Key Points
Vitamin D deficiency is pervasive and problematic in HSCT; repletion is poorly tolerated and rapidly catabolized.
We show marked, rapid, sustained improvement in vitamin D levels for 20 patients with novel, OTF cholecalciferol formulation.
Abstract
Vitamin D deficiency is common in childhood, pervasive before and after bone marrow transplant, and is associated with increased incidence of graft-versus-host disease (GVHD) and decreased survival in patients undergoing hematopoietic stem cell transplant (HSCT). Numerous barriers impede replacement, including malabsorption secondary to gut GVHD, mucositis, inability to take capsules, kidney disease, liver disease, and infection; many patients remain refractory despite vitamin D therapy. We hypothesized that a different formulation of cholecalciferol, administered on the tongue as a readily dissolving oral thin film (OTF), would ease administration and facilitate therapeutic vitamin D levels (>35 ng/mL) in patients who are refractory. In this prospective pilot study, we evaluated 20 patients after HSCT (range, day +21 - day +428 at enrollment) with serum vitamin D levels ≤35 ng/mL. Cholecalciferol OTF strips were administered for 12 weeks. Dosing was based on patient body weight and titrated per individual pharmacokinetics. Wilcoxon matched-pairs signed-rank test demonstrated marked improvement in all 20 patients who were formerly refractory, increasing from a median baseline vitamin D level of 29.2 ng/mL to 58 ng/mL at end of study (P < .0001). All patients demonstrated improvement in serum vitamin D level by week 4 on study, some of whom had been refractory for years prior. Median dose was 1 OTF strip (40 000 IU) per week. No toxicity was observed. This formulation proved to be safe, effective, efficient, and well received. We are eager to explore other patient populations, which might benefit from this promising development, and other therapeutics that might be optimized using this mode of delivery. This trial was registered at www.clinicaltrials.gov as #NCT04818957.
Introduction
The role of vitamin D in bone health and skeletal maturation has long been established because it facilitates the absorption of calcium and enables normal growth and bone remodeling. However, in more recent medical literature, it has come to light that vitamin D has a widespread and diverse set of biological actions, with receptors scattered throughout the body,1,2 playing a significant role in many pathophysiological processes. Similarly, vitamin D deficiency has been implicated in an extensive list of severe diseases, and inferior outcomes are often associated with deficiency.3-6
Specifically, in our cohort of patients undergoing hematopoietic stem cell transplant (HSCT), vitamin D deficiency is shown to be exceedingly common before, during, and after transplant.7 Perhaps even more worrisome is that the vitamin D deficiency uncovered in this vulnerable population was persistent and associated with decreased overall survival.7-11 Compared with deficient vitamin D levels, normal vitamin D levels before transplant and in the early posttransplant period have been associated with a lower incidence of graft-versus-host disease (GVHD), lower levels of proinflammatory cytokines, and improved immune reconstitution.11-13 With aggressive enteral supplementation, we have been able to significantly improve outcomes and quality of life for many of these children.7,9,10
However, the sustainability of improved serum vitamin D levels presents another challenge. The morbidity associated with HSCT is significant and, often, severely affects the health and function of the gut. Enteral vitamin D supplementation is dependent on passive diffusion of the fat-soluble vitamin across the intestine.14,15 We previously demonstrated that it is very challenging to achieve and sustain therapeutic vitamin D levels in pediatric recipients of HSCT using currently available vitamin D formulations (capsules or liquid), even after assuring patient compliance.9 Many patients with severe malnutrition, diarrhea, and/or gastrointestinal GVHD after HSCT are unable to absorb enterally administered vitamin D and are refractory to supplementation, with serum vitamin D levels poorly responsive to ongoing supplementation even after assuring compliance with prescribed therapy. Furthermore, with a high incidence of mucositis and severe nausea/vomiting, and many young patients in our population, taking medication capsules is a painful struggle, and often not possible.
We report significant improvement in vitamin D levels in patients who were previously refractory by using oral thin film (OTF) cholecalciferol, a novel, berry-flavored formulation of vitamin D, which dissolves readily when placed on the tongue and has the ability to bypass, at least in part, the need for absorption in the gut that allowed our study participants to achieve and sustain normal 25-hydroxy vitamin D (25-OH vitamin D) levels of ≥35 ng/mL. OTF formulation also significantly improves the ease of administration, making it well received by patients, and facilitating sustainable therapeutic vitamin D levels.
Methods
Study design
Our single site, prospective, pilot study to evaluate the safety, efficacy, and tolerance of OTF cholecalciferol in repleting serum vitamin D levels in patients receiving bone marrow transplant was approved by the institutional review board at Cincinnati Children’s Hospital Medical Center. This study was clinically registered (#NCT04818957). Our primary aim was to achieve and sustain serum 25-OH vitamin D levels >35 ng/mL during a 12-week study period of vitamin D supplementation. This level was based on the Endocrine Society guidelines for bone metabolism, defining 25-OH vitamin D level <30 ng/mL as insufficient, with 25-OH vitamin D levels from 30 ng/mL to 40 ng/mL required for physiologic affect via parathyroid hormone.16
Patient selection
We aimed to evaluate 20 patients who had undergone or were undergoing HSCT and had 25-OH vitamin D levels ≤35 ng/mL or who had been unable to tolerate or were refractory to standard enteral supplementation. Inclusion criteria were recipients of HSCT of any age with vitamin D levels ≤35 ng/mL, unable to tolerate, or refractory to enteral supplementation formulations of vitamin D. Inability to take prescribed vitamin D formulation or noncompliance was confirmed by reviewing nursing documentation in medical records. Patients could be undergoing HSCT or have completed HSCT at any time in the past; both inpatients and outpatients were eligible to enroll. Exclusion criteria were any patients with vitamin D level ≥100 ng/mL and any patients with clinically significant and uncontrolled hypercalcemia. Written, informed consent to participate was obtained from each enrolled patient and/or legal guardian. It is important to note that patients enrolled into this study had been on enteral supplementation and had remained vitamin D deficient despite using other vitamin D formulations in escalating doses for ∼3 to 23 months before this study. In addition, all study patients had received a single ultrahigh dose vitamin D (Stoss therapy) at the start of HSCT without adequate or sustained response.10 Stoss therapy, from the German word for “to push,” is a single megadose of vitamin D, which has been proven to be effective in repleting vitamin D levels in many chronic diseases. Stoss dose in our recipients of HSCT had been administered as a single dose based on patient weight and serum vitamin D level, with a maximum dose limit of 600 000 IU, as previously published.10
Vitamin D OTF supplementation and outcome measurement
Baseline serum vitamin D (25-OH vitamin D), calcium, and phosphorus were obtained for all patients. These levels were monitored throughout enrollment, weekly for the first 4 weeks, and at least monthly thereafter, with more frequent evaluations performed as clinically indicated. End-of-study serum vitamin D levels were obtained within 2 weeks after final OTF administration. Serum vitamin D levels were measured via chemiluminescent immunoassay, and all other blood tests were performed using biochromatic end point technique.
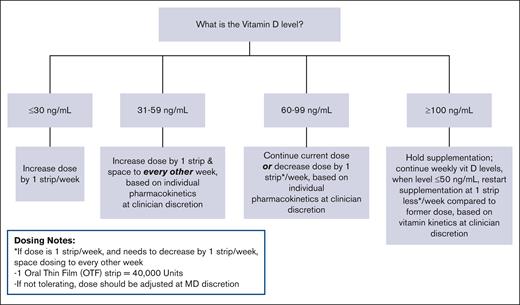
All patients stopped other forms of vitamin D supplementation at study entry when vitamin D OTF administration was started. Supplementation with cholecalciferol OTF strips (CURE Pharmaceutical, Oxnard, CA) was initiated based on patient weight, in accordance with institutional standard of care. Each OTF strip contains 40 000 IU vitamin D3 (1000 μg cholecalciferol). Patients with body weight <40 kg at the time of enrollment received 1 strip for their initial dose, and patients with body weight ≥40 kg at the time of enrollment received 2 strips. Dosing was titrated based on response and pharmacokinetics of the individual, in accordance with our dosing schema shown in Figure 1, based on prior institutional experience.
Patients in the inpatient ward or those receiving their dose at an outpatient clinic were observed by medical staff to verify ingestion. Vitamin D OTF strips were berry-flavored and dissolved readily when placed on the tongue; patients were permitted to take a small sip of water immediately before or after the strip dissolved, as desired. Outpatients taking OTF strips at home maintained a study diary to document dosing. Patients were required to complete at least 6 weeks of the 12-week study to be considered evaluable. Patients with clinically significant and/or uncontrolled hypercalcemia were excluded from the study.
Each patient received OTF strips for a maximum of 12 weeks, with the ability to stop supplementation at the discretion of the physician if vitamin D level was adequate and additional supplementation was not needed. Any patients with difficulty tolerating the OTF could have their dose adjusted or discontinued at the discretion of the physician.
Clinical care
Routine clinical care for patients during and after HSCT was continued for all patients, in accordance with institutional standards of care. All patients on study were concurrently managed by a registered dietitian.
Statistical analysis
Continuous data were summarized per their median values. Differences in outcomes were compared using a Wilcoxon matched-pairs signed-rank test. Statistical evaluation was performed using GraphPad Prism (version 9.2.0). P < 0.5 was considered statistically significant.
Results
Study population
A total of 24 patients were enrolled in this study, with the goal of 20 evaluable patients. Four patients were not evaluable: 2 patients had to take multiple OTFs for each dose and disliked the texture with repeated administration, 1 disliked the taste, and 1 discontinued because of parental noncompliance.
Demographics and disease characteristics of patients who were evaluable are summarized in Table 1. Among our patient population, some patients were refractory to Stoss therapy (n = 6) and some to Stoss therapy and enteral supplementation (n = 10), whereas others were unable to tolerate enteral supplementation formulations (n = 4). Fourteen patients had underlying clinical conditions or transplant complications predisposing them to impaired intestinal absorption: thrombotic microangiopathy (n = 6), inflammatory bowel disease (n = 3), pancreatic insufficiency (n = 2), malacoplakia (n = 1), stage 4 intestinal GVHD (n = 1), and a history of gastrointestinal vaso-occlusive crisis (n = 1).
Efficacy of OTF cholecalciferol on serum vitamin D levels in patients who received HSCT
Twenty patients were evaluable. The median number of weeks on study for these 20 patients was 12 weeks. There were 2 patients in our cohort who did not complete all 12 weeks: 1 patient unexpectedly relapsed after transplant, and family preference was to come off study at that time (8 weeks) and another patient returned to their home institution before completion (9 weeks). Serum vitamin D levels of our cohort on study are outlined in Table 1. The median age of our cohort was 8 years (range, 1-28 years).
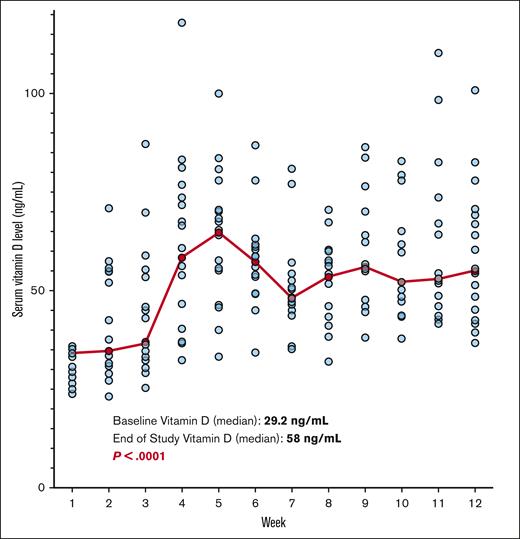
All study patients achieved the primary study end point to reach and sustain a 25-OH vitamin D serum level of ≥35 ng/mL. Study patients’ serum vitamin D levels improved from a baseline median of 29.2 ng/mL (range, 23.9-35.8 ng/mL) at enrollment to 58 ng/mL (range, 36.7-100.8 ng/mL) at the end of study (P < .0001; Figure 2).
Weekly patient vitamin D levels are shown from baseline to 12 weeks while on study. Each blue dot represents 1 patient. Median values each week shown in red.
Weekly patient vitamin D levels are shown from baseline to 12 weeks while on study. Each blue dot represents 1 patient. Median values each week shown in red.
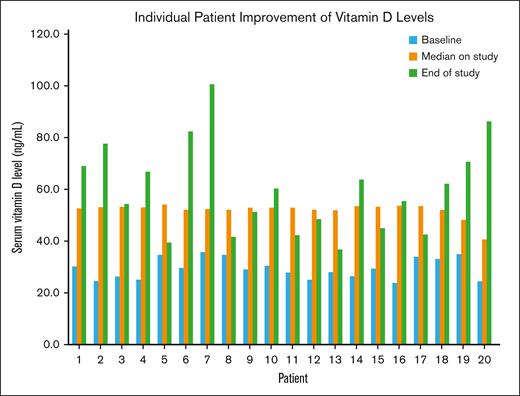
Median improvement of vitamin D levels while on study was an increase by 29 ng/mL, effectively doubling baseline vitamin D levels at the end of study. All 20 patients demonstrated improved vitamin D levels within the first 4 weeks, with consistent median values on study of 53 ng/mL (range, 40.8-54.1 ng/mL; Figure 3). Some patients’ levels escalated quickly, prompting dose reduction to maintain target levels. The dosing schema was updated after enrollment of the first few patients secondary to the observed rapid absorption. The median dose for our cohort was 40 000 IU (1 strip) per week. Doses of vitamin D during the 12 weeks on study ranged from 40 000 IU monthly to 160 000 IU weekly.
Individual patient serum vitamin D levels are shown at baseline, median while on study, and at the end of study, demonstrating marked improvement. Vitamin D levels doubled across the 12 weeks on study.
Individual patient serum vitamin D levels are shown at baseline, median while on study, and at the end of study, demonstrating marked improvement. Vitamin D levels doubled across the 12 weeks on study.
Tolerance and toxicity of OTF cholecalciferol
Ability to take vitamin D OTF and compliance with prescribed study therapy was either documented by nursing staff in medical records or reported to the study staff by caregivers for those receiving OTF at home. OTF strips were well received by patients of all ages, with palatable flavor and rapid dissolution. Patients who were enrolled in this study because of intolerance of or noncompliance with other vitamin D formulations were documented to have good compliance with OTF. Eight study patients (40%) were aged <5 years, with 4 aged <2 years, and all were able to successfully take the OTF strips, which dissolved within seconds and did not need to be swallowed. Peak median vitamin D levels for the cohort were noted at week 5 on study, at 64.7 ng/mL, and these results were well sustained over the remaining weeks on study. The maximum serum vitamin D level on study was 118 ng/mL, trending down to 65.3 ng/mL the following week. No toxicity was noted for any of the patients during the study; observed elevations of >100 ng/mL were noted for 1 week only. Concurrent monitoring of renal panels, including calcium and phosphorus levels, did not demonstrate any concerning elevations. There were no adverse events attributable to the study drug while taking the vitamin D OTF strips.
Discussion
This prospective pilot study demonstrates the safety and efficacy of a novel formulation of cholecalciferol, administered as a readily dissolvable OTF, for repletion and maintenance of therapeutic serum vitamin D levels in patients who had received HSCT who are refractory to other available vitamin D formulations. The vitamin D OTF strips were also very efficient in repleting serum vitamin D levels, with all patients achieving therapeutic vitamin D levels (>35 ng/mL) by week 4. Improved levels were likewise sustained throughout 12 weeks on study, with median serum vitamin D levels on study ranging from 40.8 to 54.1 ng/mL for all 20 patients. This was especially noteworthy for patients who had been refractory to enteral supplementation using other formulations of vitamin D supplements for months or years before this study enrollment yet rapidly achieved therapeutic vitamin D levels after switching to comparable dosing with OTF formulation.
In addition to noting marked improvement in serum vitamin D levels for all patients enrolled, OTF was easy to administer, readily dissolved without the need for swallowing, and was received well by patients of all ages on study. Those patients who reported disliking the OTF had been taking multiple OTF strips for a single dose; we then adjusted our administration strategy, giving 1 OTF at a time with a short break in between strips, which resolved this issue. There were no complaints from patients taking 1 OTF strip at a time. Numerous patients and families expressed how much they enjoyed the flavor and ease of this formulation. They also expressed a preference for the OTF formulation and an interest in being able to continue OTFs at the completion of the 12 weeks of study. The wide range of ages enrolled highlights the versatility of this mode of delivery, with our youngest patient aged 13 months tolerating strips as well as our oldest patient who was aged 28 years.
It is well-known that the bioavailability of vitamin D differs among individuals because of variable absorption or altered metabolism in the body. Oral vitamin D formulations are influenced by several factors after ingestion, including gastric pH; gastric enzymes, including pepsin and trypsin; and duodenal enzymes, such as proteases, amylase, and lipase. Our patient cohort was enriched with cases prone to prolonged impaired bowel and/or pancreatic function due to their underlying pretransplant conditions, such as Shwachman-Diamond syndrome, immune deficiencies with inflammatory bowel disorder, or transplant complications such as intestinal GVHD and intestinal thrombotic microangiopathy, that rendered these patients vitamin D deficient even later after transplant.
This new vitamin D OTF formulation is absorbed, at least in part, through the oral mucosa directly into the blood stream, bypassing the first-pass hepatic metabolism. It also does not cause additional discomfort in patients with mucositis because of its fast dissolution and no need to swallow. This is very appealing in a complex patient population, such as recipients of HSCT with altered gastrointestinal tract function. OTF also lightly adheres to the tongue while being dissolved by saliva, likely contributing to improved mucosal absorption. Administration of OTF is straightforward for caregivers, readily observed, reliably absorbed, and well tolerated. For these reasons, OTF is desirable compared with other liquid formulations, especially for young children.
As could be expected, pharmacokinetics in our study population were patient dependent, likely multifactorial, with significant variability in dosing required to achieve vitamin D repletion. In our cohort this variability was not strictly related to patient age or body weight but likely related to a patient’s clinical condition. We observed ongoing increases in serum vitamin D levels up to 2 or 4 weeks after administration of a dose. We analyzed weekly vitamin D levels for the first 4 weeks to learn vitamin D OTF kinetics in our study subjects. The majority of patients achieved and sustained therapeutic vitamin D levels by using 1 OTF strip per week (40 000 IU per week), although we noted a wide range of needs, with patients on study requiring between 1 strip per month (40 000 IU per month) and up to 4 strips per week (160 000 IU per week). This supports our prior observations that monthly monitoring of vitamin D kinetics is essential in recipients of HSCT, especially early after transplantation.
Notwithstanding the variability noted in our study in dosing amount and frequency, there were no instances of vitamin D toxicity in our cohort. There is significant variability in the literature concerning levels at which vitamin D toxicity is observed, ranging from 120 to 300 ng/mL,2,17-19 with serum levels demonstrating poor correlation with calcium levels and clinical symptomatic presentation. In general, toxicity is observed only in patients with sustained, prolonged elevations in their serum vitamin D levels. Common signs and symptoms of vitamin D toxicity include nausea, vomiting, anorexia, diarrhea, bone pain, fatigue, weakness, and nephrolithiasis.2,17,18,20,21 Severe cases involving seizure, coma, and even death have been reported.18 In our cohort, 3 of 20 patients were noted to have serum vitamin D levels >100 ng/mL, with a maximum of 118 ng/mL, although none of these levels were sustained for >7 days nor were they associated with any clinical symptoms of toxicity. Serum calcium and phosphorus levels were normal throughout.
Although our prospective pilot study includes only 20 patients, the wide range of ages represented helped to answer essential study questions about efficacy, tolerance, and compliance across different age groups. Based on the promising results of this study, vitamin D OTF has been approved as a formulary option for recipients of HSCT at our institution. We are encouraged by the efficacy and ease of administration of using an OTF formulation for treatment of vitamin D deficiency. This novel mode of delivery was especially helpful in this vulnerable population of patients who received HSCT, who experience many barriers surrounding medication absorption and compliance secondary to complications encountered after bone marrow transplant. We are eager to explore other important therapeutics for this vulnerable patient population, which might be optimized by this mode of delivery. In addition, we are working to collaborate with clinicians who care for patient populations with similar challenges and barriers to vitamin D repletion, such as patients with short gut, Crohn’s disease, celiac disease, and cystic fibrosis, to further expand the reach of this novel, exciting, and promising therapeutic.
Acknowledgments
The authors are indebted to the children and families who participated in this study for their invaluable contribution to this research. The authors are grateful for the regulatory assistance of Evelyn Nguyen, Adesuwa Ekunwe, and Jennifer Bravo. The authors appreciate the support of Vered Gigi and the team at CURE Pharmaceutical. The study drug was provided by CURE Pharmaceutical at no cost to study participants.
Authorship
Contribution: A.L.B., G.W., K.C.M., A.T-C., C.T., B.P., R.D., S.M.D., and S.J. designed the research; A.L.B., G.Z., G.W., S.M., A.T-C., C.T., and S.J. performed research; B.P. and R.D. supplied the study drug; A.L.B., G.Z., G.W., S.M., K.C.M., A.T-C., C.T., S.M.D., and S.J. analyzed data; and all authors contributed to writing the manuscript.
Conflict-of-interest disclosure: S.J. holds US patent No. US 10,815,296 B2; is the lead principal investigator for National Institutes of Health–funded multi-institutional study investigating TA-TMA (R01HD093773); and has received travel support and honoraria for lectures from Omeros, Alexion, and Sobi, all unrelated to this project. K.C.M. has an investigator-initiated clinical trial sponsored by Incyte and serves as the primary investigator of an industry-sponsored Elixirgen trial, both unrelated to this project. The remaining authors declare no competing financial interests.
Correspondence: Allison L. Bartlett, 3333 Burnet Ave, MLC 11027, Cincinnati, OH 45229; e-mail: allison.bartlett@cchmc.org.
References
Author notes
Study data will be shared in the form of scientific publication as part of our continued, systematic research on vitamin D in recipients of HSCT, with the goal to improve clinical management. Published material will have deidentified data on demographics, disease features, vitamin D levels, supplementation mode, and intervention outcomes of recipients of HSCT. This publication is immediately available to the research community.
Original data are available on request from the corresponding author, Allison L. Bartlett (allison.bartlett@cchmc.org).