μM. Photomicrographs were taken by spinning-disk confocal microscopy under 63× objective oil immersion (numerical aperture 1.47) with DIC and laser fluorescence (488, 647) channels on a Quorum multimodal imaging system equipped with 50 μm pinhole spinning disk and ORCA-Flash 4.0 V2 PLUS sCMOS camera. At least 4 images at distinct locations were taken near the center of each coverslip with Z-stacking every 0.33 μm. Z-stacked images were 3D reconstructed for analysis using Imaris v8.0.2.
Both the monoclonal (mouse IgG2a) anti-GPIIb/IIIa clone A2A9/6 and the HPA-1a alloantibody-positive human serum mediated significant macrophage phagocytosis of platelets relative to their respective PBS or normal human serum (NHS) negative controls (Figure 1B). Platelet uptake was observed and quantified by microscopy, and nonphagocytosed platelets were distinguished using a fluorescent anti-GPIX antibody incubated after stopping phagocytosis on ice and macrophage fixation with formaldehyde (Figure 1C).
Autoantibody characterization in serum from patients with ITP
We next characterized sera from a cohort of patients with ITP for antiplatelet autoantibodies. Twenty-four adult patients with a diagnosis of ITP, who had not received intravenous immunoglobulin or anti-D in the previous 4 weeks, were studied. A summary of patient characteristics is given in Table 1, and specific patient treatment information is provided in supplemental Table 1. Serum was collected from all patients with ITP and characterized for IgG autoantibodies to GPIIb/IIIa, GPIb/IX, and GPIa/IIa by the indirect MAIPA. Alloantibodies against MHC class I were detected using a Luminex assay platform.
Across all patients, 10 of 24 had detectable GPIIb/IIIa IgG autoantibodies, 10 of 24 had GPIb/IX IgG autoantibodies, 6 of 24 had GPIa/IIa IgG autoantibodies, and 7 of 24 had no detectable autoantibodies. In addition, 5 of 24 patients (23.1%) possessed alloantibodies against MHC class I. Individual indirect MAIPA results are provided in supplemental Table 2.
A theoretical problem with the MAIPA is that the antigen-immobilizing antibodies and antiplatelet autoantibodies may bind to overlapping or nearby epitopes, leading to reduced autoantibody detection. By extension, antibodies binding disparate epitopes may have improved MAIPA sensitivity. Therefore, we performed the indirect MAIPA detection of GPIIb/IIIa autoantibodies twice: once with anti-GPIIb clone GI5 and a second time with anti-GPIIIa antigen-immobilizing antibody clone AP3. Among patients with detectable anti-GPIIb/IIIa autoantibodies (n = 10), 5 patients were positive via either clone GI5 (GPIIb) or clone AP3 (GPIIIa), whereas 2 patients were positive only via clone GI5, and 3 patients were positive only via clone AP3 (supplemental Figure 1). In addition, we used clone FMC 25 to immobilize platelet GPIb/IX via GPIX. Many anti-GPIb/IX autoantibody epitopes appear localized to GPIbα,22,23 and anti-GPIX clone FMC 25 has been described to increase indirect MAIPA sensitivity for GPIb/IX autoantibodies when compared with antigen immobilization via GPIbα (clone MB45).10
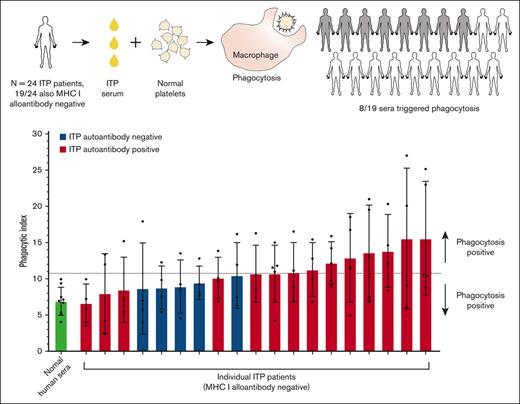
Sera from half of the patients with ITP triggered macrophage phagocytosis of platelets
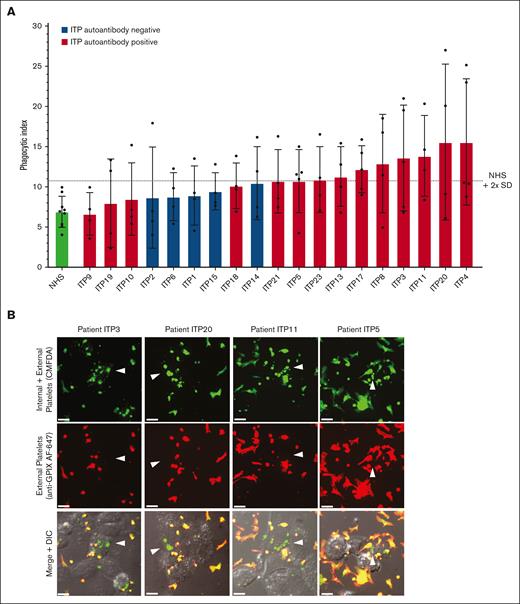
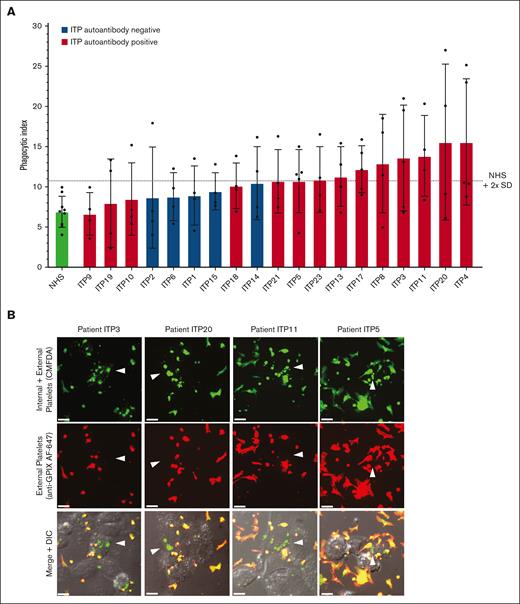
We next evaluated the ITP sera for their potential to trigger macrophage phagocytosis of platelets (Figure 2). Phagocytosis experiments were performed blinded to all information and sera characterization of patients with ITP to avoid potential bias. Normal donor platelets were incubated with an individual patient’s serum and then with macrophages for potential phagocytosis (Figure 2A). ITP sera negative for MHC class I alloantibodies (n = 19) are displayed in Figure 2A. Phagocytosis was quantified by confocal microscopy, and photomicrographs of example macrophages with successfully phagocytosed platelets are shown in Figure 2B. ITP sera are displayed in ascending order of phagocytosis magnitude. Sera were considered positive for phagocytosis if the mean phagocytic index was ≥2 standard deviations above the NHS mean.
Human ITP serum–triggered macrophage phagocytosis of platelets in vitro. (A) Serum from patients with ITP were evaluated for their potential to trigger THP-1-CD16A macrophage phagocytosis of platelets. Each serum was evaluated over 4 to 5 independent experiments. As a negative control, sera from healthy laboratory donors (NHS) were used to opsonize platelets (4 unique NHS donors used over 9 independent experiments). Phagocytosis was performed blinded to patient clinical information and all characterization of ITP sera to avoid bias. Only ITP sera negative for MHC class I alloantibodies (n = 19) are displayed. Phagocytosis was performed as described in the “Methods” and quantified from fluorescent photomicrographs. Phagocytic index: number of phagocytosed platelets per 100 counted macrophages. Dashed line indicates the phagocytic index mean of NHS plus 2 SD . Error is presented as mean ± 1 SD. (B) Example photomicrographs of THP-1-CD16A macrophages after incubation with human platelets incubated human ITP serum. White arrows point toward example macrophages with phagocytosed platelets. Platelets were labeled with the cytoplasmic dye CMFDA (green, top row). External (nonphagocytosed) platelets were identified after phagocytosis using an AlexaFluor 647 (AF647)-conjugated anti-GPIX antibody (red, middle row). Merged fluorescence and DIC images are shown in the bottom row. Areas of red and green colocalization in the merge appear as yellow-orange. Scale bar: 10 μM. Photomicrographs were taken by spinning-disk confocal microscopy under 63× objective oil immersion (numerical aperture 1.47) with DIC and laser fluorescence (488, 647) channels on a Quorum multimodal imaging system equipped with 50 μm pinhole spinning disk and ORCA-Flash 4.0 V2 PLUS sCMOS camera. At least 4 images at distinct locations were taken near the center of each coverslip with Z-stacking every 0.33 μm. Z-stacked images were 3D reconstructed for analysis using Imaris v8.0.2.
Human ITP serum–triggered macrophage phagocytosis of platelets in vitro. (A) Serum from patients with ITP were evaluated for their potential to trigger THP-1-CD16A macrophage phagocytosis of platelets. Each serum was evaluated over 4 to 5 independent experiments. As a negative control, sera from healthy laboratory donors (NHS) were used to opsonize platelets (4 unique NHS donors used over 9 independent experiments). Phagocytosis was performed blinded to patient clinical information and all characterization of ITP sera to avoid bias. Only ITP sera negative for MHC class I alloantibodies (n = 19) are displayed. Phagocytosis was performed as described in the “Methods” and quantified from fluorescent photomicrographs. Phagocytic index: number of phagocytosed platelets per 100 counted macrophages. Dashed line indicates the phagocytic index mean of NHS plus 2 SD . Error is presented as mean ± 1 SD. (B) Example photomicrographs of THP-1-CD16A macrophages after incubation with human platelets incubated human ITP serum. White arrows point toward example macrophages with phagocytosed platelets. Platelets were labeled with the cytoplasmic dye CMFDA (green, top row). External (nonphagocytosed) platelets were identified after phagocytosis using an AlexaFluor 647 (AF647)-conjugated anti-GPIX antibody (red, middle row). Merged fluorescence and DIC images are shown in the bottom row. Areas of red and green colocalization in the merge appear as yellow-orange. Scale bar: 10 μM. Photomicrographs were taken by spinning-disk confocal microscopy under 63× objective oil immersion (numerical aperture 1.47) with DIC and laser fluorescence (488, 647) channels on a Quorum multimodal imaging system equipped with 50 μm pinhole spinning disk and ORCA-Flash 4.0 V2 PLUS sCMOS camera. At least 4 images at distinct locations were taken near the center of each coverslip with Z-stacking every 0.33 μm. Z-stacked images were 3D reconstructed for analysis using Imaris v8.0.2.
Of the 19 patients negative for MHC I alloantibodies, 8 of 14 autoantibody-positive sera and 0 of 5 autoantibody-negative sera triggered phagocytosis of platelets (mean greater than NHS plus 2 standard deviations) (Figure 2A). The phagocytosis results for all patients, including those positive for MHC class I alloantibodies, are also presented in supplemental Figure 2. Interestingly, the 2 ITP sera inducing the highest magnitude of phagocytosis were both positive for MHC class I alloantibodies (supplemental Figure 2), suggesting that anti-MHC class I alloantibodies from patients with ITP can be potent triggers of macrophage phagocytosis. Consequently, ITP sera positive for MHC class I alloantibodies were excluded from subsequent phagocytosis analyses.
The magnitude of macrophage phagocytosis triggered by autoantibody-positive ITP sera correlated with the platelet count
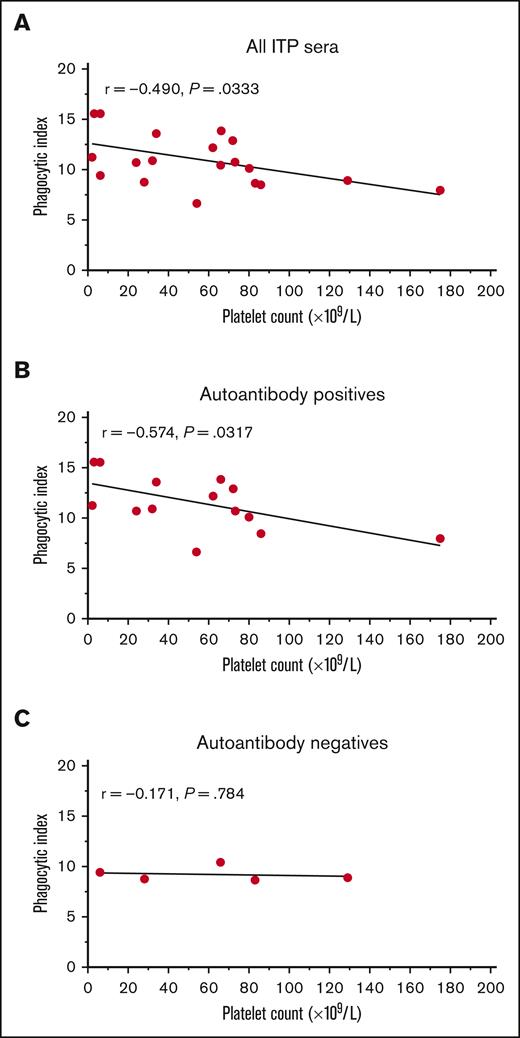
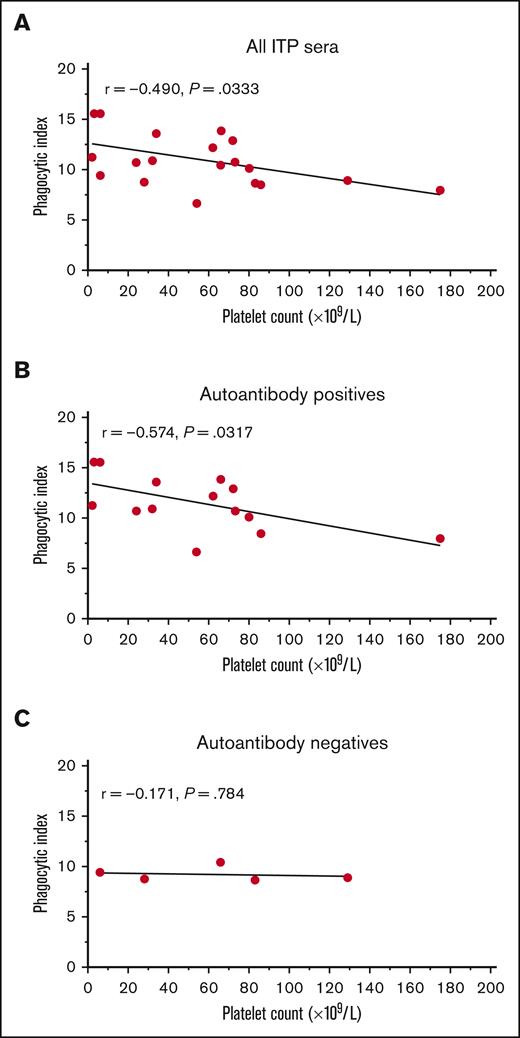
The phagocytosis induced by sera from patients with ITP varied considerably in magnitude. We hypothesized that the magnitude of in vitro phagocytosis may inversely correlate with the donor’s platelet count. Therefore, we performed an exploratory analysis correlating the magnitude of phagocytosis triggered by ITP sera and the platelet count. Across all patients with ITP, a correlation coefficient between the phagocytic index and the platelet count of r = −0.490 was obtained (P = .0333) (Figure 3A). When separated by autoantibody-positive and -negative ITP sera, the correlation was more apparent among autoantibody-positive patients (r = −0.574, P = .0317) (Figure 3B), but was seemingly absent among autoantibody-negative patients (r = −0.171, P = .784) (Figure 3C).
The magnitude of phagocytosis of platelets mediated by autoantibody-positive ITP serum related to the platelet count. The mean phagocytic index mediated for each ITP serum was explored for a potential relationship with the patient’s platelet count at the time of sera collection. All patients with ITP negative for MHC class I alloantibodies (19 total patients) were included in this analysis. (A) All ITP sera vs platelet count. (B) IgG autoantibody-positive ITP sera (any specificity by the indirect MAIPA) vs platelet counts. (C) IgG autoantibody-negative ITP sera vs platelet counts. The mean phagocytic index is shown for each patient. Data had a normal (Gaussian) distribution by the Shapiro-Wilk test and Pearson correlation coefficients were computed. A simple linear regression line for visualization is depicted.
The magnitude of phagocytosis of platelets mediated by autoantibody-positive ITP serum related to the platelet count. The mean phagocytic index mediated for each ITP serum was explored for a potential relationship with the patient’s platelet count at the time of sera collection. All patients with ITP negative for MHC class I alloantibodies (19 total patients) were included in this analysis. (A) All ITP sera vs platelet count. (B) IgG autoantibody-positive ITP sera (any specificity by the indirect MAIPA) vs platelet counts. (C) IgG autoantibody-negative ITP sera vs platelet counts. The mean phagocytic index is shown for each patient. Data had a normal (Gaussian) distribution by the Shapiro-Wilk test and Pearson correlation coefficients were computed. A simple linear regression line for visualization is depicted.
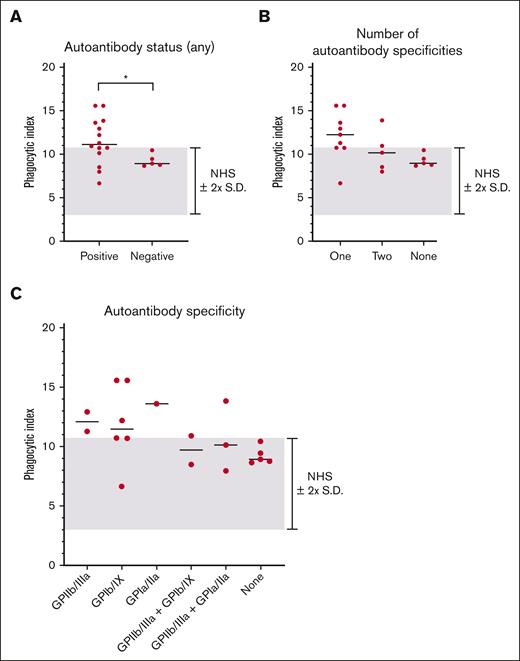
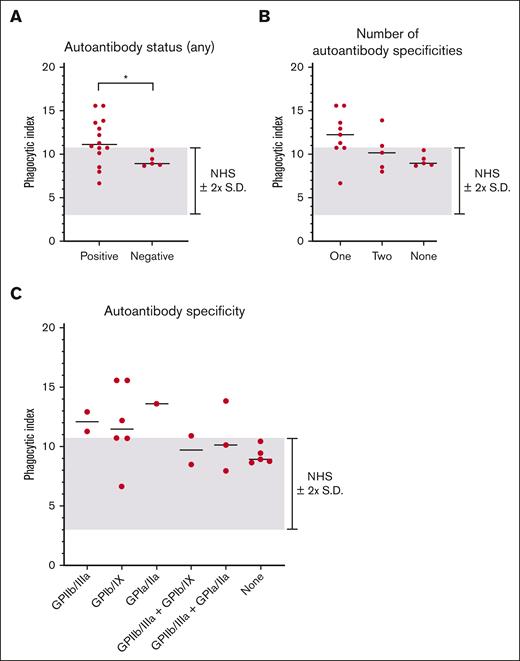
We next examined whether the magnitude of macrophage phagocytosis was related to the ITP sera autoantibody antigen specificity (Figure 4). In general, autoantibody-positive ITP sera triggered higher magnitudes of phagocytosis than autoantibody-negative ITP sera (Figure 4A). The magnitude of phagocytosis observed did not appear to be related to the number of autoantibody specificities, (Figure 4B), the magnitude of the MAIPA result (optical density) (supplemental Figure 3A-D) or particular autoantibody profile (Figure 4C). However, we caution that few patients were present in several of these assessed subgroups.
IgG autoantibody antigen specificity did not relate to the magnitude of macrophage phagocytosis of platelets mediated by patient serum. All patients with ITP negative for MHC class I alloantibodies (19 total patients) were included in the analysis. The median of each grouping is shown as a horizontal line. The NHS mean ± 2 SD cutoff is depicted by the gray-shaded area. (A) ITP sera were grouped according to the presence or absence of detectable autoantibodies against GPIIb/IIIa, GPIb/IX, or GPIa/IIa. Each dot indicates the mean phagocytic index obtained for an individual ITP serum. ∗P ≤ .05, by 2-tailed Welch’s unequal variances t test. Data was determined to have a normal (Gaussian) distribution by the Shapiro-Wilk test. (B) ITP sera were grouped by the number of antigens targeted by autoantibodies. (C) ITP sera were grouped according to their particular autoantibody profiles.
IgG autoantibody antigen specificity did not relate to the magnitude of macrophage phagocytosis of platelets mediated by patient serum. All patients with ITP negative for MHC class I alloantibodies (19 total patients) were included in the analysis. The median of each grouping is shown as a horizontal line. The NHS mean ± 2 SD cutoff is depicted by the gray-shaded area. (A) ITP sera were grouped according to the presence or absence of detectable autoantibodies against GPIIb/IIIa, GPIb/IX, or GPIa/IIa. Each dot indicates the mean phagocytic index obtained for an individual ITP serum. ∗P ≤ .05, by 2-tailed Welch’s unequal variances t test. Data was determined to have a normal (Gaussian) distribution by the Shapiro-Wilk test. (B) ITP sera were grouped by the number of antigens targeted by autoantibodies. (C) ITP sera were grouped according to their particular autoantibody profiles.
Consistent with in vitro macrophage phagocytosis, patients with ITP with a serum-detectable autoantibody had a lower median platelet count than patients without (62 × 109/L vs 83 × 109/L) (supplemental Figure 4A). However, patients with ITP in our cohort with multiple autoantibody specificities did not have a lower median platelet count (supplemental Figure 4B), and no group of patients by autoantibody profile appeared to be clearly more thrombocytopenic (supplemental Figure 4C).
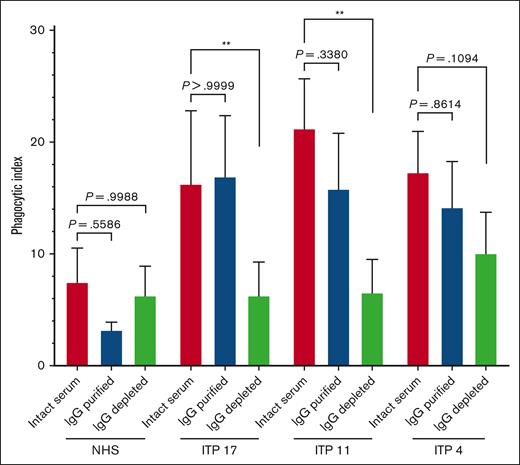
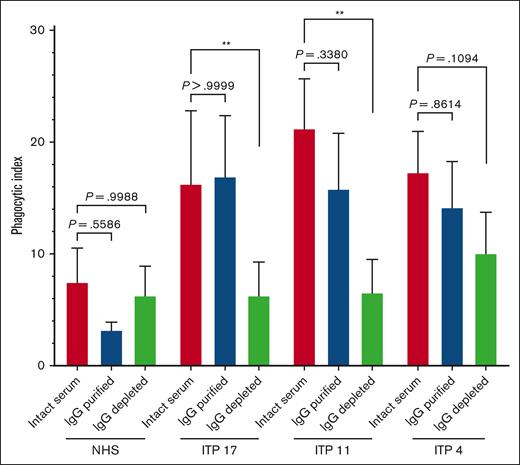
Purified IgG from ITP sera retained the capacity to trigger macrophage phagocytosis of platelets
We next asked whether purified IgG from ITP serum was sufficient to trigger macrophage phagocytosis of platelets. Three select phagocytosis-positive sera (ITP4, ITP11, and ITP17) were fractionated into purified serum IgG vs IgG-depleted serum proteins via protein G and reassessed for phagocytosis (Figure 5). Among the 3 tested patients with ITP, the phagocytosis triggered by purified serum IgG was comparable to that triggered by the respective whole patient serum. For ITP 11 and ITP17, the IgG-depleted serum protein fraction triggered a phagocytosis magnitude that was significantly lower than that of whole serum and comparable to the NHS. The phagocytosis triggered by IgG-depleted serum proteins from patient ITP 4 was decreased, with the IgG-depleted fraction retaining some marginal ability to trigger phagocytosis.
Protein G purified IgG from human ITP serum retained the capacity to trigger macrophage phagocytosis of platelets. Serum from patients with ITP or NHS were fractionated via protein G into purified IgG and IgG-depleted protein fractions. Unfractionated whole serum, IgG-purified, or IgG-depleted fractions were then incubated with normal donor platelets and assessed for the ability to trigger macrophage phagocytosis. Each serum or serum fraction was evaluated over n = 3 to 5 independent experiments and whole NHS was evaluated over n = 7 independent experiments. THP-1-CD16A macrophages were used as phagocytes. Phagocytosis was performed as described in the “Methods” and quantified from fluorescent photomicrographs. ∗∗P ≤ .01, by ordinary one-way analysis of variance (ANOVA) with Šídák’s multiple comparisons test, except for the comparisons of ITP 17 sera vs ITP 17 IgG-purified and ITP 4 sera vs ITP 4 IgG-depleted, which were computed via the nonparametric Kruskal-Wallis test with Dunn’s multiple comparisons test. Data was tested for normal (Gaussian) distributions by the Shapiro-Wilk test. Phagocytic index: number of phagocytosed platelets per 100 counted macrophages. Error is presented as mean ± 1 SD.
Protein G purified IgG from human ITP serum retained the capacity to trigger macrophage phagocytosis of platelets. Serum from patients with ITP or NHS were fractionated via protein G into purified IgG and IgG-depleted protein fractions. Unfractionated whole serum, IgG-purified, or IgG-depleted fractions were then incubated with normal donor platelets and assessed for the ability to trigger macrophage phagocytosis. Each serum or serum fraction was evaluated over n = 3 to 5 independent experiments and whole NHS was evaluated over n = 7 independent experiments. THP-1-CD16A macrophages were used as phagocytes. Phagocytosis was performed as described in the “Methods” and quantified from fluorescent photomicrographs. ∗∗P ≤ .01, by ordinary one-way analysis of variance (ANOVA) with Šídák’s multiple comparisons test, except for the comparisons of ITP 17 sera vs ITP 17 IgG-purified and ITP 4 sera vs ITP 4 IgG-depleted, which were computed via the nonparametric Kruskal-Wallis test with Dunn’s multiple comparisons test. Data was tested for normal (Gaussian) distributions by the Shapiro-Wilk test. Phagocytic index: number of phagocytosed platelets per 100 counted macrophages. Error is presented as mean ± 1 SD.
Characterization of ITP serum pentraxin levels as potential modulators of macrophage phagocytosis of platelets
There is evidence that pentraxins such as CRP can enhance antibody-mediated phagocytosis of erythrocytes11 and platelets.12 Therefore, we hypothesized that ITP sera with elevated pentraxin concentrations may trigger increased phagocytosis of platelets.
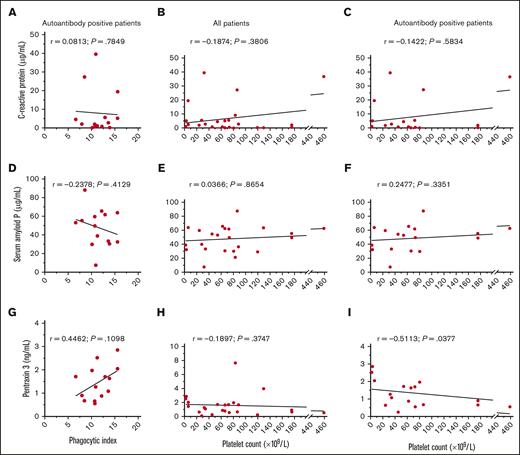
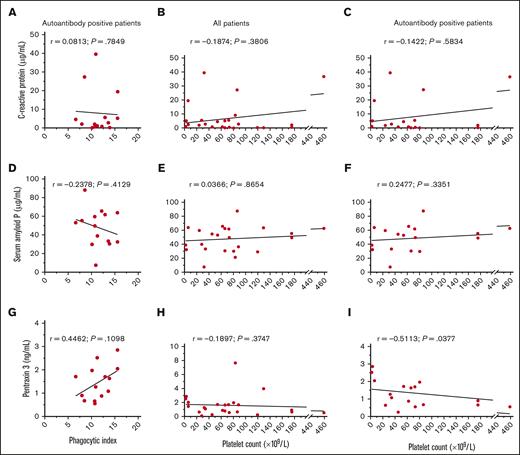
The concentrations of CRP, SAP, and PTX3 were determined for each ITP serum and explored for a relationship with the magnitude of phagocytosis or the patient’s platelet count (Figure 6). Across all patients with ITP, the median (interquartile range; minimum-maximum) concentration of CRP was 2.65 μg/mL (0.57-5.58; 0.06-39.50), the median concentration of SAP was 51.30 μg/mL (32.73-62.73; 7.60-87.60), and the median concentration of PTX3 was 1.18 ng/mL (0.66-1.91; 0.09-7.66).
CRP, SAP, and PTX3 concentrations in ITP sera and their relationship with macrophage phagocytosis and the platelet count. The concentrations of CRP, SAP, and PTX3 were determined for each ITP sera and explored for a potential correlation with the magnitude of phagocytosis and the platelet count. The concentrations of (A-C) CRP, (D-F) SAP, and (G-I) PTX3 were correlated with the phagocytic index among indirect MAIPA-positive ITP sera in panels A, D, and G the platelet count across all patients in panels B, E, and H, or the platelet count among indirect MAIPA-positive patients in panels C, F, and I. Data was assessed for a normal (Gaussian) distribution by the Shapiro-Wilk test; Pearson correlation coefficients were computed for panels with Gaussian distribution in panels D-G; Spearman correlation coefficients were computed for data with non-Gaussian distribution in panels A-C, E, F, H, and I. A simple linear regression line for visualization is depicted.
CRP, SAP, and PTX3 concentrations in ITP sera and their relationship with macrophage phagocytosis and the platelet count. The concentrations of CRP, SAP, and PTX3 were determined for each ITP sera and explored for a potential correlation with the magnitude of phagocytosis and the platelet count. The concentrations of (A-C) CRP, (D-F) SAP, and (G-I) PTX3 were correlated with the phagocytic index among indirect MAIPA-positive ITP sera in panels A, D, and G the platelet count across all patients in panels B, E, and H, or the platelet count among indirect MAIPA-positive patients in panels C, F, and I. Data was assessed for a normal (Gaussian) distribution by the Shapiro-Wilk test; Pearson correlation coefficients were computed for panels with Gaussian distribution in panels D-G; Spearman correlation coefficients were computed for data with non-Gaussian distribution in panels A-C, E, F, H, and I. A simple linear regression line for visualization is depicted.
The concentration of CRP was within the normal range (up to 10 μg/mL)24 for 20 of 24 (83%) patients with ITP in our cohort. The concentration of CRP did not appear to relate to the magnitude of phagocytosis triggered among autoantibody-positive ITP sera (P = .7849) (Figure 6A), or the degree of thrombocytopenia (P = .3806) (Figure 6B-C). The concentration of SAP also did not appear to relate to the magnitude of phagocytosis triggered by ITP sera (P = .4129) (Figure 6D) or the degree of thrombocytopenia (P = .8654) (Figure 6E-F). There was a very weak but nonsignificant trend of increased PTX3 levels and increased ITP serum–mediated macrophage phagocytosis of platelets (r = 0.4462, P = .1098) (Figure 6G). PTX3 concentrations did not relate to the platelet count across all patients with ITP (P = .3747) (Figure 6H). However, an apparent inverse correlation was observed between PTX3 concentration and the platelet count among patients positive for serum autoantibodies (r = −0.5113; P = .0377) (Figure 6I).
Discussion
We observed that serum from about half (42%) of the MHC I alloantibody-negative patients with ITP in our cohort triggered macrophage phagocytosis of normal donor platelets. The ability of a particular ITP serum to trigger phagocytosis was associated with the presence of detectable autoantibodies: 8 of 14 autoantibody-positive sera and 0 of 5 autoantibody-negative sera triggered phagocytosis of platelets. Purified serum IgG from several select phagocytosis-triggering patients with ITP also retained the ability to trigger macrophage phagocytosis of platelets. These data support the finding that IgG antiplatelet autoantibodies can trigger removal of platelets through macrophage phagocytosis in ITP. However, the presence of detectable antiplatelet autoantibodies did not guarantee the ability to trigger phagocytosis. This may suggest that additional autoantibody characteristics may be modulating serum–triggered macrophage phagocytosis of platelets, such as IgG subclass or glycosylation. Generally, IgG1 and IgG3 bind activating FcγRs with higher affinity and may be more proficient at activating the classical complement pathway than IgG2 and IgG4.25 Consistent with this, patients with ITP with platelet-associated IgG3 appear to have more severe thrombocytopenia than patients with platelet-associated IgG4.26 IgG glycosylation features such as afucosylation of the core N-acetylglucosamine can greatly increase affinity to FcγRIII.27 However, a study by Schmidt et al observed that the total IgG Fc glycosylation pattern in ITP is similar to that of healthy controls.28 The glycosylation features specific to antiplatelet autoantibodies in ITP remain unclear.
The presence of additional humoral factors such as proteins, cytokines, or hormones may potentially have modulated the uptake of platelets by macrophages triggered by ITP sera. Patient treatments may also have modified autoimmune humoral factors in ITP serum. Among the 19 MHC I alloantibody-negative patients evaluated for phagocytosis, 12 were not receiving active treatment. Among treated patients, pertinent to our study may be the influence of therapies on autoantibody presence. Patient ITP1, who received rituximab, tested negative for autoantibodies by the indirect MAIPA. Interestingly, all patients who underwent splenectomy were positive for antiplatelet autoantibodies. To reduce the effect of drugs potentially present in the serum from patients with ITP on phagocytosis, we removed the serum from platelets before addition to macrophages.
Humoral proteins augmenting phagocytosis may potentially include the complement system, as complement deposition on platelets is observed in ITP29-33 or pentraxins such as CRP. CRP is an acute-phase reactant of the innate immune system that can bind FcγRs and activate the classical complement pathway.11,34 There is evidence that CRP can enhance antibody-mediated phagocytosis of erythrocytes11 and platelets.12 We asked whether CRP concentrations may be higher in patients with more severe thrombocytopenia or whether increased serum levels trigger macrophage phagocytosis of platelets. The concentrations of SAP and PTX3, which also bind C1q and FcγRs, were also evaluated.34,35 We were unable to observe a clear effect between increased CRP and the platelet count or phagocytosis, as only 4 of 24 patients (17%) in our cohort had elevated CRP levels (above 10 μg/mL). SAP is not an acute-phase reactant in humans34 but may theoretically decrease in concentration through consumption if it is involved in platelet clearance. In our cohort, SAP levels did not apparently relate to phagocytosis or the platelet count. We did observe a weak but significant inverse correlation between increased PTX3 and decreased platelet counts among autoantibody-positive patients with ITP. We tested for a correlation within autoantibody-positive patients based on evidence that CRP is relatively inert toward normal platelets and erythrocytes but may bind after antibody opsonization.11,12 However, overall PTX3 levels of patients with ITP were generally within the normal range or slightly elevated when compared with literature-reported values for normal individuals of typically <2 ng/mL.36-38 An important caveat is that we only measured pentraxin concentrations in serum, which is not a direct measure of pentraxin activity, and therefore our correlation cannot define a mechanistic relationship. Nevertheless, our exploratory analysis may provide impetus for future investigation into the role of PTX3 in ITP pathophysiology.
The sensitivity of the indirect MAIPA is generally low, as only 10% to 20% of patients with ITP typically test positive for serum or plasma autoantibodies to either GPIIb/IIIa or GPIb/IX.9 A theoretical problem with the MAIPA is that the antigen-immobilizing antibody and antiplatelet autoantibody may bind to overlapping or nearby epitopes, which reduces autoantibody binding or detection. Porcelijn et al observed that GPIb/IX immobilization via clone FMC 25, which binds GPIX, had improved MAIPA sensitivity to detect GPIb/IX autoantibodies relative to antigen immobilization via anti-GPIbα antibody clone MB45.10 In our experiments, we also used clone FMC 25. In addition, we evaluated whether 2 complementary GPIIb/IIIa-immobilizing antibodies, clone GI5 against GPIIb and clone AP3 against GPIIIa, may increase indirect MAIPA sensitivity for GPIIb/IIIa autoantibodies. Crucially, we found that 2 patients were positive only when GPIIb/IIIa was immobilized via anti-GPIIb clone GI5, whereas 3 patients were positive only when GPIIb/IIIa was immobilized via anti-GPIIIa clone AP3. Our data suggest that the use of both anti-GPIIb and anti-GPIIIa antigen-immobilizing antibodies may increase the detection of anti-GPIIb/IIIa autoantibodies in ITP.
It is not completely clear why the direct MAIPA is more sensitive than the indirect MAIPA. A report by Smith et al observed that most autoantibodies eluted from patient platelets could be rebound to normal donor platelets, indicating lower sensitivity was not entirely because of a lack of epitopes.39 A study by Vollenberg et al identified that some patients contain plasma autoantibodies that are only detectable by more sensitive techniques (surface plasmon resonance).40 We acknowledge that our cohort of patients had a substantially higher prevalence of serum autoantibodies (71% of patients positive for at least 1 autoantibody specificity) than previously published data.9 Potential explanations may be the relatively high proportion of patients with chronic ITP in our study (78%; median ITP duration of 8 years), the assessment of autoantibodies to 3 glycoproteins (GPIIb/IIIa, GPIb/IX, and GPIa/IIa), as well as using 2 antigen-immobilizing monoclonal antibodies to GPIIb/IIIa and GPIb/IX immobilization via a GPIX-specific antibody.
There are several important limitations of our study. First, only 24 patients were evaluated in the study, limiting the strength of our analyses. Second, we did not evaluate for serum GPV autoantibodies or IgM autoantibodies because of sera volume limitation concerns. GPV autoantibodies are observed to have a similar prevalence as GPIIb/IIIa and GPIb/IX autoantibodies7-10 and GPV autoantibodies may contribute to macrophage phagocytosis of platelets.40 Third, we did not evaluate the phagocytosis of ITP platelets directly, which would likely better reflect platelet clearance pathophysiology.
In summary, the data presented here provide a clear, direct demonstration that incubating normal donor platelets with ITP sera can trigger macrophage phagocytosis of platelets. Such phagocytosis could be observed using sera from approximately half of the patients with ITP studied and was associated with the presence of IgG antiplatelet autoantibodies. Furthermore, the purified serum IgG from evaluated ITP sera retained the capacity to trigger phagocytosis. We also observed an inverse correlation between PTX3 serum concentration and platelet counts among autoantibody-positive patients, which may serve as an impetus for future investigation into PTX3 and ITP pathophysiology.
Acknowledgments
The authors are grateful for all blood donors and the patients with ITP who contributed to this research.
This research was supported by the University of Toronto, Alexandra Yeo Chair Grant in Benign Hematology (C.M.C.-G.). This research received funding support from Canadian Blood Services Intramural Research Grant Program (A.H.L.) and Graduate Fellowship Program (P.A.A.N.), funded by the federal government (Health Canada) and the provincial and territorial ministries of health.
The views herein do not necessarily reflect the views of Canadian Blood Services or the federal, provincial, or territorial governments of Canada.
Authorship
Contribution: P.A.A.N., A.H.L., C.M.C.-G., Y.L., and J.C. conceived the study; P.A.A.N., A.H.L., Z.T., M.C.-G., Y.L., J.C., and U.J.S. designed experiments; C.M.C.-G., J.C., Y.L., L.G., Y.S., and D.R.B. provided critical reagents; P.A.A.N., Z.T., L.G., Y.S., and U.J.S. performed experiments; P.A.A.N., Z.T., and A.H.L. analyzed and interpreted data; P.A.A.N. wrote the manuscript; and A.H.L., Z.T., J.C., L.G., Y.L., and P.A.A.N. edited the manuscript. This study contains data and content which has, in part, been a component of a doctoral thesis by P.A.A.N. (University of Toronto, Toronto, Canada).
Conflict-of-interest disclosure: A.H.L. has patents on the use of monoclonal antibodies as replacements for intravenous immunoglobulin and has received past research funding from Rigel Pharmaceuticals Incorporated and CSL Behring. D.R.B. has performed research supported in part by CSL Behring that provided in-kind materials as well as research funding. Y.L. has received research funding from Octapharma. The remaining authors declare no competing financial interests.
Correspondence: Alan H. Lazarus, Keenan Research Centre, St. Michael’s Hospital, Unity Health Toronto, 30 Bond St, Toronto, ON M5B 1W8, Canada; e-mail: alan.lazarus@unityhealth.to.
References
Author notes
Data are available on request from the corresponding author, Alan H. Lazarus (alan.lazarus@unityhealth.to).
The full-text version of this article contains a data supplement.