Key Points
In CLL, fixed-duration immunochemotherapy (15 months) produced deep and sustained PB MRD responses and high survival rates.
No differences were apparent in the depth and durability of PB MRD responses per the IGHV mutational status.
Abstract
In previously untreated, medically fit patients with chronic lymphocytic leukemia (CLL), research is focused on developing fixed-duration strategies to improve long-term outcomes while sparing patients from serious toxicities. The ICLL-07 trial evaluated a fixed-duration (15-month) immunochemotherapy approach in which after obinutuzumab-ibrutinib induction for 9 months, patients (n = 10) in complete remission (CR) with bone marrow (BM) measurable residual disease (MRD) <0.01% continued only ibrutinib 420 mg/day for 6 additional months (I arm), whereas the majority (n = 115) received up to 4 cycles of fludarabine/cyclophosphamide-obinutuzumab 1000 mg alongside the ibrutinib (I-FCG arm). Primary analysis at month 16 showed that 84 of 135 (62.2%) patients enrolled achieved CR with a BM MRD <0.01%. Here, we report follow-up at median 63 months. Peripheral blood (PB) MRD was assessed 6 monthly beyond the end of treatment using a highly sensitive (10-6) flow cytometry technique. In the I-FCG arm, the PB MRD <0.01% rate (low-level positive <0.01% or undetectable with limit of detection ≤10-4) in evaluable patients was still 92.5% (74/80) at month 40 and 80.6% (50/62) at month 64. No differences in the PB MRD status were apparent per to the IGHV mutational status. In the overall population, 4-year progression-free and overall survival rates were 95.5% and 96.2%, respectively. Twelve deaths occurred overall. Fourteen serious adverse events occurred beyond the end of treatment. Thus, our fixed-duration immunochemotherapy approach produced deep and sustained PB MRD responses, high survival rates, and low long-term toxicity. A randomized trial is needed to compare our immunochemotherapy approach with a chemotherapy-free strategy. This trial was registered at www.clinicaltrials.gov as #NCT02666898.
Introduction
In previously untreated, medically fit patients with chronic lymphocytic leukemia (CLL), the availability of targeted agents (notably, ibrutinib, obinutuzumab, and venetoclax) has led to research interest in achieving deep, durable remissions and improving survival outcomes.1,2
For ∼2 decades, immunochemotherapy was the backbone of first-line treatment for CLL.3-7 Six cycles of fludarabine, cyclophosphamide, and rituximab (FCR) offers patients an effective time-limited treatment and produces prolonged remissions after the end of treatment.7-10 However, this regimen carries a risk for long-term toxicities, including treatment-related myelodysplastic syndrome and acute myeloid leukemia (occurring in up to 2%-5% of patients),1,8,9,11 myelosuppression, and life-threatening infections.1,7-9,11-13 Also, associated survival outcomes are poorer in patients with immunoglobulin gene heavy variable (IGHV)–unmutated vs in those with mutated status.8,9,14 In many countries, immunochemotherapy remains a standard-of-care option for previously untreated, medically fit patients with CLL, particularly those with IGHV-mutated status.1,3 Although in some countries, chemotherapy-free combinations of targeted agents are increasingly replacing immunochemotherapy.1,15 Some targeted agents were initially developed for continuous use, as either monotherapy or in combinations, and required indefinite maintenance therapy.12,16-23 However, many patients were found to discontinue treatment because of toxicities. With continuous ibrutinib, reported serious toxicities include cardiac disorders, such as atrial fibrillation, bleeding, arthralgia, and hematologic toxicities.15,24-28
Current research is focused on developing fixed-duration, first-line approaches in CLL, with aims to achieve deep and durable remissions and high survival rates while sparing patients from serious toxicities. In this regard, randomized studies have evaluated chemotherapy-free combinations of targeted agents, such as ibrutinib-venetoclax19,29-31 or obinutuzumab-venetoclax.32-34 However, several phase 2 trials are now providing evidence to support the alternative approach of combining targeted agents with immunochemotherapy.35-38
Such trials are widely adopting the assessment of measurable residual disease (MRD) in the peripheral blood (PB) and/or bone marrow (BM) via flow cytometry, polymerase chain reaction, or next-generation sequencing.39 MRD provides a deeper measure of the treatment efficacy than clinical complete response (CR) and is a rapidly available surrogate shown to correlate with progression-free survival (PFS) and overall survival (OS). Classically, the MRD reporting threshold was 0.01%, per a flow cytometry with a sensitivity of 10-4, meaning that a patient has <1 CLL cell per 10 000 lymphocytes. With the development of more sensitive MRD assays, the reporting threshold became lower than previous measurements, and these days, it is common to use an MRD assay with better sensitivity, and cases of undetectable MRD can be categorized based on the intervals of the limit of detection (LOD).
The phase 2 ICLL-07 trial by the French Innovative Leukemia Organization (FILO) evaluated a fixed-duration (15-month) combination of ibrutinib plus obinutuzumab-based immunochemotherapy in previously untreated, medically fit patients with CLL.35,36 Patients underwent obinutuzumab and ibrutinib induction for 9 months, then an MRD-guided strategy for 6 additional months, in which all patients received ibrutinib, and immunochemotherapy (≤4 cycles of FCG) was given only to those in partial response and/or with BM MRD ≥0.01%. MRD was measured using an 8-color flow cytometry technique with sensitivity of 10-6.40 Primary analysis at the end of treatment (day 1 of month 16) showed that 62.2% of all patients (84/135; 90% confidence interval [CI], 55-69) enrolled achieved the primary end point of CR with a BM MRD <0.01%.35 The follow-up at median 37 months from the start of the treatment showed a persistent PB MRD benefit beyond the end of treatment, high survival rates, and low long-term toxicity.36
Here, we report results from the ICLL-07 trial at median 63 months from treatment start to explore the evolution of PB MRD up to 5.5 years, long-term survival rates, and safety. As well as the classical reporting threshold of MRD <0.01% used in the primary analysis, our highly sensitive MRD assay enabled the MRD status to be subdivided into categories as positive MRD >0.01%, low-level positive MRD <0.01%, or undetectable MRD. We aggregated undetectable MRD into mutually exclusive (not cumulative) intervals based on the LOD: MRD4 means undetectable MRD at > 10-5 and LOD ≤ 10-4; MRD5 means undetectable MRD at > 10-6 and LOD ≤ 10-5; and MRD6 means undetectable MRD at ≤ 10-6. We go on to discuss our results in the context of findings to date for other trials of fixed-duration first-line approaches in CLL, including combining targeted agents with immunochemotherapy and chemotherapy-free combinations.
Methods
The ICLL-07 study design (supplemental Figure 1), inclusion/exclusion criteria (supplemental Data), and methodology are published.35,36 Supplemental Figure 2 shows the patient-care flow. Briefly, between 27 October 2015 and 16 May 2017, a total of 135 previously untreated, medically fit patients with CLL were enrolled from 27 FILO centers. All of them underwent induction with obinutuzumab 1000 mg (8 infusions over 6 cycles) plus oral ibrutinib 420 mg (once a day for 9 months). After evaluation on day 1 of month 9 (n = 130 evaluable), those in CR with BM MRD <0.01% continued ibrutinib 420 mg/day for 6 additional months (I arm; n = 10); otherwise, patients received ≤4 cycles of FCG 1000 mg, alongside continuing ibrutinib 420 mg/day for 6 additional months (I-FCG arm; n = 115). The primary analysis evaluation was performed on day 1 of month 16. Ibrutinib was stopped at this time, except for patients in the I-FCG arm in CR with BM MRD ≥0.01%, who continued ibrutinib 420 mg/day until PB MRD reached <0.01%.
Beyond month 16 (the end of treatment for most patients), for 3 years (until month 64), patients were followed up for PB MRD every 6 months, response every 3 months, PFS and OS, and safety. MRD was assessed oligocentrally (4 laboratories) by 8-color flow cytometry using a highly sensitive (10-6) technique developed by the FILO group that enabled subdivision of classical MRD <0.01% into low-level positive MRD <0.01% or undetectable MRD.35,36,40 Undetectable meant MRD results not reaching positivity criteria (ie, >10 CLL cells detectable, representing >0.00003% of the total lymphocytes). To simplify the reporting, we used the following 3 mutually exclusive (not cumulative) categories defined by intervals of LOD: MRD4 for undetectable MRD at LOD >10-5 and ≤10-4; MRD5 for undetectable MRD at >10-6 and LOD ≤10-5; and MRD6 for undetectable MRD at ≤10-6. IGHV mutational status was assessed via Sanger sequencing or next-generation sequencing. Responses were assessed by investigators in accordance with the International Working Group on CLL 2008 criteria.41 Adverse events were monitored in accordance with the National Cancer Institute Common Toxicity Criteria (version 4.03).
This trial was performed in accordance with the Declaration of Helsinki, with ethical committee approval (Comité de Protection des Personnes, Lorraine, France) and with written informed consent from all participants. Data cutoff for this analysis was 20 May 2022, with a median follow-up for survival of 63.3 months (range, 5.7-76.4) from treatment start. Statistical analyses used Fisher exact test or Pearson χ2test.
Results
Patients
The ICLL-07 trial enrolled 135 patients, and the patient-care flow is shown in supplemental Figure 2. As previously reported,35 based on the evaluation on day 1 of month 9, after obinutuzumab and ibrutinib induction, only 10 patients attained CR with a BM MRD <0.01% and entered the I arm. The I-FCG arm included 115 patients treated with FC-obinutuzumab 1000 mg for up to 4 cycles, and 105 (91.3%) of these patients received all 4 cycles. Baseline patient characteristics have been described previously.35,36
A total of 123 patients (10 in the I arm and 113 in the I-FCG arm) were eligible for the primary evaluation on day 1 of month 16.35 A blinded, centralized review of those with computed tomography scans available confirmed the CR rate as 73.4% (80/109).36
On day 1 of month 16, the classical BM MRD <0.01% rates in evaluable patients were 100% (9/9) in the I arm and 94.2% (98/104) in the I-FCG arm. The undetectable BM MRD rates (with LOD ≤10-4) were 66.7% (6/9) in the I arm and 81.7% (85/104) in the I-FCG arm. The classical PB MRD <0.01% and undetectable PB MRD rates (with LOD ≤10-4) were 90.0% (9/10) and 60.0% (6/10), respectively, in the I arm and 97.2% (104/107) and 92.5% (99/107), respectively, in the I-FCG arm. Supplemental Figure 3 shows the correlations between detectable and undetectable PB MRD and BM MRD in patients with both values by arm. BM MRD was generally a more sensitive measure of residual disease than PB MRD, but some patients had detectable PB MRD yet undetectable BM MRD.
PB MRD beyond end of treatment
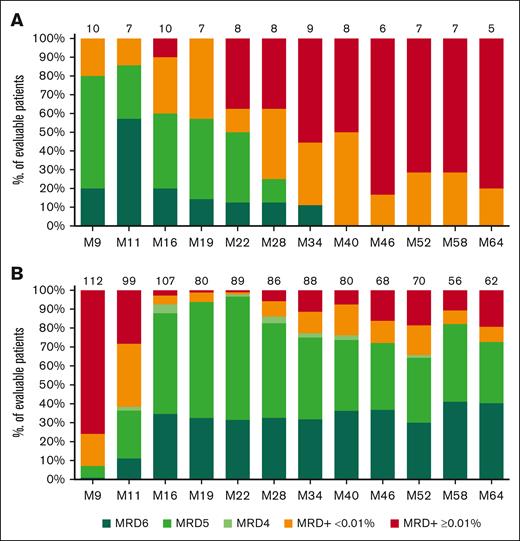
In the I arm, beyond the end of treatment, the undetectable PB MRD rate (with LOD ≤10-4) decreased rapidly and, by month 40, all evaluable patients had detectable MRD (low-level positive <0.01% or MRD ≥0.01%) (Figure 1A).
Longitudinal follow-up of PB MRD based on the arm. (A) I arm. (B) I-FCG arm. MRD is expressed as the percentage of evaluable patients, with MRD+ ≥0.01%, low-level positive MRD+ <0.01%, or undetectable MRD subdivided into mutually exclusive (not cumulative) categories depending on the LOD. Undetectable MRD was subdivided as follows: MRD4 for 10-4 ≥ LOD >10-5, MRD5 for 10-5 ≥ LOD >10-6, and MRD6 for LOD ≤10-6. PB MRD was assessed until month 64. N above each bar represents the number of evaluable patients at that time point. ND, not determined.
Longitudinal follow-up of PB MRD based on the arm. (A) I arm. (B) I-FCG arm. MRD is expressed as the percentage of evaluable patients, with MRD+ ≥0.01%, low-level positive MRD+ <0.01%, or undetectable MRD subdivided into mutually exclusive (not cumulative) categories depending on the LOD. Undetectable MRD was subdivided as follows: MRD4 for 10-4 ≥ LOD >10-5, MRD5 for 10-5 ≥ LOD >10-6, and MRD6 for LOD ≤10-6. PB MRD was assessed until month 64. N above each bar represents the number of evaluable patients at that time point. ND, not determined.
In the I-FCG arm, the classical PB MRD <0.01% and undetectable PB MRD rates (with LOD ≤10-4) in evaluable patients were 92.5% (74/80) and 76.3% (61/80), respectively, at month 40 and 80.6% (50/62) and 72.6% (45/62), respectively, at month 64 (Figure 1B; supplemental Figure 4).
At data cutoff for this long-term follow-up, of the 115 patients in the I-FCG arm, 21 were nonevaluable at month 64 because of either death (7 patients), progressive disease (6), withdrawal by the patient (3) or investigator (2), or loss to follow-up (3). Two patients had CR with BM MRD ≥0.01% and continued to receive ibrutinib. Of the 92 patients potentially eligible for evaluation at month 64, a total of 62 were evaluated for PB MRD (data were missing for 30 patients). Of these 62 patients, 72.6% had undetectable PB MRD (with LOD ≤10-4); none had MRD4 (undetectable MRD with 10-4 ≥ LOD >10-5), 32.3% had MRD5 (with 10-5 ≥ LOD >10-6), and 40.3% had MRD6 (with LOD ≤10-6). Furthermore, 8.1% of patients had low-level positive MRD <0.01%, whereas 14.5% had MRD >0.01 to 1%, and 4.8% had MRD >1%.
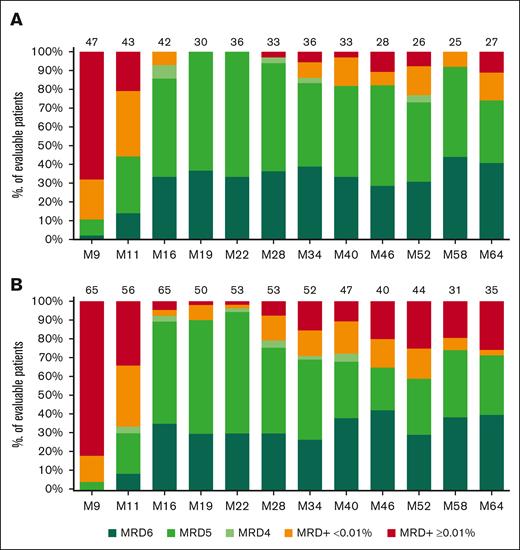
Throughout follow-up of the I-FCG arm, no statistically significant differences in PB MRD status were apparent per the IGHV mutational status (Figure 2; supplemental Figure 5). At month 64, the classical PB MRD <0.01% and undetectable PB MRD rates (with LOD ≤10-4) in evaluable patients were 88.9% (24/27) and 74.1% (20/27), respectively, in IGHV-mutated cases and 74.3% (26/35) and 71.4% (25/35), respectively, in IGHV-unmutated cases (P = .26).
Longitudinal follow-up of PB MRD in the I-FCG arm, based on the IGHV mutational status. (A) IGHV-mutated status. (B) IGHV-unmutated status. MRD is expressed as percentage of evaluable patients, with MRD+ ≥0.01%, low-level positive MRD+ <0.01%, or undetectable MRD subdivided into mutually exclusive (not cumulative) categories depending on the LOD. Undetectable MRD was subdivided as follows: MRD4 for 10-4 ≥ LOD >10-5, MRD5 for 10-5 ≥ LOD >10-6, and MRD6 for LOD ≤10-6. PB MRD was assessed until month 64. N above each bar represents the number of evaluable patients at that timepoint.
Longitudinal follow-up of PB MRD in the I-FCG arm, based on the IGHV mutational status. (A) IGHV-mutated status. (B) IGHV-unmutated status. MRD is expressed as percentage of evaluable patients, with MRD+ ≥0.01%, low-level positive MRD+ <0.01%, or undetectable MRD subdivided into mutually exclusive (not cumulative) categories depending on the LOD. Undetectable MRD was subdivided as follows: MRD4 for 10-4 ≥ LOD >10-5, MRD5 for 10-5 ≥ LOD >10-6, and MRD6 for LOD ≤10-6. PB MRD was assessed until month 64. N above each bar represents the number of evaluable patients at that timepoint.
PFS and OS
In the overall trial population (n = 133 from induction treatment start), 4-year PFS and OS rates (95% CI) were 95.5% (92.0%-99.1%) and 96.2% (93.0%-99.5%), respectively, and 5-year PFS and OS rates were 92.8% (88.3%-97.4%) and 93.6% (89.3%-98.0%), respectively. Median PFS and OS were not yet reached (Figure 3).
Survival outcomes in the overall trial population from induction treatment start (n = 133 evaluable). (A) PFS (14 progression events). (B) OS (12 deaths). The dashed lines represent the CIs.
Survival outcomes in the overall trial population from induction treatment start (n = 133 evaluable). (A) PFS (14 progression events). (B) OS (12 deaths). The dashed lines represent the CIs.
A total of 14 progression events occurred at median times to event of 49.7 months (95% CI, 5.7-76.4) from treatment start (Figure 3A). Four relapses occurred beyond the end of treatment, including 2 CLL transformations (both Richter’s syndrome). No statistically significant differences in PFS were apparent according to the PB MRD status at the time of primary evaluation (day 1 of month 16) (supplemental Figure 6A). It should be noted, however, that only 7.1% (8/113) of patients had any detectable PB MRD at the time of primary evaluation. There were also no correlations between PFS and PB MRD status at months 28 or 48 (supplemental Figure 6B,C).
A total of 12 deaths occurred at median times to event of 49.7 months (95% CI, 5.7-68.3) since the start of the treatment (Figure 3B; Table 1). Seven deaths occurred beyond the end of treatment. Since the last publication,36 deaths were due to Richter syndrome at month 50, COVID-19 at month 50, lung cancer at month 50, myelodysplastic syndrome at month 60, and acute myeloid leukemia at month 69.
Safety
Overall, since the start of the treatment, 78 serious adverse events occurred, including 64 during treatment (supplemental Table 1) and 14 beyond the end of treatment (Table 2). Since the last publication,36 nonfatal serious adverse events were metastatic prostate adenocarcinoma at month 50 and suspicion of cerebrovascular accident at month 65.
Discussion
These 5.5-year results from the ICLL-07 trial in previously untreated, medically fit patients with CLL demonstrated that our fixed-duration (15-month) immunochemotherapy approach produced deep and sustained PB MRD responses and high survival rates, without excess toxicity. In our I-FCG arm: 50 of 62 (80.6%) evaluable patients still had PB MRD <0.01% at month 64 (4 years beyond the end of treatment). The high sensitivity of our 8-color flow cytometry MRD technique enabled subdivision of the PB MRD <0.01% based on the depth of response (low-level positive MRD <0.01%, MRD4, MRD5, or MRD6), and we found that 45 of 62 (72.6%) evaluable patients still had undetectable PB MRD (with LOD ≤10-4) at month 64.
Three phase 2 studies of fixed-duration combinations of ibrutinib with immunochemotherapy in medically fit patients with CLL have now consistently shown high BM MRD response rates at the end of treatment using somewhat different approaches, as displayed in supplemental Table 2.35-38,42,43 These approaches have also yielded high survival rates at 3 years or longer. In our study, the BM MRD <0.01% rate at month 16 (end of treatment for most patients) in all enrolled patients was 79%,35,36 and at a median of 63 months since the start of the treatment, the overall 4-year PFS and OS rates were 95.5% and 96.2%, respectively. In a US study led by the Dana-Farber Cancer Institute (DFCI), 85 patients were treated with 6 cycles of I-FCR followed by 24 months of ibrutinib maintenance.37,42 Unlike our study, in which no del(17p)/TP53 mutation was an inclusion criterion, patients in the DFCI study were eligible regardless of genomic risk factors, and 6% had CLL with del(17p) and/or TP53 mutation. Regardless, the best BM MRD response rate (<10-4) was 84%, and at median follow-up of 40 months, 3-year PFS and OS rates were 97% and 99%, respectively. No long-term PB MRD data are available from this study. A single-center study at the MD Anderson Cancer Center enrolled 45 patients with CLL and genomic criteria of IGHV-mutated and no del(17p)/TP53 mutation.38,43 Patients were treated with 3 cycles of I-FCG, then an MRD-guided strategy, in which those who achieved CR/CR with incomplete blood count recovery with a BM MRD response (<10-4) received an additional 9 cycles of ibrutinib with 3 cycles of obinutuzumab, whereas all others received an additional 9 cycles of ibrutinib and obinutuzumab. The best BM MRD response rate in these patients with low genomic risk was 98%. At a median follow-up of 57 months, the 5-year PFS and OS rates were 98% and 98%, respectively. Six patients experienced a PB MRD recurrence (2 consecutive values of ≥0.01%) without clinical progression beyond the end of treatment; the follow-up PB MRD data could not be subdivided by depth of response. All 3 of these studies suggested improvements in outcomes compared with recent long-term data for FCR alone. For example, in the phase 3 Eastern Cooperative Group 1912 study at median follow-up of 5.8 years, 5-year PFS and OS rates in the FCR arm were 51% and 89%, respectively.17 In the phase 3 FLAIR study at median follow-up of 53 months, the median PFS was 67 months, and the 4-year OS rate in the FCR arm was 94.5%.44
Given the differences in patient inclusion criteria and study designs, it is not possible to directly compare the findings for fixed-duration ibrutinib plus immunochemotherapy with findings for fixed-duration, chemotherapy-free strategies such as ibrutinib-venetoclax and obinutuzumab-venetoclax (supplemental Table 2).19,29,45-48 However, the long-term PB MRD response rates in our I-FCG arm compare well with the chemotherapy-free strategy findings. By contrast, in our I arm, comprising the 10 patients in CR with BM MRD <0.01% at month 9 who continued only ibrutinib for 6 additional months, the undetectable PB MRD rate decreased rapidly beyond the end of treatment. In the phase 2 CAPTIVATE-MRD cohort, 164 patients with CLL and no genomic restrictions were treated with 3 cycles of ibrutinib then 12 cycles of ibrutinib-venetoclax; then the 86 patients with confirmed undetectable PB and BM MRD (<10-4) at 3 months after treatment were randomized to double-blinded treatment with placebo (ie, a fixed-duration regimen) or continued ibrutinib.19,45 At a median follow-up of 56 months for those randomized, the 3-year undetectable MRD rates were similar between placebo and ibrutinib (58% vs 63%), 4-year PFS rates were 88% vs 95%, and 4-year OS rates were 100% vs 98%. Separately, a CAPTIVATE-FD cohort of 159 patients received the same 15-month treatment schedule.29,46 The best BM MRD response rate (<10-4) was only 60% overall and 62% in patients with CLL and no del(17p). At median follow-up of 39 months, 3-year PFS and OS rates were 88% and 98%, respectively. Of patients with undetectable PB MRD (<10-4) at 3 months after treatment, 78% (66/85) maintained this through 12 months after treatment. In both the CAPTIVATE-MRD and CAPTIVATE-FD cohorts, the follow-up MRD data were not subdivided by the depth of response. The phase 3 CLL13 study in CLL and no del(17p)/TP53 mutation included 231 patients treated with obinutuzumab-ibrutinib-venetoclax (GIVe) for at least 12 cycles (and continued until cycle 36 if MRD was detectable) and 229 patients treated with obinutuzumab-venetoclax (GVe) for 6 cycles.47,48 At month 15, the BM MRD response rates (<10-4) were 78% with GIVe and 73% with GVe, and the PB MRD response rates (<10-4) were 92% and 87%, respectively. At median follow-up of 38.8 months, 3-year PFS rates were 91% with GIVe and 88% with GVe. No long-term PB MRD data are available from this study.
Our long-term PB MRD response rates also compare well with findings for first-line, chemotherapy-free combinations requiring continuous use in medically fit patients with CLL. Notably, in the phase 3 FLAIR study, in 136 patients with CLL and no del(17p), continuous ibrutinib-venetoclax yielded BM and PB MRD response rates <10-4 at 24 months for 65% and 71% of patients treated, respectively.49
With the standard 6 cycles of FCR in medically fit patients with CLL, previous studies found better outcomes in those with IGHV-mutated status than in those with IGHV-unmutated status.8,9,14 We found that patients in our I-FCG arm had deep and sustained PB MRD responses irrespective of their IGHV mutational status, suggesting that benefits of our fixed-duration approach of ibrutinib plus FC-obinutuzumab may extend to those with IGHV-unmutated status. Similarly, the DFCI study found no apparent differences in PB MRD response rates over time according to the IGHV mutational status with their fixed-duration approach of ibrutinib plus FCR.42
The deep and sustained PB MRD response rates obtained in our study were underpinned by quality assessments, using a highly sensitive (10-6) 8-color flow cytometry technique.40 Our study provides a long follow-up of PB MRD data for a first-line, fixed-duration approach in CLL of a median of 63 months since the start of the treatment. Our undetectable PB and BM MRD rates in the I-FCG arm at month 16 (with LOD ≤10-4) of 92.5% and 81.7%, respectively, reflect the multicompartment nature of CLL and that MRD may differ in different tissues for the same patient.39,40 Our observation that BM MRD was generally a more sensitive measure of residual disease than PB MRD, but that some patients had detectable PB MRD yet undetectable BM MRD is in line with previous observations.19,40 Assessment in the follow-up of PB MRD using only a highly sensitive assay avoids needing multiple BM samples. At month 64, of 115 patients in the I-FCG arm, 21 were nonevaluable for PB MRD because of either death, progressive disease, or patient withdrawal, and 2 had CR with BM MRD ≥0.01% and continued to receive ibrutinib. Sixty-two patients were evaluated for PB MRD at month 64, meaning data were missing in 30 of the 92 patients potentially eligible for evaluation at this time point.
We found that our fixed-duration (15-month) immunochemotherapy approach (≤4 cycles of FC-obinutuzumab) carries a low risk for long-term toxicities. Two patients experienced treatment-related myelodysplastic syndrome or acute myeloid leukemia beyond the end of treatment (1 case each). Such treatment-related myeloid neoplasms were similarly reported at low rates with the other fixed-duration ibrutinib with immunochemotherapy approaches.38,42,43 Any secondary malignancies occurred in 7 patients beyond the end of treatment. Secondary malignancies were also reported with the fixed-duration chemotherapy-free strategies,31-34,47,48 including myeloid neoplasms.31,34 Serious infections occurred in 3 patients beyond the end of treatment, which again is similar to reports for other fixed-duration strategies.29-34,37,38,42,47,48
The evidence in support of fixed-duration, first-line approaches in medically fit patients with CLL is clearly strengthening, whether through combining targeted agents with immunochemotherapy or through chemotherapy-free combinations. However, to our knowledge, no randomized trial to date has directly compared these 2 strategies. The long-term PB MRD response rates with our fixed-duration (15-month) immunochemotherapy approach compare well with findings for the new targeted agent combinations of ibrutinib-venetoclax or obinutuzumab-venetoclax. A randomized trial is needed to compare our approach with a chemotherapy-free strategy. It will be critical for the randomized trial to evaluate outcomes beyond the end of treatment and long-term toxicities. Our finding of deep and sustained PB MRD responses even in patients with IGHV-unmutated status supports the inclusion of patients regardless of their IGHV mutational status. We advocate to rigorously follow up PB MRD longitudinally by using quality MRD assessments with high sensitivity and by proactively ensuring completeness of PB MRD data collection over the long-term.
Acknowledgments
The authors thank the patients who participated in this trial and their families, the trial investigators, and other support staff at the clinical centers. The authors also thank Cath Carsberg (Stockport, UK) for medical writing assistance in the preparation of this manuscript, Roche and Janssen provided funding for this assistance, and the FILO for its participation in this study. This trial was sponsored by Roche and Janssen.
Authorship
Contribution: V.R. was the head of project and was responsible for regulatory authorization and following data collection; P.F. and A.-S.M. performed a literature search; P.F., A.-S.M., L.Y., V. Leblond, O.T., R.L., V. Lévy, F.C., and A.D. contributed to the study design; V.R., P.F., and A.-S.M. collected data; S.M., A.-S.M., and P.F. analyzed and interpreted the data; A.-S.M. and P.F. drafted the report; and all authors revised the report for content, approved the final version, and had access to the primary clinical trial data.
Conflict-of-interest disclosure: R.L. reports personal fees from AbbVie and AstraZeneca; nonfinancial support from Janssen; and personal fees and nonfinancial support from Alexion. M.L.G.-T. reports personal fees from Alexion Pharma France. M.-S.D. reports personal fees and nonfinancial support from Janssen and AbbVie. K.L. reports grants from Takeda; personal fees from Amgen, Gilead, and Janssen; and grants and personal fees from AbbVie, Novartis, Roche, and Sandoz. E.F. reports personal fees and nonfinancial support from AbbVie, Janssen, and AstraZeneca. O.T. reports personal fees from Takeda, and personal fees and nonfinancial support from AbbVie, Gilead, Janssen, Roche, and Sandoz. A.D. reports personal fees and nonfinancial support from AbbVie, Gilead, Janssen, and Roche. V. Leblond reports personal fees and nonfinancial support from AbbVie, Gilead, Janssen and Roche. P.C. reports personal fees from Novartis and Roche; nonfinancial support from Gilead and Sandoz; and personal fees and nonfinancial support from Takeda. G.C. reports personal fees from Celgene, Gilead, Janssen, and Roche. L.M.F. reports personal fees from Gilead, Janssen, Roche, and Takeda. L.Y. reports grants from AbbVie, Janssen, Gilead, and Roche. C.D. reports personal fees from Janssen; nonfinancial support from Gilead; and personal fees and nonfinancial support from AbbVie and Roche. F.C. reports grants and personal fees from Sunesis, and personal fees and nonfinancial support from AbbVie, Gilead, Janssen, and Roche. V. Lévy reports personal fees from AbbVie, Gilead, Janssen, and Roche. P.F. reports personal fees and nonfinancial support from AbbVie, Gilead, Janssen, and Roche. The remaining authors declare no competing interests.
Correspondence: Anne-Sophie Michallet, Department of Hematology, Centre Léon Bérard, FR-69008 Lyon, France; e-mail: anne-sophie.michallet@lyon.unicancer.fr.
Appendix
List of participating FILO centers and lead investigators
| Participating center . | Lead investigator . | Number of patients . |
|---|---|---|
| Lyon Bérard CLCC | A.-S.M. | 17 |
| Nantes CHU | B.M. | 16 |
| Nancy CHU | P.F. | 10 |
| Bordeaux Pessac CHU | M.-S.D. | 9 |
| Marseille IPC CLCC | T.A.S. | 7 |
| Rennes CHU | S.d.G. | 7 |
| La Roche-sur-Yon CH | B.V. | 6 |
| Le Mans CH | K.L. | 6 |
| Reims CHU | A.D. | 6 |
| St Priest en Jarrez CHU | C. Portois | 5 |
| Clermont-Ferrand CHU | O.T. | 4 |
| Lyon-Sud CHU | G. Salles | 4 |
| Metz CH | P.C. | 4 |
| Paris Bobigny CHU | V. Lévy | 4 |
| Strasbourg CHU | L.M.F. | 4 |
| Grenoble CHU | B. Pegourie | 3 |
| Paris Pitié Salpêtrière CHU | V. Leblond | 3 |
| Poitiers CHU | C.T. | 3 |
| Toulouse CHU | L.Y. | 3 |
| Annecy CH | F.O.P. | 2 |
| Bayonne CH | A.B. | 2 |
| Caen CHU | J.-P.V. | 2 |
| Montpellier CHU | G.C. | 2 |
| Rouen Becquerel CLCC | S.L. | 2 |
| Tours CHU | C.D. | 2 |
| Angers CHU | M.T.-G. | 1 |
| Lille CHU | C. Herbaux | 1 |
| Participating center . | Lead investigator . | Number of patients . |
|---|---|---|
| Lyon Bérard CLCC | A.-S.M. | 17 |
| Nantes CHU | B.M. | 16 |
| Nancy CHU | P.F. | 10 |
| Bordeaux Pessac CHU | M.-S.D. | 9 |
| Marseille IPC CLCC | T.A.S. | 7 |
| Rennes CHU | S.d.G. | 7 |
| La Roche-sur-Yon CH | B.V. | 6 |
| Le Mans CH | K.L. | 6 |
| Reims CHU | A.D. | 6 |
| St Priest en Jarrez CHU | C. Portois | 5 |
| Clermont-Ferrand CHU | O.T. | 4 |
| Lyon-Sud CHU | G. Salles | 4 |
| Metz CH | P.C. | 4 |
| Paris Bobigny CHU | V. Lévy | 4 |
| Strasbourg CHU | L.M.F. | 4 |
| Grenoble CHU | B. Pegourie | 3 |
| Paris Pitié Salpêtrière CHU | V. Leblond | 3 |
| Poitiers CHU | C.T. | 3 |
| Toulouse CHU | L.Y. | 3 |
| Annecy CH | F.O.P. | 2 |
| Bayonne CH | A.B. | 2 |
| Caen CHU | J.-P.V. | 2 |
| Montpellier CHU | G.C. | 2 |
| Rouen Becquerel CLCC | S.L. | 2 |
| Tours CHU | C.D. | 2 |
| Angers CHU | M.T.-G. | 1 |
| Lille CHU | C. Herbaux | 1 |
References
Author notes
Presented in abstract form at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 10-13 December 2022.
All data are available on request from the author, Valérie Rouille (v-rouille@chu-montpellier.fr).
The full-text version of this article contains a data supplement.
A list of participating FILO centers and lead investigators appears in “Appendix.”