Key Points
Patients with CLL receiving B-cell directed therapy, including BTK and BCL2 inhibitors, are less likely to seroconvert with COVID vaccines.
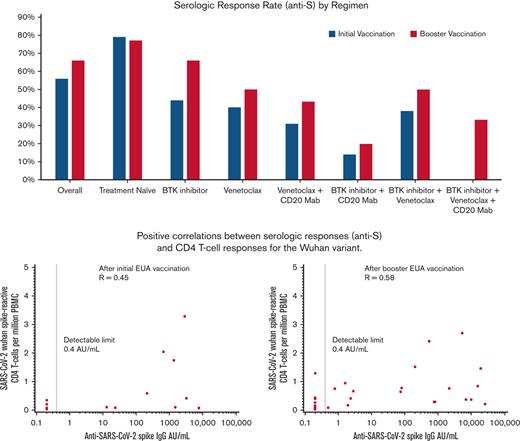
Positive correlations were observed between serologic responses and CD4 T-cell responses in the Wuhan variant.
Abstract
Previous studies have demonstrated low rates of seroconversion to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) messenger RNA (mRNA) vaccines in patients with chronic lymphocytic leukemia (CLL). In this national collaboration of 11 cancer centers in the United States, we aimed to further characterize and understand vaccine-induced immune responses, including T-cell responses, and the impact of CLL therapeutics (#NCT04852822). Eligible patients were enrolled in 2 cohorts (1) at the time of initial vaccination and (2) at the time of booster vaccination. The serologic response rates (anti-S) from 210 patients in the initial vaccination cohort and 117 in the booster vaccination cohort were 56% (95% confidence interval [CI], 50-63) and 68% (95% CI, 60-77), respectively. Compared with patients not on therapy, those receiving B-cell-directed therapy were less likely to seroconvert (odds ratio [OR], 0.27; 95% CI, 0.15-0.49). Persistence of response was observed at 6 months; anti-S titers increased with the administration of booster vaccinations. In the initial vaccination cohort, positive correlations were observed between the quantitative serologic response and CD4 T-cell response for the Wuhan variant and, to a lesser degree, for the Omicron variant (Spearman P = 0.45 Wuhan; P = 0.25 Omicron). In the booster vaccination cohort, positive correlations were observed between serologic responses and CD4 T-cell responses for both variants (P = 0.58 Wuhan; P = 0.57 Omicron) and to a lesser degree for CD8 T-cell responses (P = 0.33 Wuhan; P = 0.22 Omicron). Although no deaths from coronavirus disease 2019 (COVID-19) have been reported after booster vaccinations, patients should use caution as newer variants emerge and escape vaccine-induced immunity. This trial was registered at www.clinicaltrials.gov as #NCT04852822.
Introduction
Patients with chronic lymphocytic leukemia (CLL) suffer from an impaired immune system characterized by defects in B-cell and T-cell numbers and function, hypogammaglobulinemia, and neutropenia, which can be further exacerbated by anticancer therapy. As such, patients with CLL are at an increased risk of infection, which is known to be a leading cause of death due to this disease. Risk factors for coronavirus disease 2019 (COVID-19), a syndrome caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, overlap with many clinical features of CLL, such as older age, immunodeficiency, and medical comorbidities. We previously demonstrated that COVID-19 is associated with a high mortality rate of up to 37% in patients with CLL before the implementation of SARS-CoV-2 vaccines; therefore, prevention of SARS-CoV-2 viral infection and its associated inferior outcomes especially in vulnerable populations are of critical importance.1 Four vaccines have been authorized for administration in the United States: BNT162b2 messenger RNA (mRNA) vaccine (Pfizer/BioNTech, Berlin, Germany), mRNA-1273 mRNA vaccine (Moderna, Cambridge, MA), Ad26.COV2.S viral vector vaccine (Johnson & Johnson, New Brunswick, NJ), and NVX-CoV2373 subunit protein vaccine (Novavax, Gaithersburg, MD). mRNA vaccines are particularly potent in eliciting coordinated CD4 and CD8 T-cell responses. CD4 T cells are required for potent and durable antibody responses, whereas both CD4 and CD8 provide a second line of defense against viral infection.2,3 Unfortunately, due to the same immune challenges and suboptimal experiences with other types of vaccines, there has been significant concern regarding the efficacy of these vaccines in the CLL population.4 Decreased rates of seroconversion have previously been noted with the influenza and pneumococcal vaccines in patients with CLL.5,6 Additionally, impaired T-cell responses have been noted with the recombinant zoster vaccine in those receiving Bruton tyrosine kinase (BTK) inhibitors.7 Researchers have demonstrated serologic response rates of 40%-52% with the first 2 doses of the mRNA vaccines, with considerably lower serologic conversion rates in patients with CLL receiving B-cell directed therapy.8,9 Recent data have also indicated that some patients with CLL do not develop memory B-cell or T-cell responses after SARS-CoV-2 vaccination.10 We aimed to further characterize and understand the vaccine-induced immune response in patients with CLL, with particular interest in the influence of CLL therapeutics and in the T-cell responses.
Methods
Study design and sample collections
In this national, prospective, observational study, patients were enrolled from 11 academic cancer centers (#NCT04852822). This study was approved by the Institutional Review Board (IRB) and was conducted in accordance with the Declaration of Helsinki. The eligibility criteria were diagnosis of CLL or small lymphocytic lymphoma (SLL), age ≥18 years, and no known history of SARS-CoV-2 infection. The patients may have received any of the Food and Drug Administration (FDA) authorized vaccines. The primary objective was to evaluate the immune response to SARS-CoV-2 vaccine in patients with CLL/SLL. The secondary objectives were to understand the predictors of vaccine immune responses and COVID infection rates.
The patients were enrolled in 2 cohorts (1) at the time of the initial 2-dose mRNA vaccine or 1-dose adenoviral vector vaccine, and (2) at the time of the subsequently authorized initial (first) booster vaccine. Patients enrolled in the first cohort must have enrolled within 4 months of receiving their initial vaccination, whereas those enrolled in the second cohort must have enrolled within 3 months of booster vaccination. The patients in the initial cohort were permitted to enroll in the booster cohort. Demographic information, vaccine details, and disease information were collected. The patients underwent serologic assessment at enrollment and at 6 and 12 months. Clinical assessments of SARS-CoV-2 infection, booster vaccinations, and therapeutic interventions were performed at 6, 12, 18, and 24 months.
A subset of patients underwent peripheral blood mononuclear cell (PBMC) T-cell assays. Patient selection was based on clinical site PBMC capabilities and per-patient medical suitability for phlebotomy to supportsupport this assay. Heparin-anticoagulated blood was processed for PBMC isolation using routine Ficoll-Hypaque density gradient centrifugation and was cryopreserved. PBMC were shipped to the immune monitoring laboratory on dry ice and were transferred to liquid nitrogen.
Antibody responses
The gold standard and key method for assessing functional immune response in the original trials that led to SARS-CoV-2 vaccine approvals was neutralization assays; however, these tests are not readily available to patients. We have recently shown that in patients with CLL, use of the Roche Elecsys Anti-SARS-CoV-2 S anti-Spike (anti-S) immunoglobulin G (IgG) assay, a semiquantitative total antibody assay against the spike protein receptor binding domain (anti-S), is a reasonable surrogate for the SARS-CoV-2 spike D614G pseudotyped lentivirus neutralization assay.11 In this study, serologic response was assessed in all via the Roche Elecsys Anti-SARS-CoV-2 S assay. The reference range for negative results was <0.8 AU/mL. The Roche Elecsys Anti-SARS-CoV-2 Nucleocapsid (anti-N) assay was also used to assess prior COVID infection. Reference range for a negative result was <1.0 cutoff index. Patients with serologic evidence of infection were excluded from analysis. A subset of patients was assessed via SARS-CoV-2 spike D614G pseudotyped lentivirus neutralization assays (based on the Wuhan variant).12,13 The serum was heated at 56°C for 30 minutes, diluted 1:10, followed by six 1:3 serial dilutions to 1:7290, and run in duplicate to determine neutralizing dilution 50 (ND50) and ND80 values. External quality assurance was performed using the EQAPOL SARS-CoV-2 antibody assay monitoring program. Reference interval for a negative result was ND50 <20.
T-cell responses
Responses to SARS-CoV-2 S were assessed by intracellular cytokine staining (ICS) method modified from those used to study a recombinant zoster vaccine in persons with CLL.7 Stimuli included pools of overlapping peptides (OLP), 15 amino acids long overlapping by 11 amino acids, which covered the full length of S protein from SARS-CoV-2 strain Wu-1 (PM-WCPV-S-2; JPT, Berlin, Germany) or SARS-CoV-2 Omicron variant (PM-SARS2-SMUT08-2; JPT). The Wu-1 OLP was used as 2 subpools covering the N- and C-terminal halves of the S protein, whereas the Omicron OLP comprised of only one pool. Net data from the 2 Wuhan subpools were summed for statistical analysis. For all peptide pools, the final concentration of each peptide was 1 μg/mL in 0.2% dimethyl sulfoxide (DMSO). The negative control was 0.2% DMSO and the positive control was 1.6 μg/mL phytohemagglutinin (Remel, Lenexa, KS). Omicron OLP was limited to samples acquired later in the study owing to dynamic changes during the SARS-CoV-2 pandemic. PBMC were thawed and resuspended to 2 × 106 PBMC per mL of T-cell medium (RPMI-1640; TCM; containing 4% defined fetal bovine serum (Hyclone), 4% human serum (Valley Biomedical), 2 mM L-glutamine (Hyclone), and 100 U/mL penicillin/streptomycin (Gibco)). Approximately 1 × 106 PBMC were removed to estimate the percentages of T, B, and natural killer (NK) (TBNK) cell, and the remainders were rested overnight at 37°C.
For TBNK testing, cells were stained with LIVE/DEAD Fixable Near-IR (ThermoFisher), anti-CD45-FITC (clone 2D1; Biolegend), anti-CD19-PE (clone SJ25C1; Biolegend), anti-CD3-ECD (clone UCHT1; Beckman Coulter), anti-CD16-PacificBlue (clone 3G8; Biolegend), and anti-CD56-APC (clone HCD56; Biolegend). Events captured using a four-laser BD Canto II flow cytometer (University of Washington Cell Analysis Facility) were evaluated using FlowJo (v10 for Mac; BD).
ICS testing was performed as described in reference 7.7 Events were recorded with BD Fortessa, analyzed with FlowJo, and processed for detection of the activation markers interferon- γ (IFN-γ), interleukin-2 (IL-2), TNF-α, and CD40L (CD154) in either gated live CD3+CD4+CD8- (CD4 T cells) or live CD3+CD4-CD8+ (CD8 T cells) cells.5 The frequencies of activated CD4+ T cells with ≥2 activation markers (CD42+) and activated CD8 T cells (total IFN-γ positive) were determined. Specimens unreactive to PHA or with <10 000 CD4 T cells in peptide exposed wells were excluded from analysis. Serial specimens were tested in the same assay run, whenever possible. The results are reported as the percentage of activated CD4 or CD8 T cells in response to S peptide stimulation after subtraction of responses to DMSO per sample.
Statistical methods
Scatter plots between T-cell and serologic responses were created, with serologic responses plotted on a log scale due to the wide range of values. Spearman’s rank correlation coefficient was estimated between the 2 responses; logistic regression was used to assess the association between various factors and serologic response modeled as a binary outcome. Linear regression was used to assess the association between various factors and T-cell response, where response levels were replaced by their ranks due to the wide range of observed values, with rank used as the outcome variable. The factors examined included gender, race, absolute lymphocyte count (ALC), years since diagnosis, months from CD20 monoclonal antibody use, treatment-naïve status, Rai stage, type of therapy, treated vs untreated, and vaccine type.
Results
Patient characteristics
The enrollment consisted of 210 patients in the initial cohort and 117 patients in the booster cohort (Table 1). At the time of data cut-off, 60 patients from the initial cohort were enrolled in the booster cohort. In all, 11 patients were excluded from the analysis due to serologic evidence indicating COVID infection before enrollment (8 from the initial cohort and 3 from the booster cohorts), resulting in 202 and 114 evaluable patients for analysis, respectively. The patient characteristics are detailed in Table 1. The median ALC was 2.96 × 103/μL and 5.06 × 103/μL. Most patients did not receive anti-CLL therapy at the time of vaccination (58%, 55%); 35% and 39% were treatment-naïve. For patients in the initial cohort who were previously treated, the median time off therapy before vaccination was 20 months (range, 1-184 months).
Serologic responses
Of the 202 patients enrolled in the initial cohort, the serologic response rate (anti-S ≥0.8 AU/mL) assessed within 4 months of vaccination was 56% (95% confidence interval [CI], 50-63). The median time between the initial vaccination (second dose for the 2-dose mRNA vaccine) and the first serologic response assessment was 59 days (range, 8-124). Among the 114 patients enrolled in the booster cohort, the serologic response rate assessed within 3 months of the first booster vaccination was 68% (95% CI, 60-77). The median time between the booster and first serologic laboratory assessment was 40 days (range, 7-128). The median time between the initial vaccination and the first booster vaccination was 169 days (range, 34-282). Among treatment-naïve patients, the serologic response rates were 79% for the initial cohort and 77% for the booster cohort. Among patients receiving B-cell directed therapies, including anti-CD20 monoclonal antibodies, BTK inhibitors, B-cell lymphoma 2 (BCL-2), inhibitor, and venetoclax, we observed lower serologic response rates and anti-S levels (Table 2). Notably, none of the patients enrolled in this study received cytotoxic chemoimmunotherapy at the time of vaccination.
By univariate logistic regression models, clinical features that correlated with a positive serologic response in the initial cohort were female gender (odds ratio [OR], 1.96; 95% CI, 1.10-3.50) and treatment naïve status (OR, 4.70; 95% CI, 2.41-9.15). Increasing age in years (OR, 0.96; 95% CI, 0.93-0.99; age modeled as a continuous linear variable in units of one year) and Rai Stage (global P = .03, with increasing stage associated with a decreased probability of response) were associated with a lower likelihood of response, whereas greater time (months) since anti-CD20 monoclonal antibody was associated with a greater chance of response (OR, 1.02; 95% CI, 1.00-1.03; time modeled as a continuous linear variable in units of one month). Compared with patients not on therapy, those receiving B-cell-directed therapy were also less likely to respond (OR, 0.27; 95% CI, 0.15-0.49). A multivariable model among these factors was fit, and the individual factors of treatment-naïve status and receipt of B-cell-directed therapy were combined because all patients who received B-cell-directed therapy were not treatment naïve. This resulted in a new variable with 3 categories, as summarized in Table 3. In addition, Rai stage was not included in the multivariable model, as it did not show sufficient evidence of an association after including other factors (global P = .56). Months since anti-CD20 receipt were also not included, as 55% of the patients were missing such data.
Clinical features that correlated with a numerically positive serologic response in the booster cohort were female gender (OR, 1.58; 95% CI, 0.68-3.66) and treatment naïve status (OR, 2.01; 95% CI, 0.85-4.73). Increasing age in years was associated with a lower likelihood of response (OR, 0.97; 95% CI, 0.93-1.01); further time from anti-CD20 monoclonal antibody exposure was associated with greater chance of response (OR, 1.02; 95% CI, 1.00-1.04). Including each of these factors in a multivariable model caused each association to draw closer to the null (ie, the OR became closer to 1, Table 3). There was no demonstrable correlation between the type of vaccination and the serologic response in either cohort.
Serial assessments were available for 83 patients in the initial cohort to assess the persistence of serologic responses. Of the 47 patients who had a positive serologic response after the initial vaccination, 46 remained positive at 6 months. The median time between the initial vaccination and serologic assessment was 179 days (range, 153-237). Anti-S titers decreased in 20 patients by a median value of 121 AU/mL (range, 0.1-13684). One patient who had a very weak positive response (1.14 AU/mL) decreased to a negative value of 0.62 AU/mL by 6 months. Of the 36 patients who had no initial serologic response, 9 had detectable anti-S levels at 6 months. Three COVID-19 infections were reported during this timeframe. Subsequent nucleocapsid antibody testing was not performed.
Seventy-five patients had serologic assessments (anti-S) available before and after booster vaccination. Of the 42 patients who had positive serologic responses before the booster, 36 noted an increase in the serologic titer after the booster. The median increase in anti-S level was 11238 AU/mL (range, 18-24697). Of the 33 patients who had no evidence of serologic response to the primary vaccination series, 12 patients responded to the booster (36%), with a wide variability in anti-S titer: median, 48.6 AU/mL (range, 1.2-5228).
Pseudotyped lentivirus neutralization assays were performed on 20 patients in the initial cohort. Of the 9 patients with positive responses (ND50 value ≥20), 8 had positive coinciding anti-S responses (median, 2973 AU/mL; (range, 84.7-16950)) (supplementary Table 1). Of the 11 patients with negative neutralization assay results, 9 had negative anti-S results and 2 had weakly positive responses (12.7 and 22.9 AU/mL).
T-cell responses
T-cell correlative analyses were performed on 17 patients in the initial cohort and 34 patients in the booster cohort (Table 4). In the initial cohort, 59% of the patients were not on treatment, whereas 41% were receiving BTKi monotherapy (n = 5) or in combination with venetoclax (n = 1). The booster cohort was characterized by 44% of patients not receiving therapy and 56% receiving B-cell directed therapy. Pre and postvaccination samples were available for 8 patients in the initial cohort and 5 patients in the booster cohort, whereas only postvaccination samples were available for the remaining 9 and 29 patients, respectively. All patients were evaluated for their response to the Wuhan variant; 10 patients in the initial cohort and 30 patients in the booster cohort were also evaluated for their response to the Omicron variant.
Because there is no consensus for what numerical T-cell level constitutes a “response,” we evaluated T-cell response as a continuous quantitative outcome, using the rank of the response level as the outcome in various simple linear regression models. The factors examined included gender, race (Caucasian vs other), ALC, age, years since diagnosis (each of the latter 3 was modeled as a continuous linear variable), and treatment status. In the initial cohort, increasing years since diagnosis and increasing age were each associated with a decreased CD8 T-cell response (P = .043 for years and the Wuhan variant, P = .030 for age and the Omicron variant), and females had an increased CD8 T-cell response to the Omicron variant (P = .035). Other factors that showed some evidence of an association with response level but were not definitive included years since diagnosis and CD4 T-cell response level for the Wuhan variant (increasing years, decreased response (P = .064)) and ALC and CD8 T-cell response level for the Omicron variant (increase ALC, increased response (P = .072)). There was no apparent association with other factors (P > .163). In the booster cohort, age and years since diagnosis showed associations that should be considered for further study for CD4 and CD8 T cells and both variants (increasing age/years, decreased T-cell response for all; P = .065 for age and CD4 Wuhan, P = .053 Omicron; P = .007 for years and CD4 Wuhan, P = .014 Omicron; P = .036 for age and CD8 Wuhan, P = .060 Omicron; P = .001 for years and CD8 Wuhan, P = .011 Omicron). In addition, white patients had a lower numerical CD8 T-cell response for the Omicron (P = .083) and Wuhan variants (P = .094). All other associations in the booster cohort were insignificant (P > .133).
In multivariable models, years since diagnosis was the only factor that was demonstrably associated with the outcome of CD4 response to the Wuhan variant for both initial and booster vaccines, as well as CD4 and CD8 responses to the Omicron variant for booster vaccines. supplementary Table 2 summarizes the multivariable models for CD8 response to each variant for initial vaccines, as well as for the Wuhan variant for booster vaccines.
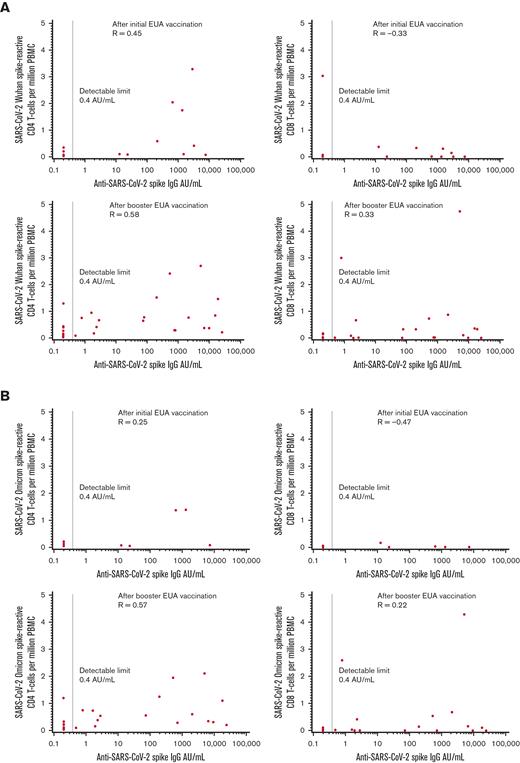
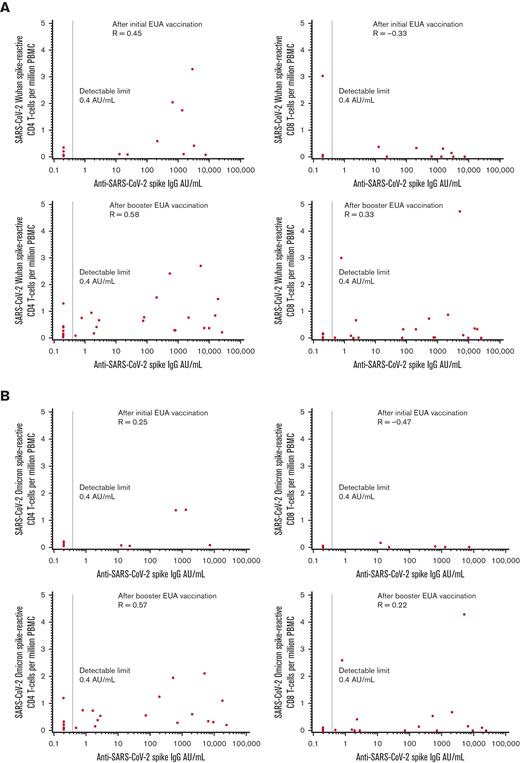
The cognate antigen theory of CD4 T cell/B cells suggests that CD4 T cell and antibody levels may be positively correlated. CD4 and CD8 T-cell responses to the Wuhan and Omicron variants were compared with anti-S values (Figure 1A-B). In the initial cohort, positive correlations were seen between quantitative serologic response and CD4 T-cell response for each variant, but the magnitude of the correlation was modest, at best, for the fewer number of patients evaluated for the Omicron variant (Spearman P = 0.45 for Wuhan; Spearman P = 0.25 for Omicron). The correlations between serologic response and CD8 T-cell response were negative for each variant (Spearman P = -0.33 for Wuhan; Spearman P = -0.47 for Omicron). In the booster cohort, positive correlations were seen between serologic response and CD4 T-cell responses for both variants (Spearman P = 0.58 Wuhan; Spearman P = 0.57 Omicron) and to a lesser degree with CD8 T-cell responses (Spearman P = 0.33 Wuhan; Spearman P = 0.22 Omicron).
Correlations between anti-S serologic response and T-cell response. (A) Wuhan and (B) Omicron variants in the initial and booster vaccination cohorts.
Correlations between anti-S serologic response and T-cell response. (A) Wuhan and (B) Omicron variants in the initial and booster vaccination cohorts.
COVID-19 infections
After enrollment, 12 patients in the initial cohort reported a new SARS-CoV-2 infection after vaccination. Only 4 had seroconverted to the vaccine before their reported infection (median anti-S, 173; range, 2.7-666 AU/mL). The time frame of the infections in patients who seroconverted correlated with the delta and omicron variants in the United States; however, the variant testing results were not available. Three deaths occurred due to COVID-19, and none of these patients experienced seroconversions. The median time from onset of symptoms to death was 15 days (range, 13 days-2 months). Prophylactic tixagevimab with cilgavimab was not FDA-authorized before the onset of symptoms. None of the patients received therapeutic antibodies, but 2received remdesivir. In the booster cohort, 24 patients developed a SARS-CoV-2 infection after the first booster vaccine. Sixteen of these patients had detectable anti-S after the booster vaccination (median, 448; range, 0.87->25 000 AU/mL) before the development of COVID-19. The time frame for these infections was correlated with the emergence of the omicron variant. All the infected patients in both cohorts received mRNA vaccines only. Four patients in the booster cohort received prophylactic tixagevimab with cilgavimab before the infection. At the time of this analysis, no deaths had been reported in the booster cohort.
Discussion
Here, we present the results of the largest multicenter academic collaboration in the United States that prospectively evaluated the immunogenicity of SARS-CoV-2 vaccines in CLL/SLL. Our data confirm those of previous studies, noting decreased serologic response rates in all subgroups, notably in those receiving B-cell directed therapy (Table 1).8,9 Although not formally compared, we found that the anti-S levels measured in the initial cohort appeared lower than those previously reported in vaccinated health care workers using the same Roche assay.14 Specifically, the median anti-S titer in responding patients in our study was 202 AU/mL, whereas it was noted that uninfected health care workers mounted levels of 1100 to 2800. Unfortunately, our data set could not clarify the clinical impact and protective benefits of lower anti-S titers. We found that the serologic response persisted over time and could be augmented in responding patients with the addition of a booster vaccination. The median anti-S titer after booster vaccination was 5350 AU/mL, which primarily reflected patients not receiving CLL-directed therapy. Among those who did not respond to initial vaccination, 36% showed seroconversion. Although ongoing therapy could explain much of this, it is not yet clear whether additional boosters or further administration of heterologous vaccines could influence the outcomes. Similar to our previous findings, the anti-S Roche assay appeared to be a reasonable surrogate for testing for the neutralization assay, at least for the Wuhan variant.11
mRNA and adenovirus vaccines are newer formats with the ability to stimulate CD8 T cells, in addition to coordinated CD4 T-cell and antibody responses. The relative importance of antibodies and T cells may vary with regard to the prevention of infection and disease, but both likely contribute to overall clinical efficacy. CD4 T-cells are known to contribute to the antibody response to foreign antigens. Consistent with this, we observed significant positive associations between CD4 T-cell responses to the S antigen, as measured by ICS, and IgG responses to the S protein. As CD4 T-cells also provide factors such as IL-2 to support CD8 T-cell expansion, the direct correlation observed between CD8 T-cell responses and anti-S IgG is interpreted as a consequence of the CD4 T-cell response assisting both antibodies and CD8 T cells. Given the relatively preserved sequences of Wuhan and Omicron variants and the multiple CD4 T-cell epitopes within S typically recognized per person, it is not surprising that the CD4-IgG association was observed for T-cell responses to both viral strains. Strong CD8 T-cell responses after SARS-CoV-2 vaccinations have recently been reported in lymphoma patients after receipt of rituximab.15
One limitation of this study was that most patients did not undergo laboratory assessment before vaccination. This was in part due to the rapid rollout of vaccinations, as well as the successful identification and education of a vulnerable population. Second, there were some limitations to assessing SARS-CoV-2 infections noted in this study. After baseline labs were performed at enrollment, anti-N was not assessed at subsequent visits; therefore, asymptomatic infections that patients were unaware of were not captured. Additionally, variant testing of the infected individuals was not performed in this study. Lastly, only 6 patients underwent heterologous vaccination; therefore, we were unable to draw any meaningful conclusions regarding the potential benefit of this strategy.
Given the ongoing pandemic, patients with CLL, particularly those receiving current standard-of-care therapy with BTK inhibitors +/− anti-CD20 antibody or venetoclax + anti-CD20 antibody therapy, remain a vulnerable population of concern. Although prophylactic anti-SARS-CoV-2 Mab are beneficial, they are not a permanent solution as they have been shown to lose efficacy with newer SARS-CoV-2 variants.16 It is important to continue modifying our approach for these patients. As BTK inhibitors are administered indefinitely, using combinations with BCL-2 inhibitors to achieve shorter courses of therapy may be beneficial. However, in addition to confirming comparable long-term efficacy, we need to first understand how much time off therapy would allow for a successful immune response and whether resumption of therapy would affect previously achieved immunity. Given that anti-S levels decrease over time, necessitating the need for boosters, as well as the immune escape seen as the virus continues to mutate, brief interruptions in therapy are unlikely to be a viable long-term option. Notably, only 35 patients developed COVID-19 over the course of this study, and only 3 deaths were observed, all of the patients who did not generate an immune response. This highlights the importance of attaining some degree of vaccine-mediated immunity as well as the importance of ongoing mitigation strategies and therapeutics.
To this end, patients should be educated about the increased morbidity and mortality associated with COVID-19 and the need for precautions at every level, including masking high-risk situations and utilizing preventive interventions. Patients should be educated about the potential clinical implications of lower anti-S titers as well as the variability in thresholds for positive serologic response among the commercially available assays. As newer variants emerge that escape vaccine-induced immunity, patients should continue to use caution. Future studies should continue to define the role of additional vaccines, especially for specific viral subtypes, and the impact of Mab and antiviral medications.
Acknowledgments
The authors thank University of Washington Center for Emerging and Re-emerging Infectious Diseases GLP Flow Cytometry Laboratory. The authors also thank the University of Washington Cell Analysis Facility.
This research was supported by a grant from CLL Global Research Foundation and National Institutes of Health contract 75N93019C00063 (D.M.K.).
Authorship
Contribution: C.U., P.A.T., S.E.S., D.M.S., C.L., C.M.B., S.O., A.M.W., G.P., M.S.D., M.S., T. Siddiqi, K.A.R., A.V.D., and J.A.H. designed the research; C.U., P.A.T., K.J.L., D.M.K., A.L.G., A.S., G.Q., C.J.B., B.T., and H.Z. performed the research; C.U., P.A.T., S.E.S., D.M.S., C.L., C.M.B., S.O., A.M.W., G.P., M.S.D., M.S., A.S., G.Q., and T. Sorensen. collected the data; T.A.G. performed the statistical analysis; K.L. performed the research regulatory work; and C.U., P.A.T., T.A.G., K.J.L., D.M.K., A.L.G., S.E.S., D.M.S., C.L., C.M.B., S.O., A.M.W., G.P., M.S.D., M.S., T. Siddiqi, K.A.R., A.V.D., and J.A.H. analyzed and interpreted the data and wrote the manuscript.
Conflict-of-interest disclosure: C.U. has received consulting fees from Atara Biotherapeutics, AbbVie, AstraZeneca, Beigene, Curio Science, Epizyme, Genentech, Incyte, Janssen, Loxo/Lilly, Pharmacyclics, and research support from AbbVie, Adaptive Biotechnologies, AstraZeneca, Loxo/Lilly, Pharmacyclics. S.O. has received consulting fees from AbbVie, Alexion, Amgen, Aptose Biosciences Inc., Astellas, AstraZeneca, Autolus, Beigene, Bristol Myers Squibb (BMS), Celgene, Dynamed, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Johnson & Johnson, Juno Therapeutics, MEI Pharma, Merck, Nova Research, Pfizer, Pharmacyclics, TG Therapeutics, Vaniam, Verastem, Vida Ventures, and research support from Acerta, Alliance, Beigene, Caribou Biosciences, Gilead, Kite, Loxo, Mustang, Nurix, Pfizer, Pharmacyclics, Regeneron, and TG Therapeutics. A.M.W. has received consulting fees from Janssen, Seattle Genetics, and ADC Therapeutics. M.S.D. has received consulting fees from AbbVie, Adaptive Biosciences, Aptitude Health, Ascentage Pharma, AstraZeneca, Beigene, BMS, Celgene, Curio Science, Eli Lilly, Genentech, Janssen, Merck, Ono Pharmaceuticals, Research to Practice, TG Therapeutics, Takeda, and research support from AbbVie, AstraZeneca, Ascentage Pharma, Genentech, MEI Pharma, Novartis, Surface Oncology, and TG Therapeutics. K.A.R. received consulting fees from AstraZeneca, Genentech, Pharmacyclics, AbbVie, Innate Pharma, and Beigene, travel funding from AstraZeneca; and research support from Genentech, AbbVie, Novartis, and Janssen. A.L.G. has received contract testing from Abbott, Cepheid, Novavax, Pfizer, Janssen, Hologic, and research support from Gilead and Merck, outside of the described work. D.M.K. has received research support from Merck, Oxford Immunotec, Sensei Biotherapeutics, Sanofi Pasteur; is a member of Scientific Advisory Board for Curevo Vaccine and MaxHealth LLC; and has received in-kind contribution of free immunosequencing assays as a component of collaborative research on COVID-19 illness and vaccination with Adaptive Biotechnologies, Inc. J.A.H. received consulting fees from Gilead Sciences, Amplyx, Allovir, Allogene Therapeutics, CRISPR Therapeutics, CSL Behring, OptumHealth, Octapharma, Takeda, and research funding from Takeda, Allovir, Karius, Merck, Deverra, and Gilead Sciences. M.S. has received consulting fees from AbbVie, Genentech, AstraZeneca, Sound Biologics, Pharmacyclics, Beigene, BMS, Morphosys/Incyte, TG Therapeutics, Innate Pharma, Kite Pharma, Adaptive Biotechnologies, Epizyme, Eli Lilly, Adaptimmune, Mustang Bio, Regeneron, Merck, Fate Therapeutics, MEI Pharma and Atara Biotherapeutics, and research support from Mustang Bio, Celgene, BMS, Pharmacyclics, Gilead, Genentech, AbbVie, TG Therapeutics, Beigene, AstraZeneca, Sunesis, Atara Biotherapeutics, Genmab, Morphosys/Incyte, and Vincerx. P.A.T. has received consulting fees from AbbVie, Genentech, Pharmacyclics, Janssen, Adaptive Biotechnologies, Beigene, and Lilly, and research support from AbbVie, Genentech, Lilly, Adaptive Biotechnologies, and Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Chaitra Ujjani, Fred Hutchinson Cancer Center, 825 Eastlake Ave, Seattle, WA 98109; e-mail: ujjani@uw.edu.
References
Author notes
Data are available upon request from the corresponding author, Chaitra Ujjani (ujjani@uw.edu).
The full-text version of this article contains a data supplement.